SUMMARY
Immune responses in diverse tissue sites are critical for protective immunity and homeostasis. Here, we investigate how tissue localization regulates the development and function of human natural killer (NK) cells, innate lymphocytes important for anti-viral and tumor immunity. Integrating high-dimensional analysis of NK cells from blood, lymphoid organs, and mucosal tissue sites from 60 individuals, we identify tissue-specific patterns of NK cell subset distribution, maturation, and function maintained across age and between individuals. Mature and terminally differentiated NK cells with enhanced effector function predominate in blood, bone marrow, spleen, and lungs and exhibit shared transcriptional programs across sites. By contrast, precursor and immature NK cells with reduced effector capacity populate lymph nodes and intestines and exhibit tissue-resident signatures and site-specific adaptations. Together, our results reveal anatomic control of NK cell development and maintenance as tissue-resident populations, whereas mature, terminally differentiated subsets mediate immunosurveillance through diverse peripheral sites.
Graphical Abstract
In Brief
A study of the distribution and function of natural killer cells across various human tissues reveals anatomic control of their development as well as populations that mediate immunosurveillance systemically.
INTRODUCTION
Natural killer (NK) cells are innate lymphocytes that can directly kill target cells without any prior exposure while not damaging healthy ‘‘self’’ cells. Unlike T and B cells that recognize antigen through clonally distributed, somatically rearranged receptors, NK cells recognize their targets through integrating signals from multiple germline-encoded activating and inhibitory receptors that recognize major histocompatibility complex class I (MHC class I) molecules (Colonna et al., 1999; Lanier et al., 1986). In humans, NK cells (CD3−CD56+) are relatively abundant, comprising 5%–20% of lymphocytes in blood and other sites (Freud et al., 2017), and play key roles in anti-viral and anti-tumor immunity. NK cells are required for control of acute and persistent viral infections such as Herpesviruses, and in cancer, NK cells promote direct lysis of multiple tumor types, and patrol diverse sites to prevent metastasis (Cerwenka and Lanier, 2001; Imai et al., 2000; Kelly et al., 2002; Morvan and Lanier, 2016; Orange, 2006; Scalzo et al., 2007; Vivier et al., 2012). These multiple aspects of NK cell functionality indicate their dynamic role in immune-mediated protection and homeostasis.
It has become increasingly clear that tissue localization is a critical determinant of the function and in vivo role of lymphocytes. Tissue-resident memory T cells (Trm) have been shown in mouse models to populate barrier, mucosal, and peripheral sites following antigen encounter, and are required for optimal protective immunity to pathogens in situ (Jiang et al., 2012; Masopust and Soerens, 2019; Muruganandah et al., 2018; Schenkel et al., 2014; Teijaro et al., 2011). In mice and humans, Trm exhibit cell surface phenotypes and transcriptional profiles distinct from circulating memory T cells (Gebhardt et al., 2018; Hombrink et al., 2016; Kumar et al., 2017; Mackay et al., 2016; Szabo et al., 2019), and Trm are the predominant T cell subset in humans (Thome et al., 2014). NK cells also populate multiple tissue sites including liver, lung, skin, kidneys, and bone marrow (BM) and a proportion express Trm markers CD69 and/or CD103 (Freud et al., 2017; Gaynor and Colucci, 2017; Geissmann et al., 2005; Hudspeth et al., 2016; Marquardt et al., 2017; Melsen et al., 2018; Montaldo et al., 2016; Shi et al., 2011; Sojka et al., 2014; Victorino et al., 2015). The role of tissue localization in human NK cell development and function, and how circulating NK cells relate to those in different sites, are not well understood. Human NK cells can be subdivided based on the level of expression of CD56 and the antibody binding-Fc receptor CD16 into two major subsets with distinct maturation and functional properties (Lanier et al., 1986; Nagler et al., 1989; Yu et al., 2013). CD56dimCD16+ NK cells are potent cytolytic effector cells, rapidly secreting pro-inflammatory cytokines (interferon [IFN]-γ, tumor necrosis factor alpha [TNF-α]) and cytotoxic mediators (granzymes and perforin) following receptor-mediated activation. By contrast, CD56brightCD16− NK cells exhibit reduced lytic capacity, although can produce IFN-γ when stimulated with cytokines, interleukin (IL)-12, and IL-18 (Aste-Amezaga et al., 1994; Carson et al., 1994; Cooper et al., 2001; Fehniger et al., 1999). Following stimulation with viruses, cytokines, or haptens, NK cells can also acquire memory-like properties and enhanced functional responses similar to adaptive memory T cells (Cooper et al., 2009; O’Leary et al., 2006; O’Sullivan et al., 2015; Sun et al., 2009, 2010, 2012). Memory-like NK cells responsive to cytomegalovirus (CMV) have been identified in mouse models of MCMV infection and in humans (Arase et al., 2002; Daniels et al., 2001; Dokun et al., 2001; Gumá et al., 2004, 2006; Lopez-Vergès et al., 2011). Understanding the distribution, function, and development of human NK cell subsets across multiple tissue sites, age, and individuals will provide essential insights into their role in immune responses and surveillance.
Here, we investigate how NK cells are distributed across tissues, ages, and individuals and the impact of tissue site on NK development and function, using a human tissue resource we established to obtain blood and multiple tissue sites from individual organ donors spanning nine decades of human life (Carpenter et al., 2018; Gordon et al., 2017; Granot et al., 2017; Kumar et al., 2017). We show that NK cells comprise a sizeable fraction of the lymphocyte compartment in blood, BM, spleen, and lung, where they are predominantly CD56dimCD16+; significantly lower frequencies of NK cells populate lymph nodes (LNs), tonsils, and intestines, and are mostly CD56bright CD16−. This tissue-intrinsic profile of NK cell frequency, subset distribution, and differentiation states is conserved between diverse individuals over a lifetime. Using high-dimensional transcriptional and phenotypic profiling, we show that precursor and immature NK cells in the lymph nodes and intestines exhibit tissue-resident molecular signatures and site-specific properties, whereas mature and terminally differentiated NK cells have similar effector function and shared gene expression profiles between sites. Our findings reveal tissue-mediated segregation of NK cell development and function and provide a blueprint for how NK cells are seeded, maintained, and surveil tissues across the body.
RESULTS
Tissue-Intrinsic Distribution of Human NK Cell Subsets
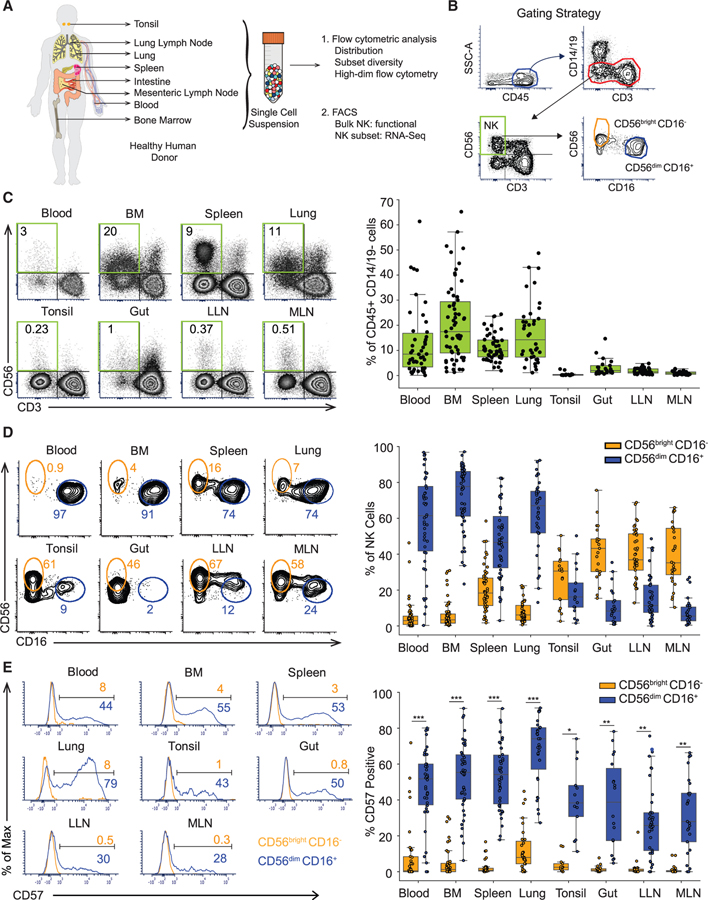
To analyze human NK cell distribution, maturation, function, and transcriptional profiles across different anatomic sites and compartments, we obtained blood, BM, secondary lymphoid organs (spleen, lung-draining lymph nodes [LLN], and mesenteric lymph nodes [MLN]), mucosal tissues (lungs, gut [small and large intestine]), and tonsils from human organ donors as described previously (Carpenter et al., 2018; Gordon et al., 2017; Granot et al., 2017; Kumar et al., 2017; Thome et al., 2014; Figure 1A). Tissues were obtained from 60 donors ranging in age from 5–92 years and representing a racially and ethnically diverse population (Table S1). NK cells were distinguished from other lymphocytes and myeloid lineage cells based on gating for CD45+CD3−CD14−CD19−CD56+ cells and further delineated into the major NK subsets, CD56brightCD16− and CD56dimCD16+ (Figure 1B). The tissue distribution of NK cells among CD45+ CD14−CD19− cells was consistent between donors with blood, BM, spleen, and lung having significantly higher frequencies of NK cells (5%–50%) compared to low frequencies (<0.1%–2%) of NK cells in the LNs, tonsil, and gut (Figure 1C and see Figure S1A, left panel for individualized data for each site per donor). BM contained a broader range of NK cell frequencies compared to other sites (Figure 1C), and donors with high NK cell content in BM also showed higher NK frequencies in blood, spleen, and/or lung (Figure S1A, left).
Figure 1. Human NK Cell Subset Distribution Is a Function of Tissue Site.
(A) Schematic diagram of the human tissues obtained and experimental workflow presented in this study.
(B) Gating strategy used for identification of NK cells and subsets by flow cytometry for analysis and sorting.
(C) NK cell distribution in different human tissue compartments depicted in representative flow cytometry plots (left, D344) showing NK cell (CD45+CD14−19−CD3−CD56+) frequency, and boxplots of compiled data from 18–55 donors (right). See also Figure S1A, left panel.
(D) Distribution of CD56brightCD16− (light orange) and CD56dimCD16+ (blue) NK cell subsets in multiple sites shown in representative flow cytometry plots (left, D337 and D344), and boxplots of compiled data from 18–55 donors for each site (right).
(E) CD57 expression by CD56bright CD16− (light orange) and CD56dimCD16+ (blue) NK cell subsets shown in representative flow cytometry plots (left, D344 and D345) and compiled data from 13–43 donors (right). See also Figure S1A, right panel. Dots on the boxplots show data collected for each individual donor. ***p ≤ 0.001; **p ≤ 0.01; *p ≤ 0.05. Gut refers to both large and small intestines, as we found similar NK cell and subset frequencies for these two sites (Figures S1B and S1C). BM, bone marrow; LLN, lung-draining lymph node; MLN, mesenteric lymph node.
The distribution of CD56brightCD16− and CD56dimCD16+ NK cell subsets was also a feature of the tissue site; CD56brightCD16− NK cells predominated in LNs, tonsil, and gut while the majority of NK cells in blood, BM, spleen, and lung were CD56dimCD16+ as shown in compiled data (Figure 1D) and for each individual donor (Figure S1A, middle panel). CD56dimCD16+ NK cells can be further subdivided into a terminally differentiated subset identified by CD57 expression, resulting from persistent stimulation by antigens or inflammatory signals (Björkström et al., 2010; Lopez-Vergès et al., 2010). The extent of CD57 expression by CD56dimCD16+ NK cells varied between sites: substantial frequencies (50%–70%) were CD57+ in blood, BM, spleen, and lung, while lower frequencies of CD57-expressing CD56dimCD16+ NK cells were present in LNs, tonsils, and gut (Figures 1E and S1A, right). These data further indicate that terminal differentiation of NK cells is more prevalent in sites containing an abundance of CD56dimCD16+ NK cells (blood, BM, spleen, and lungs), and excluded from sites such as LN and intestines.
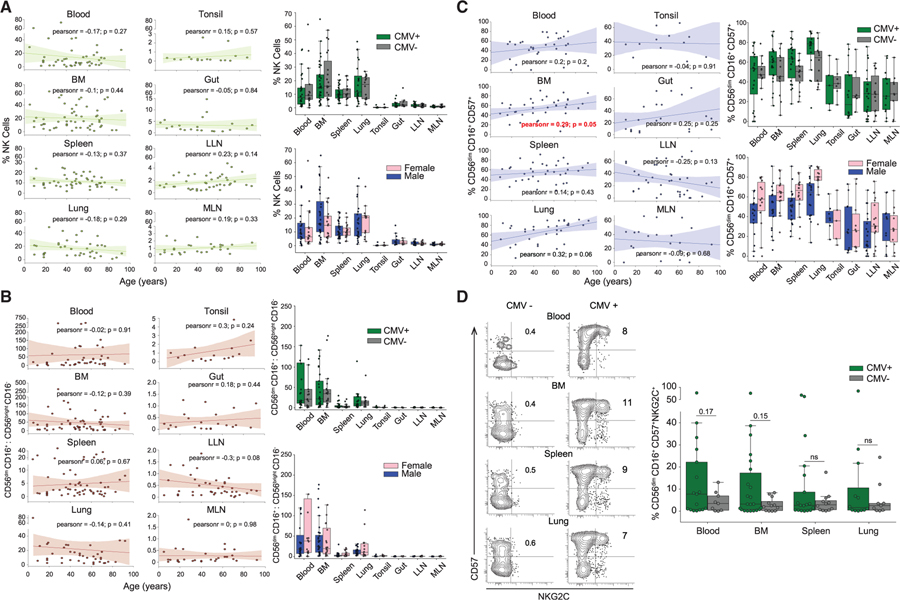
Role of Age, Sex, and CMV Status on NK Cell Subset Distribution
We investigated whether the overall distribution of NK cells or NK cell subsets in tissues varied according to age or CMV serostatus, which are known to affect lymphocyte subset differentiation and immunosenescence (Nikolich-Žugich, 2018; Pawelec and Derhovanessian, 2011). For determining age-associated effects, we performed a correlation analysis of NK cell and subset frequencies as a function of age, revealing consistent, site-specific frequencies of total NK cells and CD56brightCD16−:CD56dimCD16+ subset ratios from childhood to advanced ages (Figures 2A and 2B). Accordingly, the frequency and tissue distribution of NK cells and CD56dim/bright subsets did not vary significantly with CMV serostatus or sex of the donor (Figures 2A and 2B), consistent with NK cells being a component of innate immunity. The frequency of terminally differentiated CD56dimCD16+CD57+ NK cells showed a positive correlation with age in blood, BM, spleen, and lung, which achieved significance in the BM (p = 0.05; lung, p = 0.06), but showed no significant correlation with sex or CMV serostatus (Figure 2C). These results show minimal overt effects of age (over nine decades) and CMV serostatus on the global and site-specific distribution of NK cell subsets, but age-related effects on the accumulation of terminally differentiated NK cells in certain key sites.
Figure 2. NK Cell Subset Distribution in Tissues Is Independent of Age, Sex, and CMV Serostatus.
(A) Scatterplots showing frequency of NK cells in blood and tissue sites as a function of age for each individual donor. The line of best fit was determined by Pearson correlation; the Pearson correlation coefficient for each comparison is designated as ‘‘pearsonr’’ in each plot (left). Right: NK cell frequency in different sites in donors stratified by CMV serostatus (CMV+, seropositive; CMV−, seronegative) and sex (male or female).
(B) Scatterplots showing the ratio of CD56dimCD16+:CD56brightCD16− NK cells in blood and tissue sites as a function of age for each donor, with line of best fit and Pearson coefficient indicated as in (A). See also Figure S1A, middle panel. Right: the ratio of CD56dimCD16+:CD56brightCD16− NK cells in different sites in donors stratified by CMV serostatus (top) and sex (bottom).
(C) Scatterplots showing the distribution of terminally differentiated NK cells (CD56dimCD16+CD57+) in blood and tissue sites as a function of age for each donor; the line of best fit and Pearson correlation coefficient indicated in each plot (left). See also Figure S1A, right panel. Right: the distribution of terminally differentiated NK cells in different sites in donors stratified by CMV serostatus (top) and sex (bottom).
(D) Representative flow cytometry plots (left) showing the distribution of CMV-responsive (CD56dimCD16+CD57+NKG2C+) memory-like NK cells in a representative CMV-seronegative (CMV−, D344) and CMV-seropositive donor (CMV+, D349). Right: boxplots showing compiled distribution of CMV-responsive NK cells from HLA: C1, Bw4/C1, Bw4/C2, and Bw4/C1/C2 CMV+ (n = 12–17) and CMV− (n = 9–12) donors, data between the groups was compared using Mann-Whitney U test. See also Figure S2A. Dots on the boxplots show data collected for each individual donor. **p ≤ 0.01; *p ≤ 0.05; ns, non-significant. BM, bone marrow; LLN, lung-draining lymph node; MLN, mesenteric lymph node.
To further investigate effects of CMV seropositivity on NK cell subsets across sites, we probed for the presence of memory-like NK cells exhibiting CD56dimCD16+CD57+NKG2C+ phenotypes that were previously shown to delineate CMV-responsive NK cells (Hendricks et al., 2014; Lopez-Vergès et al., 2011). CMV-responsive NK cells were detected reliably in blood, BM, spleen, and lung (but not LN, tonsil, or gut) of CMV-seropositive donors but not significantly in CMV-seronegative donors, (Figure 2D). The presence of CD57+NKG2C+ NK cells was also associated with certain HLA types, with a strong association with HLA-Bw4 and HLA-C1/C2 group antigens (Faridi and Agrawal, 2011; Figure S2A). These data indicate that the accumulation of CMV-responsive memory-like NK cells occurs in blood and blood-rich sites (BM, spleen, and lung) in a subpopulation of seropositive individuals.
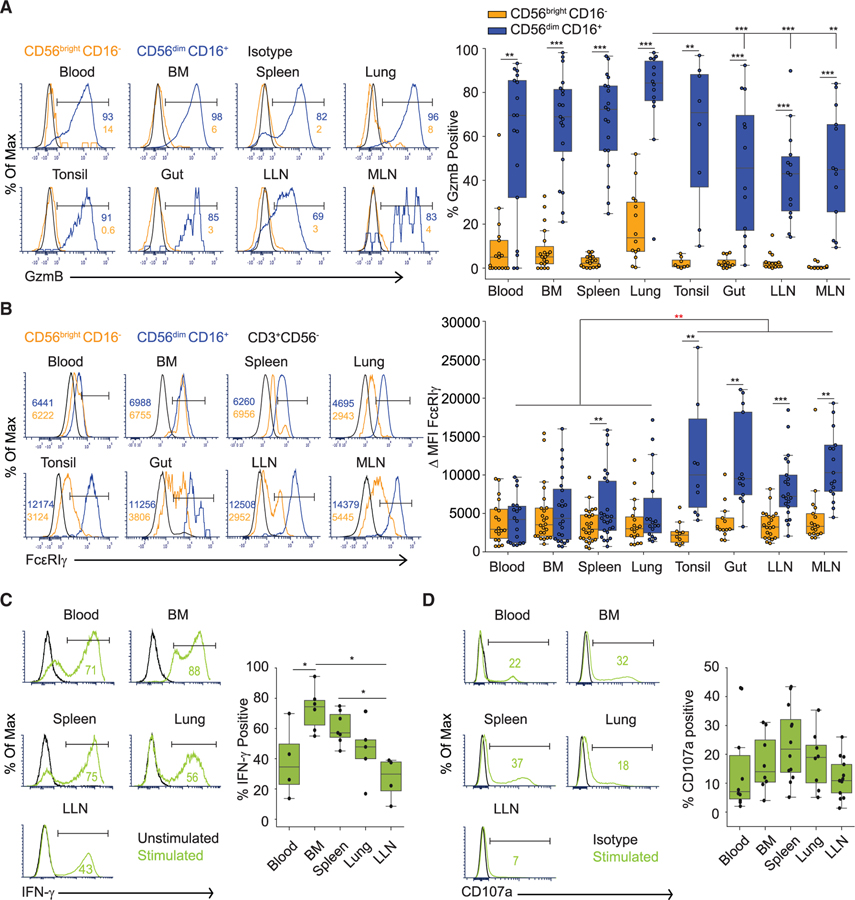
Tissue Site Shapes the Functional Potential of NK Cell Subsets
NK cells acquire granzyme B (GzmB) expression during the maturation process, and GzmB is one of the main cytolytic effector molecules produced by activated NK cells (Fehniger et al., 2007). When stained directly ex vivo, there was biased expression of GzmB (40%–80% GzmB+) in CD56dimCD16+ compared to CD56brightCD16− NK cells across blood and all tissue sites examined (Figure 3A). Between sites, the frequency of GzmB+ in CD56dimCD16+ NK cells is significantly higher in the lung compared to LNs and gut (Figure 3A). GzmB expression by CD56dimCD16+ NK cells is unaffected by age of the donors (Figure S2B). These results suggest conserved functional dichotomies between NK cell subsets across tissues, but tissue-specific variations in cytotoxic potential.
Figure 3. Tissue Localization Shapes the Functionality of NK Cells.
(A) Expression of granzyme B (GzmB) by NK cell subsets in different tissue compartments shown in representative histograms (left, D455 and D456) and graphs of compiled frequency of GzmB+ NK cell subsets from 8–19 donors (right). See also Figure S2B.
(B) Expression of the CD16 adaptor molecule FcεRIγ by NK cell subsets in different sites shown in representative histograms (left, D344); numbers in each plot represent median fluorescence intensity (MFI) of FcεRIγ for each subset (light orange, CD56brightCD16−; blue, CD56dimCD16+). Boxplots (right) show ∆MFI FcεRIγ (compiled from 10–25 donors) calculated by subtracting the FcεRIγ MFI of control (CD3+) cells from the FcεRIγ MFI of each NK cell subset. Comparison of ∆MFI from CD56dimCD16+ cells between LN and gut versus blood, BM, spleen, and lung indicated by red asterisk.
(C) IFN-γ secretion following culture of sorted NK cells from indicated sites with cytokines (see STAR Methods) shown as representative histograms (left, D388), and boxplots showing frequency of IFN-γ+ NK cells for each site compiled from 4–7 donors (right).
(D) CD107a expression following activation of NK cells from indicated sites by co-culture with K562 cells for 4 h (see STAR Methods) shown as representative histograms (left, D454), and boxplots showing frequency of CD107a+ NK cells for each site compiled from 8–13 donors (right). Dots on the boxplots show data collected for each individual donor. ***p ≤ 0.001; **p ≤ 0.01; *p ≤ 0.05. BM, bone marrow; LLN, lung-draining lymph node; MLN, mesenteric lymph node.
Antibody-mediated crosslinking of CD16 on NK cells elicits antibody-dependent cellular cytotoxicity (ADCC) through association of CD16 with the signaling adaptors FcεRIγ or CD3z (Lanier et al., 1989; Wang et al., 2015). The level of FcεRIγ expression is an indicator of ADCC capacity; NK cells that downregulate FcεRIγ and upregulate CD3ζ expression exhibit the most potent ADCC effector function (Lanier et al., 1989; Zhang et al., 2013). In all sites, there was a higher frequency of FcεRIγ+ cells within the CD56dimCD16+ compared to CD56brightCD16− NK subset (Figure S2C, left panel), consistent with the coordinated expression of FcεRIγ and CD16 in NK cells (Hibbs et al., 1989; Lanier, 2008). However, CMV seropositivity was associated with a slight increase in the frequency of functionally mature (CD56dimCD16+FcεRIγ−) NK cells (Figure S2C, right panel), consistent with the known effects of CMV on NK cells (Lee et al., 2015; Schlums et al., 2015). There were tissue-specific differences in the level of FcεRIγ expression; tonsils, gut, and LN CD56dimCD16+ NK cells express substantially higher levels of FcεRIγ compared to CD56dimCD16+ NK cells in blood, BM, spleen, and lungs (Figure 3B). Site-specific FcεRIγ expression did not vary with age (Figure S2D), suggesting that mature NK cells in blood, BM, spleen, and lung exhibit higher ADCC capacity compared to those in LN and intestines.
NK cells can also elicit effector function in response to cytokine stimulation (Zwirner and Ziblat, 2017). Culturing NK cells from blood, BM, spleen, lung, and LLNs with a cytokine cocktail (IL-12, IL-15, and IL-18) induced IFN-γ production with site-specific differences; BM NK cells had the highest frequency of IFN-γ producers followed by spleen, lung, and blood, with LN having the lowest frequency (Figure 3C). We also tested the functional potential of NK cells activated in the presence of a MHC class I-negative tumor cell line in vitro by staining for the expression of lysosomal-associated membrane protein 1 (LAMP-1: CD107a), a marker for degranulation (Alter et al., 2004). NK cells from all the sites showed similar CD107 expression and degranulation potential. Together, these functional analyses indicate site-specific variations in NK cell effector function, particularly in the capacity for cytokine production.
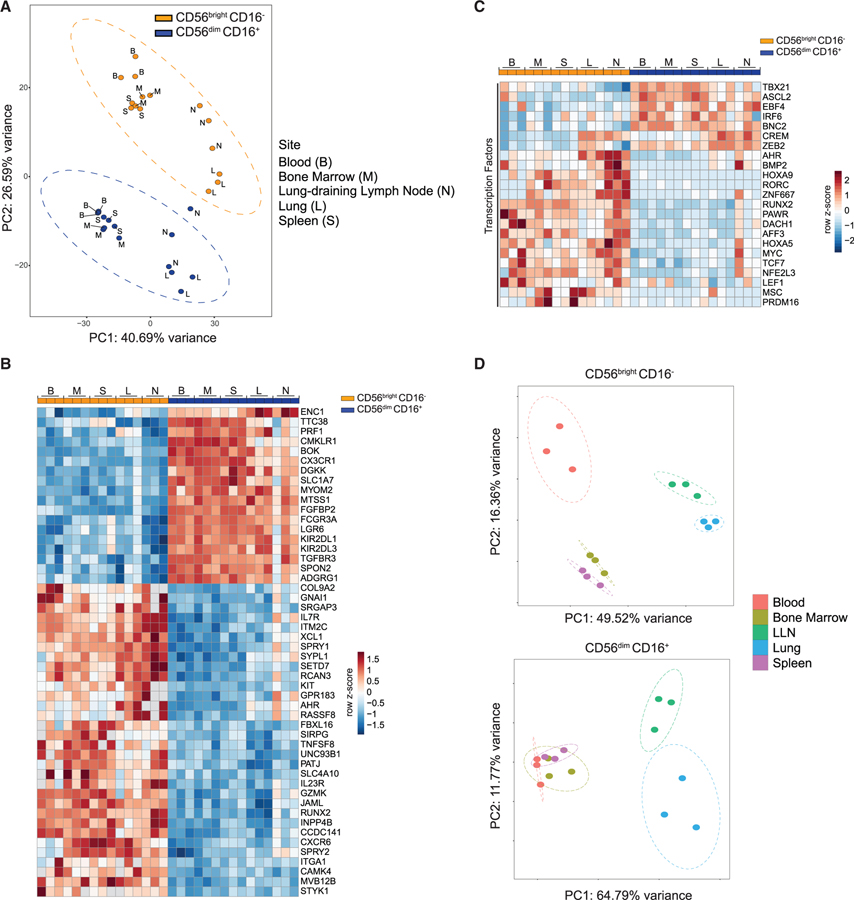
Whole Transcriptome Profiling Uncovers Subset- and Tissue-Specific Features of NK Cells
To identify the molecular basis of differential tissue distribution and functionality between NK cell subsets, we performed whole transcriptome profiling by RNA sequencing (RNA-seq) of CD56brightCD16− and CD56dimCD16+ NK cells sorted from blood, BM, spleen, lung, and LLN. Analysis of the whole transcriptome identified global signatures of 1,083 genes differentially expressed (DE) between CD56brightCD16− and CD56dimCD16+ NK cells (702 upregulated and 381 downregulated; Table S2) from these sites. Principal component analysis (PCA) shows subset and tissue-specific distinctions. Along PC2, the transcriptional profile of CD56brightCD16− NK cells clustered separately from that of CD56dimCD16+ NK cells in all the sites sampled, indicating distinct transcriptional profiles for each subset across sites (Figure 4A; see Figure S3A right for top PC2 loadings). Along PC1 within each subset, blood, BM, and spleen samples clustered together and distinct from lung and LLN samples (Figure 4A). The top genes within the common global signature of DE genes between CD56bright CD16− and CD56dimCD16+ NK cells from all sites is depicted in the heatmap in Figure 4B and includes genes associated with immune signaling and function as identified by Gene Ontology analysis (Table S3).
Figure 4. Transcriptional Analysis of CD56brightCD16− and CD56dimCD16+ NK Cells across Sites.
(A) PCA plot generated from top 500 differentially expressed (DE) genes between CD56brightCD16− and CD56dimCD16+ NK cell subsets isolated from 5 sites (blood, BM, spleen, lung, and lung-draining LN). See Table S2 for complete gene list.
(B) Heatmap of top 50 globally DE genes between CD56brightCD16− and CD56dimCD16+ NK cell subsets from all sites.
(C) Heatmap showing the top DE transcription factors between CD56brightCD16− and CD56dimCD16+ NK cells from all sites.
(D) PCA plot for CD56brightCD16− (top) and CD56dimCD16+ (bottom) NK cells isolated from five sites. Each dot in the PCA plots is a separate donor sample, and ellipses around samples indicate 95% confidence ellipses (see STAR Methods). B, blood; M, bone marrow; S, spleen; L, lung; N/LLN, lung-draining lymph node. See also Figures S3 and S4.
The global gene expression differences we identified between CD56brightCD16− and CD56dimCD16+ NK cells were conserved in all sites, revealing subset-specific profiles consistent with previous studies of NK subsets from single sites (Collins et al., 2019; Koopman et al., 2003; Marquardt et al., 2019; Melsen et al., 2018). Genes upregulated in CD56dimCD16+ NK cells include those associated with effector function (GZMB, GZMH, IFNG), killer cell immunoglobulin-like receptors (KIR), and tissue egress and circulation (S1PR5, S1PR1, CXCR1, CXCR2, and CX3CR1), while CD56brightCD16− NK cells express elevated levels of genes associated with immune regulation (CD27, TNFSF4, TNFSF8, TNFSF13, and TNFSF13B) and tissue homing (CCR7, SELL, CXCR3, and CCR5) (Figure S3B). CD56brightCD16− NK cells expressed genes encoding transcription factors involved in cellular stemness and quiescence (Kuo and Leiden, 1999; Zhou et al., 2010) including LEF1 and TCF7 (TCF-1), while CD56dimCD16+ NK cells, expressed genes encoding transcription factors associated with terminal differentiation and effector function including TBX21 (T-bet), ZEB2, and IRF6 (Figure 4C). Differential expression of TCF-1 and T-bet in CD56brightCD16− compared to CD56dim CD16+ cells in different sites was confirmed by flow cytometry (Figure S3C, top and bottom, respectively). Together, these analyses demonstrate that the core transcriptional identities of CD56brightCD16− and CD56dimCD16+ NK subsets are maintained in diverse anatomical sites.
NK cell subsets also exhibited site-specific variations in their transcriptional profile. Comparing the transcriptional profiles of tissue CD56brightCD16− and CD56dimCD16+ NK cells to their counterparts in blood identifies unique DE genes in tissue NK cell subsets (Figure S4A; Tables S4 and S5). Gene set enrichment analysis (GSEA) further reveals subset- and tissue-specific gene expression programs; both NK cell subsets in LLN were enriched for regulatory and tissue retention pathways, while in the lung, CD56brightCD16− NK cells were enriched for cell-adhesion and chemotaxis pathways, and CD56dimCD16+ NK cells were enriched for effector functional programs compared to other sites (Figures S4B and S4C). Moreover, PCA analysis for the individual subsets revealed more site-specific differences among CD56brightCD16− NK cells compared to CD56dimCD16+ NK cells. In particular, the transcriptional profile of CD56brightCD16− NK cells from blood, BM, and spleen clustered separately on the PCA plot, whereas CD56dimCD16+ NK cells from these sites clustered together (Figure 4D). In contrast, the transcriptional profile of both CD56brightCD16− and CD56dimCD16+ NK cell subsets in the lung and LLN were distinct from the corresponding subsets in blood and other tissue sites (Figure 4D) and showed increased sharing of transcriptional programs compared to these sites (Figures S4D and S4E). These results suggest a common origin for NK cells that populate the lung and the LLN. Taken together, our findings reveal that although NK cell subset transcriptional identity is preserved across sites, tissue localization drives further subset-specific transcriptional programs in NK cells.
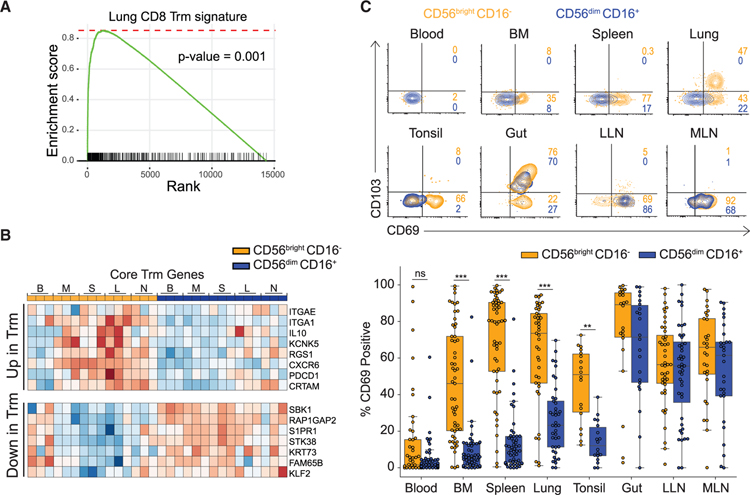
CD56brightCD16− NK Cells in Tissues Exhibit a Tissue-Resident Gene Expression Signature
We hypothesized that the tissue-adapted gene expression programs of CD56brightCD16− NK cells derives from their residence in tissues. GSEA comparing the transcriptome of CD56brightCD16− NK cells to the Trm gene signatures we previously identified in human tissue sites (Kumar et al., 2017) revealed significant enrichment of core signature genes of human lung and spleen CD8+Trm within CD56brightCD16− NK cells in tissues (Figure 5A). Core Trm gene expression signatures including upregulation of the integrin ITGAE (CD103), ITGA1, and the chemokine receptor CXCR6 and downregulation of tissue egress molecules S1PR1 and KLF2 (Kumar et al., 2017), distinguished tissue CD56brightCD16− NK cells from CD56brightCD16− NK cells in blood and CD56dimCD16+ NK cells in all sites (Figure 5B). There were also site-specific variations in the extent of expression of certain core genes incusing CXCR6, ITGA1, and ITGAE (Figure S5A), suggesting tissue-specific effects on the establishment of residency programs.
Figure 5. Tissue Residence Profile of CD56brightCD16− NK Cells.
(A) GSEA plot showing the enrichment of core tissue resident-memory T (Trm) cell genes within CD56brightCD16− NK cells from all tissue sites. See also Figure S5A.
(B) Heatmap showing the expression of select core Trm signature genes differentially expressed in tissue CD56brightCD16− NK cells and not expressed in blood CD56brightCD16− or CD56dimCD16+ NK cells in all sites.
(C) Cell surface expression of canonical tissue residence markers CD69 and CD103 on CD56brightCD16−(light orange) and CD56dimCD16+ (blue) NK cell subsets in different tissue compartments shown in representative flow cytometry plots (top, D337 and D351) and boxplots showing percent CD69+NK cells within each subset in different tissue compartments compiled from 18–40 donors. Dots on the boxplots show data collected for each individual donor. See also Figure S5B. ***p ≤ 0.001; **p ≤ 0.01. B, blood; M/BM, bone marrow; S, spleen; L, lung; N/LLN, lung-draining lymph node; MLN, mesenteric lymph node.
The expression of canonical TRM surface markers CD69 and CD103 by tissue NK cells showed subset- and site-specific variations. In BM, spleen, lungs, and tonsils, CD69 was expressed by significantly higher frequencies (35%–90%) of CD56bright CD16− compared to CD56dimCD16+ NK cells; however, in gut and LN, CD56brightCD16− and CD56dimCD16+ NK cell subsets exhibited comparable CD69 expression (Figure 5C). In mucosal sites (lungs, intestines) substantial frequencies of CD69+ NK cells co-expressed CD103 (Figure 5C). The frequency and distribution of CD69 expression by CD56brightCD16− NK cells in tissues was not altered with age (Figure S5B), suggesting that tissue residence is an intrinsic property. These findings show that CD56brightCD16− NK cells exhibit features of tissue residency in multiple sites, whereas CD56dimCD16+ NK cells bear signatures of circulating cells in blood, BM, and spleen, but can adopt resident phenotypes in LN and mucosal sites.
Tissue-Specific Properties and Heterogeneity of Tissue-Resident Cells
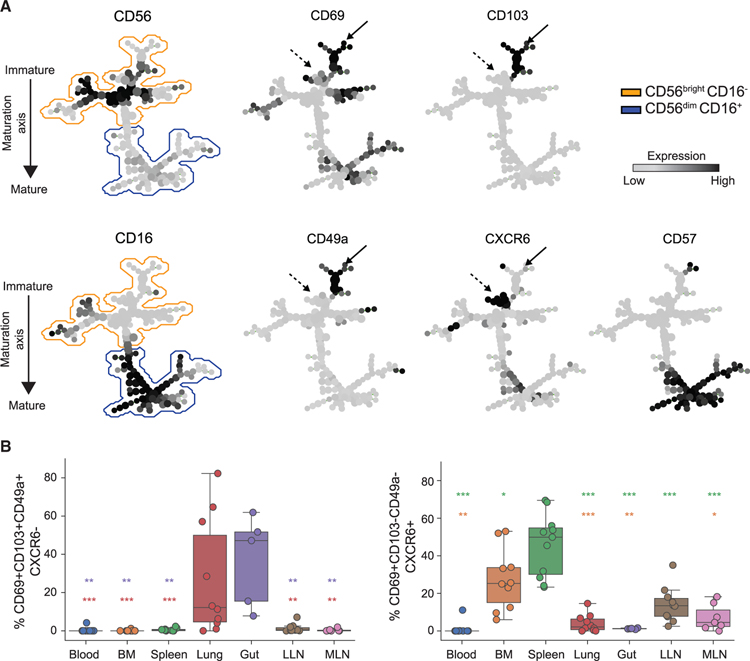
We assessed coordinate expression of key tissue residency markers CD69, CD103, CD49a, and CXCR6 (Kumar et al., 2017; Peng and Tian, 2017) to further investigate site-specific differences as suggested by the transcriptional data (Figure S5A) and define potential heterogeneity of putative tissue-resident NK (trNK) cells in different anatomical sites. Spanning-tree progression analysis of density-normalized events (SPADE) (Qiu et al., 2011) projection of NK cells from blood, BM, spleen, lung, gut, LLN, and MLN identified distinct branches of CD56brightCD16− NK cells that exhibit site-specific variations in expression of tissue-resident markers, consistent between five individual donors (Figures 6A and S5C). CD69 expression was detected in the majority of CD56+ NK cells, except for a specific branch of cells expressing the highest levels of CD56 that lack CD69 (Figures 6A and S5C), and may represent a circulating subset of immature NK cells. Other tissue-resident markers were differentially expressed; CXCR6 expression was exclusive of CD103 and CD49a expression and was mostly confined to BM, spleen, and LNs, while CD49a and CD103 were expressed together on subsets of CXCR6−CD69+NK cells in the lung and gut (Figures 6A, 6B, and S5C). These site-specific variations in CXCR6, CD49a, and CD103 expression in trNK subsets are consistent with variations in expression of the corresponding genes between sites (Figures 5B and S5A) and reveal tissue-specific segregation of trNK subtypes.
Figure 6. Heterogeneity of Tissue Resident NK Subsets across Sites.
(A) Representative SPADE plots of NK cells from blood, BM, spleen, lung, gut, LLN, and MLN stained with tissue-residence markers. The two plots on the left show the distribution NK cells along the maturation axis. The plots on the right show the expression and distribution of select residence markers and marker for terminal differentiation on NK cells along the maturation axis. Solid black arrow identifies CD103- and CD49a-expressing NK cell cluster and dashed black arrow identifies the CXCR6-expressing NK cell cluster. See also Figure S5C.
(B) Boxplots showing the distribution of CD69+CD103+CD49+CXCR6− (left) and CD69+CD103−CD49a−CXCR6+ (right) CD56brightCD16−, NK cells in indicated tissue sites compiled from 5–10 donors. Dots on the boxplots show data collected for each individual donor. Red asterisk, comparison between lung and tissue site; purple asterisk, comparison between gut and tissue site; orange asterisk, comparison between BM and tissue site; green asterisk, comparison between spleen and tissue site. ***p ≤ 0.001; **p ≤ 0.01; *p ≤ 0.05. BM, bone marrow; LLN, lung-draining lymph node; MLN, mesenteric lymph node.
Maturation and Developmental States of NK Cells across Tissues
NK cells undergo progressive development from CD56bright into CD56dim NK cells, involving expression of specific receptors associated with distinct maturation states (Abel et al., 2018; Scoville et al., 2017). For example, CD161 is one of the earliest markers expressed on NK cells during maturation from CD34+ hematopoietic stem cell precursors; inhibitory and activating receptors CD94/NKG2A, NKp46, NKp44, CD160, and NKG2D can be variably expressed in different maturation states, while expression of CD16 and multiple KIRs mark mature NK subsets, and CD57 indicates terminal differentiation. In order to trace the developmental origin of NK cell subsets in tissues, we implemented a high-dimensional flow cytometry panel incorporating multiple NK functional and developmental markers (Figure S6; Key Resources Table).
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Conventional flow cytometry | ||
| Anti-Human CD45 AF700 | BioLegend | RRID: AB_493761 |
| Anti-Human CD45 BV510 | BioLegend | RRID: AB_2561940 |
| Anti-Human CD14 BUV737 | BD-Biosciences | RRID: AB_2744285 |
| Anti-Human CD19 BUV737 | BD-Biosciences | Cat# 564304 |
| Anti-Human CD3 BV650 | BioLegend | RRID: AB_2563352 |
| Anti-Human CD56 PE-Cy7 | BioLegend | RRID: AB_2563927 |
| Anti-Human CD56 APC-Vio770 | Miltenyi Biotec | Cat# 130–100-690 |
| Anti-Human CD16 BV605 | BioLegend | RRID: AB_2562990 |
| Anti-Human CD57 PE Dazzle | BioLegend | RRID: AB_2564063 |
| Anti-Human CD159c NKG2C PE | R&D System | Cat# FAB138P-100 |
| Anti-Human FcεR1γ FITC | Millipore | RRID: AB_11203492 |
| Anti-Human GzmB AF700 | BD-Biosciences | RRID: AB_1645453 |
| Anti-Human Ifng BB700 | BD-Biosciences | RRID: AB_2744484 |
| Anti-Human CD107a BUV395 | BD-Biosciences | RRID: AB_2739073 |
| Anti-Human CD69 BV711 | BioLegend | RRID: AB_2566466 |
| Anti-Human CD103 BUV395 | BD-Biosciences | RRID: AB_2738759 |
| Anti-Human CXCR6 BV510 | BD-Biosciences | RRID: AB_2741610 |
| Anti-Human CD49a BV786 | BD-Biosciences | RRID: AB_2740720 |
| Anti-Human Tbet BV421 | BioLegend | RRID: AB_10896427 |
| Anti-Human TCF1 PE | Cell Signaling Technologies | RRID: AB_2798483 |
| Anti-Human CD127 BV421 | BioLegend | RRID: AB_10960140 |
| Anti-Human RORγt AF647 | BD-Biosciences | RRID: AB_2738324 |
|
High-dimensional flow cytometry | ||
| Anti-Human CD160 AF488 | BD Bioscience | RRID: AB_11153688 |
| Anti-Human CD27 BB660 | BD Bioscience | Clone:M-T271 |
| Anti-Human CD28 BB700 | BD Bioscience | Clone:CD28.2 |
| Anti-Human CD161 BB790 | BD Bioscience | Clone:DX12 |
| Anti-Human CD159c NKG2C PE | R&D System | Cat# FAB138P-100 |
| Anti-Human CD56 PE-CF594 | BD Bioscience | RRID: AB_2738983 |
| Anti-Human CD85J PE-Cy5 | BD Bioscience | RRID: AB_394021 |
| Anti-Human CD159a NKG2A PE-Vio770 | Miltenyi | RRID: AB_2726172 |
| Anti-Human CD158b KIRsDL2/3 APC | Miltenyi | RRID: AB_871609 |
| Anti-Human CD158b KIR2DL1/DS1,3,5 APC | BioLegend | RRID: AB_2565577 |
| Anti-Human CD158e KIR3DL1 APC | BD Bioscience | Clone:DX9 |
| Anti-Human CD45 AF700 | BioLegend | RRID: AB_2566374 |
| Anti-Human CD3 APC-H7 | BD Bioscience | RRID: AB_1645476 |
| Anti-Human TCRγδ BV421 | BD Bioscience | RRID: AB_2737655 |
| Anti-Human CD294 BV480 | BD Bioscience | RRID: AB_2743703 |
| Anti-Human TCRVd2 BV605 | BD Bioscience | RRID: AB_2741719 |
| Anti-Human CD137 (4–1BB) BV650 | BD Bioscience | RRID: AB_2738586 |
| Anti-Human CD314 NKG2D BV711 | BD Bioscience | RRID: AB_2738377 |
| Anti-Human CD127 BV750 | BD Bioscience | Clone:HIL-7R-M21 |
| Anti-Human CD336 NKp44 BV786 | BD Bioscience | RRID: AB_2742134 |
| Anti-Human CD8 BUV395 | BD Bioscience | RRID: AB_2722501 |
| Anti-Human CD14 BUV496 | BD Bioscience | Clone:M5E2 |
| Anti-Human CD19 BUV496 | BD Bioscience | RRID: AB_2744311 |
| Anti-Human CD337 NKp30 BUV563 | BD Bioscience | Clone:P30–15 |
| Anti-Human CD57 BUV615 | BD Bioscience | Clone:NK1 |
| Anti-Human CD335 NKp46 BUV661 | BD Bioscience | Clone:9E2/Nkp46 |
| Anti-Human CD279 PD-1 BUV737 | BD Bioscience | RRID: AB_2739167 |
| Anti-Human CD16 BUV805 | BD Bioscience | Clone:3G8 |
|
Cell sorting | ||
| Anti-Human CD45 BV510 | BioLegend | RRID: AB_2561940 |
| Anti-Human CD14 APC | BioLegend | RRID: AB_830681 |
| Anti-Human CD19 APC | Tonbo | Cat#20–0199-T100 |
| Anti-Human CD3 FITC | BioLegend | RRID: AB_571907 |
| Anti-Human CD56 PE-Cy7 | BioLegend | RRID: AB_2563927 |
|
Chemicals, Peptides, and Recombinant Proteins | ||
| Recombinant Human IL-12 | PeproTech | Cat#200–12 |
| Recombinant Human IL-15 | PeproTech | Cat#200–15 |
| Recombinant Human IL-18 | MBL International Corp. | Cat#B001–5 |
| Human TrueStain FcX | BioLegend | Cat#422302 |
| Fixable Viability Dye eFluor 780 | eBioscience | Cat#65–0865-14 |
| Live/Dead BV570 | BD Bioscience | Cat#FVS575V |
| RPMI 1640 | Corning | Cat#10–040-CM |
|
Deposited Data | ||
| Raw and analyzed data | This paper | GEO: GSE133383 |
|
Experimental Models: Cell Lines | ||
| K652 cell line | ATCC | Cat#ATCC CCL-243 |
|
Software and Algorithms | ||
| STARaligner | https://github.com/alexdobin/STAR | Dobin et al., 2013 |
| DEseq2 | N/A | Love et al., 2014 |
| Enrichr | https://amp.pharm.mssm.edu/Enrichr/ | Chen et al., 2013 |
| WebGestalt | http://www.webgestalt.org/ | Wang et al., 2017 |
| Rstudio version 1.2.1335 | RStudio, Inc. (2019) | https://rstudio.com |
| R version 3.5 | R Foundation for StatisticalComputing (2017) | https://www.R-project.org |
|
Other | ||
| EasySep Human NK Cell Enrichment Kit | Stem Cell Tech | Cat#19055 |
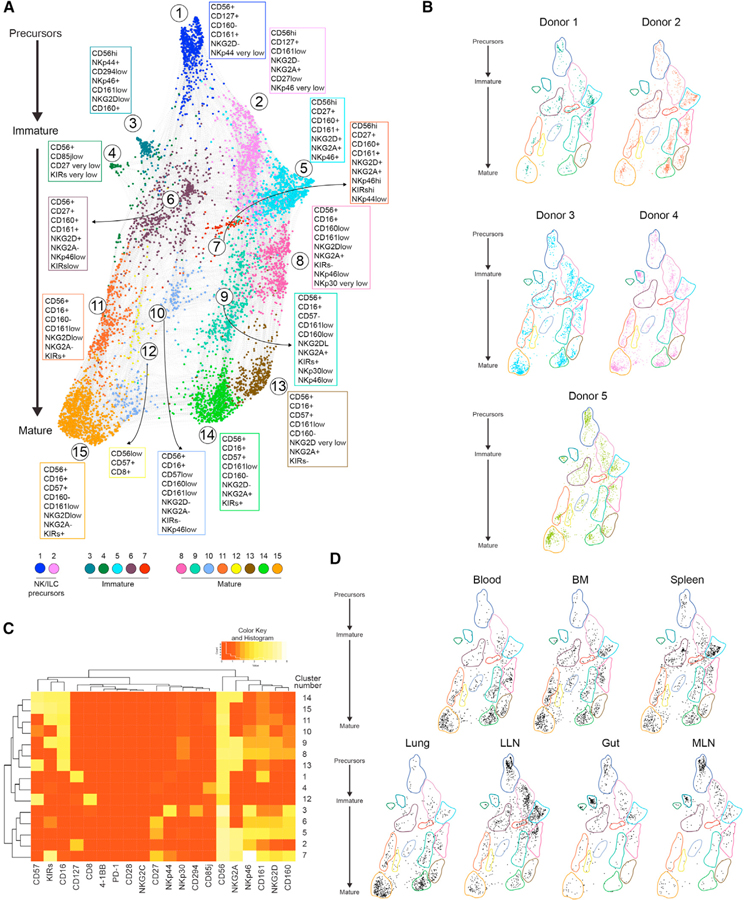
Force-directed clustering analysis of NK marker expression data from seven sites (blood, BM, spleen, lung, gut, LLN, and MLN) of five individuals generated 15 distinct NK cell clusters that were consistent between donors (Figures 7A and 7B; Table S6). There were 7 clusters within the CD56bright population: cells in clusters 1 and 2 express CD56, CD127, and CD161. Cluster 1 cells also express very low levels of activating receptor NKp44 and resemble NK-precursor-like cells described previously (Di Santo and Vosshenrich, 2006; Freud et al., 2005; Scoville et al., 2017), while cluster 2 cells exhibit increased expression of CD56 and NKG2A (Figures 7A and 7C). The remaining CD56bright cells in clusters 3–7 lack CD127 expression and show reduced NKp44 expression (except in cluster 3) but express the activating receptor NKG2D, the inhibitory receptor NKG2A, and variable levels of NKp46, CD160, and CD161 (Figures 7A, 7C, and S6). CD56dimCD16+ (mature) NK cells partition into 8 clusters (clusters 8–15) showing a progressive loss of CD160, CD161, NKp46, NKG2D, and NKG2A expression, and concomitant upregulation of CD57 and KIRs along the maturation axis (Figure 7A). The more mature CD56dim NK cells (clusters 13,14,15) resemble terminally differentiated NK cells exhibiting upregulated expression of CD57, KIRs, and downregulation of CD27 (Figures 7A and 7C). Therefore, multiple states of NK development and differentiation can be detected across blood and tissues.
Figure 7. High-Dimensional Flow Cytometry Profiling Identifies Tissue-Specific Patterns of NK Development and Maturation.
NK cells from blood, bone marrow (BM), spleen, lung, gut, lung-draining LN (LLN), and mesenteric LN (MLN) were stained with the high-dimensional antibody panel specific for multiple NK cell maturation and function markers (see STAR Methods). See also Figure S6.
(A) Force-directed plot showing the relationship between different NK cell communities identified by multi-dimensional analysis (data compiled from 5 donors). Each node is an aggregation of similar cells resulting from downsampling of data, and collection of similar colored nodes forms 15 distinct NK cell clusters. Colored text boxes show markers used to differentiate each cluster (1–15).
(B) Plots showing the distribution of NK cell communities across the five donors.
(C) Heatmap showing the differential expression of cell surface markers used for clustering and community identification in the multi-dimensional analysis in (A).
(D) Scatterplots showing distribution of NK cell states in each tissue among the clusters identified by multi-dimensional analysis. For (B) and (D), colored shapes delineate boundaries corresponding to each cluster identified in (A).
The distribution of NK cell maturation states was site-specific. BM and spleen contained the most heterogeneous composition of NK cells, comprising multiple maturation states, including precursor NK cells (clusters 1 and 2), three immature subsets (clusters 5–7), and all eight clusters that defined the mature CD16+ population (Figure 7D). Conversely, NK subsets in blood and lung consisted largely of mature and terminally differentiated states, particularly in the lung where clusters 13–15 predominated. By contrast, immature subsets populating LLN and MLN contained the two precursor subsets (CD56brightCD127+CD161+) along with lower frequencies of five immature NK cell states (clusters 3–7) (Figure 7D; Table S6). Although CD127 is also a marker for innate lymphoid cells (ILC) (Vivier et al., 2018), we did not detect substantial frequencies of CD127+ RORγt+ ILC3s (Li et al., 2018; Yudanin et al., 2019) among CD56+ cells from LLN, gut, and spleen or the other sites examined (Figure S7A). Moreover, the transcriptional profile of spleen and LLN NK cell subsets (Figure 4) was distinct from that of human ILC subsets in different sites recently reported (Yudanin et al., 2019; Figure S7B). Together, these data provide further evidence that the prevalent NK populations in LN likely represent precursor NK cells and NK subsets maintained in less differentiated states compared to other peripheral sites.
Last, we identified only two NK cell states in the gut (clusters 3 and 4) that were not found in the other sites except for low frequencies, specifically in the gut-draining MLN, and not in LLN. Cluster 3 cells exhibited a distinct NKp44+NKp46+CD160+CD294loNKG2A− phenotype, whereas cluster 4 cells lacked expression of NKp44, NKp46, and CD160, but exhibited upregulated expression of CD85j, an MHC class I-binding immunoglobulin-like molecule with inhibitory activity (Colonna et al., 1997). These distinct NK cell subsets appear to be specific or enriched within intestinal sites (and intestinal LN) because in the donors from whom we did not obtain intestinal tissues (donors 1–3; Figure 7B), these clusters were not observed. This high-dimensional analysis of NK cells across multiple tissues within individual donors demonstrate tissue-specific distribution of developmental and functional states of NK cells and identifies the LN and intestines as reservoirs for specific populations of precursor and immature NK cells, respectively.
DISCUSSION
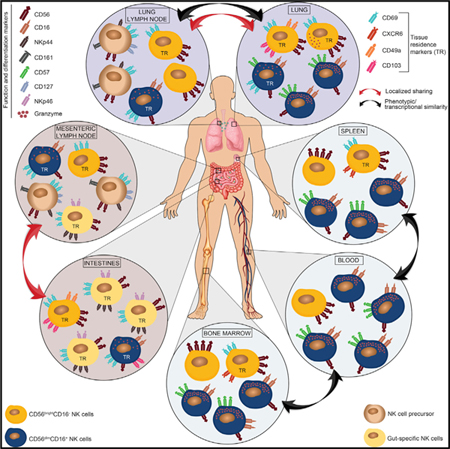
We reveal here the role of tissue localization in the development, function, and transcriptional program of NK cells, critical innate lymphocytes that mediate control of viral infections and tumors. Analysis of primary and secondary lymphoid organs as well as mucosal sites obtained from 60 organ donors shows that the tissue distribution of human NK cells and major NK cell subsets is a function of the tissue site and is unaffected by age, sex, and CMV serostatus. NK cells are most abundant in blood, BM, spleen, and lung, which are dominated by mature (CD56dimCD16+), terminally differentiated (CD56dimCD16+CD57+), and CMV-responsive (CD56dim CD16+CD57+NKG2C+) NK cell subsets with high effector function. Significantly lower frequencies of NK cells are found in LN, tonsils, and intestines comprising mostly immature (CD56brightCD16−) subsets with reduced effector capacity, which exhibit features of tissue-resident lymphocytes and tissue-specific adaptations. Moreover, developmental lineages of NK cells exhibit site-specific distribution and phenotypes; NK cell precursors and immature tissue-adapted subsets localize in LN and intestines; spleen and BM contain all developmental stages, whereas lung has prevalent mature and terminally differentiated NK cells. Together, our results provide evidence for tissue-driven segregation of NK cell development, function, and immunosurveillance.
Our tissue resource enables assessment of immune cells across sites within and between individuals, for unambiguous determination of attributes that are tissue-intrinsic versus those that vary due to age, sex, or between individuals. As shown here, the distribution of NK cells and subsets and their intrinsic function are features of the tissue site and are remarkably consistent between donors independent of age or sex, consistent with their role as an innate immune cell. Notably, the majority of NK cells localize in the blood, and blood-rich sites such as BM, spleen, and lungs where they are largely CD56dimCD16+ cells with high effector capacity—a subset predominance consistent with previous studies of human NK cells (Castriconi et al., 2018; Freud et al., 2017; Robertson and Ritz, 1990). Terminally differentiated and memory-like NK cells, including CMV-responsive NK cells, also predominate in these blood-rich sites. By contrast, NK cells are present only at low frequencies in LNs, tonsils, and throughout the GI tract where they are predominantly CD56brightCD16− cells, while mature and terminally differentiated NK cells are largely excluded from these sites. This compartmentalization of NK cell subsets may occur due to seeding of distinct subsets directly from the BM or differential migration of NK populations during maintenance.
We show here that CD56brightCD16− NK cells in lymphoid and mucosal tissues exhibit key transcriptional and functional features of Trm cells (Kumar et al., 2017; Mackay et al., 2016; Masopust and Soerens, 2019). In contrast to T cells where tissue residency is acquired during differentiation to memory T cells, tissue residency in NK cells is a feature of the less differentiated subset, consistent with recent results in human BM and lung (Marquardt et al., 2019; Melsen et al., 2018). By contrast, CD56dimCD16+ and memory-like NK cells exhibit features of circulating cells and are prevalent in blood-rich sites. We also show that trNK cells exhibit site-specific transcriptional and phenotypic adaptations. In particular, CD56brightCD16− cells from LN and lung were transcriptionally distinct compared to BM and other sites, due in part to differential expression of residency markers CD49a, CD103, and CXCR6 and multiple chemokine and homing receptors. Together, these data provide strong evidence for differential requirements for NK cell homing and/or retention in distinct tissue sites.
The diversity in NK cell phenotypes is driven by the differential expression of developmentally regulated receptors, which also govern the functional responsiveness of NK cells (Colonna et al., 1999; Lanier et al., 1986; Pegram et al., 2011). Our high-dimensional analysis of multiple NK cell lineage markers revealed tissue-specific patterns of NK development and function. At the earliest differentiation states, CD127+NKp44low NK precursor-like cells were predominant in LNs and were similar to those identified as stem cell-derived and thymus-derived NK cells (Freud et al., 2005; Luther et al., 2011; Vosshenrich et al., 2006). LN also contain additional immature subsets (CD16−CD160lowKIRlow), which express transcripts encoding chemokines (e.g., XCL1, XCL2, and CCL5) for recruitment of lymphocytes and myeloid cells (Bottcher et al., 2018), and resemble the NK2 subset recently described (Crinier et al., 2018). Intestines also harbor immature subsets, but maintain predominantly two specific types not found in blood or other tissues that are also found in gut-associated LN. One of the intestine-specific subsets exhibits co-expression of NKp44 and NKp46 and may resemble NK cell phenotypes associated with inflammatory bowel disease (Poggi et al., 2019). Interestingly, the blood, BM, and spleen contain a range of NK cell subsets and differentiation states expressing a range of KIRs, NCRs, and activating receptors, consistent with previously defined heterogeneity among blood NK cells (Bengsch et al., 2018; Horowitz et al., 2013), while lung is enriched in highly differentiated NK cells.
Together, our results suggest a model for anatomic control of NK cell development and function. Specifically, precursor and immature NK cells are seeded into LNs and intestines where they take up residence, and these sites may therefore serve as reservoirs for NK cell maintenance. Activation and differentiation of NK cells triggers dispersal into other sites including spleen, blood, and lung, where they conduct immunosurveillance and mediate effector function. In this way, NK cells control virus infections and tumors in sites readily accessible to circulation such as BM and lungs, while intestines and secondary lymphoid sites may be less amenable to NK cell-mediated immune surveillance. This site-specific segregation of NK cell maintenance and differentiation is consistent with the preferential role of NK cells in controlling the dissemination of infected cells and tumor cell metastasis (Morvan and Lanier, 2016).
In conclusion, tissue-intrinsic modes of NK cell maintenance, differentiation, and functional regulation revealed here can drive future efforts to develop anti-tumor and anti-viral immunotherapies to leverage these distinct features of NK cell immunity.
STAR★METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for reagents should be directed to and will be fulfilled by lead author Donna L. Farber (df2396@cumc.columbia.edu)
MATERIALS AVAILABILITY STATEMENT
This study did not generate new unique reagents.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Human samples
Tissues were obtained from deceased organ donors as part of organ acquisition for clinical transplantation through an approved protocol and material transfer agreement with LiveOnNY as described previously (Carpenter et al., 2018; Gordon et al., 2017; Granot et al., 2017; Kumar et al., 2017; Miron et al., 2018; Senda et al., 2019). Donors were free of cancer, chronic diseases, seronegative for hepatitis B, C, and HIV, and represented diverse ages (Table S1). This study does not qualify as ‘‘human subjects’’ research, as confirmed by the Columbia University IRB as tissue samples were obtained from brain-dead (deceased) individuals.
METHOD DETAILS
Isolation and preparation of single cell suspensions from tissue samples
Tissue samples were maintained in cold saline or CoStorSol® (University of Wisconsin (UW) solution (Preservation Solutions, Elkhorn, WI, Cat# PS004)), and transported to the laboratory within 2–4 hours of organ procurement. Lymphocytes were isolated from blood and BM samples by density centrifugation using lymphocyte separation medium (Corning cat# 25–072-CI) for recovery of mononuclear cells. Spleen, lung, intestinal, tonsil, and LN samples were processed using enzymatic and mechanical digestion, resulting in high yields of live leukocytes, as previously described (Carpenter et al., 2018; Gordon et al., 2017; Granot et al., 2017; Kumar et al., 2017; Miron et al., 2018; Senda et al., 2019).
Flow cytometry analysis and cell sorting
For flow cytometric analysis, cells were stained in 96-well U-bottom plates in the dark using antibody panels (see Key Resources Table). Surface staining was done for 20min at room temperature (RT). For intracellular staining, surface stained cells were fixed for 25min at RT in fixing buffer (Invitrogen cat# 00–5123-43), followed by staining in permeabilization buffer (Invitrogen cat# 00–8333-56) at RT for 30 min. Controls were isotype stained and single-fluorochrome stained samples. Flow cytometry data were acquired on a BD LSRII and analyzed using FCS express 6 (De Novo Software) and custom Python scripts. Spanning-tree progression analysis of density-normalized events (SPADE) analysis of the NK cell data collected from blood, BM, spleen, lung, gut, LLN, and MLN was done using MATLAB with no down-sampling and number of clusters set to 150–200 (Qiu et al., 2011).
NK cells were purified from blood, BM, spleen, lung, and LN using EasySep Human NK Cell Enrichment Kit (Stem Cell Tech cat# 19055), followed by sorting. Enriched cells were stained with the antibody panel described in the Key Resources Table. The stained cells were sorted using a BD Influx Cell Sorter. Sorted cells were used either for functional assays or RNA isolation.
High-dimensional parameter flow cytometric analysis of NK cell subsets
For high parameter analysis using the BD Symphony panels, 5x106 cells from each site were stained in 96 well v-bottom plates using an optimized antibody panel as described in the Key Resources Table. Briefly, cells were washed with PBS, re-suspended in 1 mL of viability dye and incubated at RT in the dark for 10 min. Cells were washed once with cold FACS-buffer (PBS with 2% heat-inactivated FCS and 2 mM EDTA) and stained in a two-step process. First, the cells were resuspended in a staining cocktail that included the anti-γδ TCR antibody (Key Resources Table), human TrueStain FcX (BioLegend, cat: 422302), and mouse serum (Jackson Immuno Research Labs, cat: 015–000-001) and incubated on ice for 10 min. Second, following incubation, antibodies in the Key Resources Table were added together with 50 μL of Horizon Brilliant Stain buffer (BD Bioscience, cat: 56379), incubated for 25 min on ice, washed with FACS-buffer, and fixed in 100 μL of Fluorofix Buffer (BioLegend, cat# 422101). Samples were analyzed on the LSR Fortessa X50 (‘‘Symphony’’) cytometer (BD Bioscience). Data were analyzed with FlowJo 10.2. NK cell populations (defined as live, CD45+CD14−CD19−CD3−CD56+ lymphocytes) were exported and high-dimensional analyses were performed using the R-based workflow publicly available at https://github.com/ParkerICI/flow-analysis-tutorial and https://github.com/ParkerICI.
In vitro cytokine stimulation and functional assays of NK cells
NK cells (CD45+CD56+CD3−) sorted from freshly processed tissue sites (see above) were cultured in 96-well u-bottom plates in RPMI-1640 medium containing fetal bovine serum (10%) with or without 10ng/ml IL-12 (PeproTech, cat# 200–12), 100ng/ml IL-15 (PeproTech, cat# 200–15) and 100ng/ml IL-18 (MBL International Corp. cat# B001–5) for 18 hours. Vesicular transport was inhibited by GolgiStop (BD Biosciences, cat# 554724) and GolgiPlug (BD Bioscience cat# 555029) during the last 6 hours of the incubation. Cell surface and intracellular staining was performed as described above.
For measuring degranulation by NK cells, 30,000–50,000 sorted, overnight rested NK cells were incubated with K562 cells (ATCC: CCL-243; provided by Emily Mace, Columbia University) at a 2:1 effector to target (NK: K562) ratio in a 96-well tissue culture plate at 37°C at 5% CO2 for 4 hours. Anti-CD107a antibody was added to the co-culture at the beginning of the assay. Following incubation, the cells were washed once with FACS-buffer before acquiring the data. Data were acquired on a BD LSRII and analyzed using FCS express 6 (De Novo Software).
QUANTIFICATION AND STATISTICAL ANALYSIS
Whole transcriptome profiling of NK cell subsets
RNA was isolated from sorted CD56brightCD16− and CD56dimCD16+ NK cells using the RNA Easy kit (QIAGEN cat # 74004) and library preparation was done using the SMART-Seq® ultra-low input RNA kit (Takara bio). Samples were sequenced at Genewiz (South Plainfield, NJ) using a HiSeq2500 system (Illumina). Trimmed paired-end reads were mapped to the Homo sapiens GRCh38 reference genome available on ENSEMBL using the STAR aligner v.2.5.2b. The gene list was filtered to exclude any non-coding genes, following which genes with read counts below 5 copies in at least 9 of 30 samples were excluded from the analysis. Sequencing batch differences were removed using sva package (Leek et al., 2019) and differential expression (DE) gene analysis was done using the DESeq2 (Love et al., 2014) package in R. Genes were considered as significantly differentially expressed if absolute log2fold change was ≥ 1 and p value was below the 0.05 threshold. Heatmaps were generated using the pheatmap (Kolde, 2012) package, and PCA plots and log2 fold change bi-plots were generated by use of the ggplot2 package (Wickham, 2016). Clustering groups within PCA was predicted by pcaplot function of the pcaExplorer library (Marini and Binder, 2019). The hit counts table for the ILC study (GEO: GSE126107) (Yudanin et al., 2019) was downloaded and used to compare the transcriptional profile of ILC subsets to CD56brightCD16− NK cell subsets from this study.
Gene Set Enrichment Analysis
For gene set enrichment analysis (GSEA) of unique tissue-specific transcriptional programs, differentially expressed genes with value of log2-fold change ≥ 1 for comparison between tissue CD56brightCD16− and CD56dimCD16+ NK cells, and their blood counterparts were used as input to run pre-ranked GSEA using WebGestalt web application (Wang et al., 2017). To test for enrichment of tissue-residence gene signature, genes ranked by absolute value of log2-fold change between CD56brightCD16− and CD56dimCD16+ NK cells was used as input to run pre-ranked GSEA using the Broad Institute GSEA Java web application. Gene sets were created from lung and spleen CD8+Trm transcription profiles, as previously determined (Kumar et al., 2017).
Statistical Analysis
Graphs were generated using the Python matplotlib library (Hunter, 2007). To compare expression of surface and intracellular markers across different tissue sites, a one-way ANOVA test was run followed by Tukey’s correction to correct for multiple testing. For comparing expression of markers between 2 groups within a tissue site we performed either a paired t test, or multiple comparison t tests with unequal variance. Nonparametric Mann-Whitney U test was used where data showed deviation from normal distribution as indicated in figure legends. P-values below 0.05 were considered as statistically significant. The statistical analysis was run using custom scripts based on Python SciPy library (Jones et al., 2001) and Prizm (GraphPad). For all figures ***p ≤ 0.001, **p ≤ 0.01, and *p ≤ 0.05.
DATA AND CODE AVAILABILITY
The processed data and transcriptome dataset generated during this study is available on NCBI GEO with the accession number GEO: GSE133383.
Supplementary Material
Highlights.
High-resolution map of human NK cells shows tissue-driven distribution across ages
Differentiated NK cells predominate in blood, bone marrow, spleen, and lungs
Tissue-resident NK cells exhibit specific adaptations in mucosal and lymphoid sites
Lymph nodes and intestines are reservoirs for precursor and immature NK cells
ACKNOWLEDGMENTS
This work was supported by an IOF grant from the National Institutes of Health (NIH) Human Immunology Project Consortium (HIPC) awarded to D.L.F. and L.L.L., NIH grants AI128949 and AI06697 awarded to D.L.F., and U19AI128913 to L.L.L. P.D. was supported by a Cancer Research Institute (CRI) Irvington Postdoctoral Fellowship. P.A.S was supported by an American Associate of Immunologists (AAI) Intersect Fellowship. M.T. was supported by Hungarian Campus Mundi program. D.C. was supported by T32 grant HL007854. Research reported in this publication was performed in the CCTI Flow Cytometry Core, supported in part by NIH S10RR027050. We thank our colleagues Aaron Tyznik and Bob Balderas at BD Biosciences for invaluable help in developing the NK receptor high-parameter FACS panel used in this study, Federico Gherardini at the Parker Institute for Cancer Immunotherapy for developing data analysis tools, Drs. David Artis and Laurel Monticelli for helpful discussions on ILC, and Ms. Emma Idzikowski for help with figures. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We wish to gratefully acknowledge the generosity of the donor families and the exceptional efforts of Dr. Amy Friedman and LiveOnNY transplant coordinators and staff for
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.cell.2020.01.022.
A video abstract is available at https://doi.org/10.1016/j.cell.2020.01.022#mmc5.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Abel AM, Yang C, Thakar MS, and Malarkannan S (2018). Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol 9, 1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter G, Malenfant JM, and Altfeld M (2004). CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 294, 15–22. [DOI] [PubMed] [Google Scholar]
- Arase H, Mocarski ES, Campbell AE, Hill AB, and Lanier LL (2002). Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296, 1323–1326. [DOI] [PubMed] [Google Scholar]
- Aste-Amezaga M, D’Andrea A, Kubin M, and Trinchieri G (1994). Cooperation of natural killer cell stimulatory factor/interleukin-12 with other stimuli in the induction of cytokines and cytotoxic cell-associated molecules in human T and NK cells. Cell. Immunol 156, 480–492. [DOI] [PubMed] [Google Scholar]
- Bengsch B, Ohtani T, Herati RS, Bovenschen N, Chang KM, and Wherry EJ (2018). Deep immune profiling by mass cytometry links human T and NK cell differentiation and cytotoxic molecule expression patterns. J. Immunol. Methods 453, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkström NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, Björkström AT, Flodström-Tullberg M, Michaëlsson J, Rottenberg ME, et al. (2010). Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood 116, 3853–3864. [DOI] [PubMed] [Google Scholar]
- Bottcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, Rogers NC, Sahai E, Zelenay S, and Reis e Sousa C (2018). NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 172, 1022–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter DJ, Granot T, Matsuoka N, Senda T, Kumar BV, Thome JJC, Gordon CL, Miron M, Weiner J, Connors T, et al. (2018). Human immunology studies using organ donors: Impact of clinical variations on immune parameters in tissues and circulation. Am. J. Transplant 18, 74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, and Caligiuri MA (1994). Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med 180, 1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castriconi R, Carrega P, Dondero A, Bellora F, Casu B, Regis S, Ferlazzo G, and Bottino C (2018). Molecular Mechanisms Directing Migration and Retention of Natural Killer Cells in Human Tissues. Front. Immunol 9, 2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerwenka A, and Lanier LL (2001). Natural killer cells, viruses and cancer. Nat. Rev. Immunol 1, 41–49. [DOI] [PubMed] [Google Scholar]
- Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, and Ma’ayan A (2013). Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PL, Cella M, Porter SI, Li S, Gurewitz GL, Hong HS, Johnson RP, Oltz EM, and Colonna M (2019). Gene Regulatory Programs Conferring Phenotypic Identities to Human NK Cells. Cell 176, 348–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, Navarro F, Bellón T, Llano M, García P, Samaridis J, Angman L, Cella M, and López-Botet M (1997). A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J. Exp. Med 186, 1809–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, Nakajima H, Navarro F, and López-Botet M (1999). A novel family of Ig-like receptors for HLA class I molecules that modulate function of lymphoid and myeloid cells. J. Leukoc. Biol 66, 375–381. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, and Caligiuri MA (2001). Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood 97, 3146–3151. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, and Yokoyama WM (2009). Cytokine-induced memory-like natural killer cells. Proc. Natl. Acad. Sci. USA 106, 1915–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinier A, Milpied P, Escaliere B, Piperoglou C, Galluso J, Balsamo A, Spinelli L, Cervera-Marzal I, Ebbo M, Girard-Madoux M, et al. (2018). High-Dimensional Single-Cell Analysis Identifies Organ-Specific Signatures and Conserved NK Cell Subsets in Humans and Mice. Immunity 49, 971–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels KA, Devora G, Lai WC, O’Donnell CL, Bennett M, and Welsh RM (2001). Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J. Exp. Med 194, 29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Santo JP, and Vosshenrich CA (2006). Bone marrow versus thymic pathways of natural killer cell development. Immunol. Rev 214, 35–46. [DOI] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, and Yokoyama WM (2001). Specific and nonspecific NK cell activation during virus infection. Nat. Immunol 2, 951–956. [DOI] [PubMed] [Google Scholar]
- Faridi RM, and Agrawal S (2011). Killer immunoglobulin-like receptors (KIRs) and HLA-C allorecognition patterns implicative of dominant activation of natural killer cells contribute to recurrent miscarriages. Hum. Reprod 26, 491–497. [DOI] [PubMed] [Google Scholar]
- Fehniger TA, Shah MH, Turner MJ, VanDeusen JB, Whitman SP, Cooper MA, Suzuki K, Wechser M, Goodsaid F, and Caligiuri MA (1999). Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J. Immunol 162, 4511–4520. [PubMed] [Google Scholar]
- Fehniger TA, Cai SF, Cao X, Bredemeyer AJ, Presti RM, French AR, and Ley TJ (2007). Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity 26, 798–811. [DOI] [PubMed] [Google Scholar]
- Freud AG, Becknell B, Roychowdhury S, Mao HC, Ferketich AK, Nuovo GJ, Hughes TL, Marburger TB, Sung J, Baiocchi RA, et al. (2005). A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity 22, 295–304. [DOI] [PubMed] [Google Scholar]
- Freud AG, Mundy-Bosse BL, Yu J, and Caligiuri MA (2017). The Broad Spectrum of Human Natural Killer Cell Diversity. Immunity 47, 820–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor LM, and Colucci F (2017). Uterine Natural Killer Cells: Functional Distinctions and Influence on Pregnancy in Humans and Mice. Front. Immunol 8, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt T, Palendira U, Tscharke DC, and Bedoui S (2018). Tissue-resident memory T cells in tissue homeostasis, persistent infection, and cancer surveillance. Immunol. Rev. 283, 54–76. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, Dustin ML, and Littman DR (2005). Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol 3, e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CL, Miron M, Thome JJ, Matsuoka N, Weiner J, Rak MA, Igarashi S, Granot T, Lerner H, Goodrum F, and Farber DL (2017). Tissue reservoirs of antiviral T cell immunity in persistent human CMV infection. J. Exp. Med 214, 651–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot T, Senda T, Carpenter DJ, Matsuoka N, Weiner J, Gordon CL, Miron M, Kumar BV, Griesemer A, Ho SH, et al. (2017). Dendritic Cells Display Subset and Tissue-Specific Maturation Dynamics over Human Life. Immunity 46, 504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumá M, Angulo A, Vilches C, Gómez-Lozano N, Malats N, and López-Botet M (2004). Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood 104, 3664–3671. [DOI] [PubMed] [Google Scholar]
- Gumá M, Budt M, Sáez A, Brckalo T, Hengel H, Angulo A, and López-Botet M (2006). Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood 107, 3624–3631. [DOI] [PubMed] [Google Scholar]
- Hendricks DW, Balfour HH Jr., Dunmire SK, Schmeling DO, Hogquist KA, and Lanier LL (2014). Cutting edge: NKG2C(hi)CD57+ NK cells respond specifically to acute infection with cytomegalovirus and not Epstein-Barr virus. J. Immunol 192, 4492–4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs ML, Selvaraj P, Carpén O, Springer TA, Kuster H, Jouvin MH, and Kinet JP (1989). Mechanisms for regulating expression of membrane isoforms of Fc gamma RIII (CD16). Science 246, 1608–1611. [DOI] [PubMed] [Google Scholar]
- Hombrink P, Helbig C, Backer RA, Piet B, Oja AE, Stark R, Brasser G, Jongejan A, Jonkers RE, Nota B, et al. (2016). Programs for the persistence, vigilance and control of human CD8+ lung-resident memory T cells. Nat. Immunol 17, 1467–1478. [DOI] [PubMed] [Google Scholar]
- Horowitz A, Strauss-Albee DM, Leipold M, Kubo J, Nemat-Gorgani N, Dogan OC, Dekker CL, Mackey S, Maecker H, Swan GE, et al. (2013). Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci. Transl. Med 5, 208ra145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth K, Donadon M, Cimino M, Pontarini E, Tentorio P, Preti M, Hong M, Bertoletti A, Bicciato S, Invernizzi P, et al. (2016). Human liver-resident CD56(bright)/CD16(neg) NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J. Autoimmun 66, 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JD (2007). Matplotlib: A 2D graphics environment. Comput. Sci. Eng 9, 90–95. [Google Scholar]
- Imai K, Matsuyama S, Miyake S, Suga K, and Nakachi K (2000). Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 356, 1795–1799. [DOI] [PubMed] [Google Scholar]
- Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, and Kupper TS (2012). Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 483, 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E, Oliphant T, and Peterson P (2001). SciPy: Open source scientific tools for Python. http://www.scipy.org/.
- Kelly JM, Darcy PK, Markby JL, Godfrey DI, Takeda K, Yagita H, and Smyth MJ (2002). Induction of tumor-specific T cell memory by NK cell-mediated tumor rejection. Nat. Immunol. 3, 83–90. [DOI] [PubMed] [Google Scholar]
- Kolde R (2012). Pheatmap: pretty heatmaps. R Package Version. https://CRAN.R-project.org/package=pheatmap
- Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, and Strominger JL (2003). Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J. Exp. Med 198, 1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar BV, Ma W, Miron M, Granot T, Guyer RS, Carpenter DJ, Senda T, Sun X, Ho SH, Lerner H, et al. (2017). Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep 20, 2921–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CT, and Leiden JM (1999). Transcriptional regulation of T lymphocyte development and function. Annu. Rev. Immunol 17, 149–187. [DOI] [PubMed] [Google Scholar]
- Lanier LL (2008). Up on the tightrope: natural killer cell activation and inhibition. Nat. Immunol 9, 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier LL, Le AM, Civin CI, Loken MR, and Phillips JH (1986). The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J. Immunol 136, 4480–4486. [PubMed] [Google Scholar]
- Lanier LL, Yu G, and Phillips JH (1989). Co-association of CD3 zeta with a receptor (CD16) for IgG Fc on human natural killer cells. Nature 342, 803–805. [DOI] [PubMed] [Google Scholar]
- Lee J, Zhang T, Hwang I, Kim A, Nitschke L, Kim M, Scott JM, Kamimura Y, Lanier LL, and Kim S (2015). Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity 42, 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek JT, Johnson WE, Parker HS, Fertig EJ, Jaffe AE, Storey JD, Zhang Y, and Torres LC (2019). sva: Surrogate Variable Analysis. R package version 3321, https://bioconductor.org/packages/release/bioc/html/sva.html.
- Li N, van Unen V, Höllt T, Thompson A, van Bergen J, Pezzotti N, Eisemann E, Vilanova A, Chuva de Sousa Lopes SM, Lelieveldt BPF, and Koning F (2018). Mass cytometry reveals innate lymphoid cell differentiation pathways in the human fetal intestine. J. Exp. Med 215, 1383–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Vergès S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, Norris PJ, Nixon DF, and Lanier LL (2010). CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood 116, 3865–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Vergès S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang S-M, Norris PJ, et al. (2011). Expansion of a unique CD57+NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 108, 14725–14732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, and Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther C, Warner K, and Takei F (2011). Unique progenitors in mouse lymph node develop into CD127+ NK cells: thymus-dependent and thymus-independent pathways. Blood 117, 4012–4021. [DOI] [PubMed] [Google Scholar]
- Mackay LK, Minnich M, Kragten NA, Liao Y, Nota B, Seillet C, Zaid A, Man K, Preston S, Freestone D, et al. (2016). Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 352, 459–463. [DOI] [PubMed] [Google Scholar]
- Marini F, and Binder H (2019). pcaExplorer: an R/Bioconductor package for interacting with RNA-seq principal components. BMC Bioinformatics 20, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt N, Kekalainen E, Chen P, Kvedaraite E, Wilson JN, Ivarsson MA, Mjosberg J, Berglin L, Safholm J, Manson ML, et al. (2017). Human lung natural killer cells are predominantly comprised of highly differentiated hypofunctional CD69(−)CD56(dim) cells. J. Allergy Clin. Immunol 139, 1321–1330. [DOI] [PubMed] [Google Scholar]
- Marquardt N, Kekäläinen E, Chen P, Lourda M, Wilson JN, Scharenberg M, Bergman P, Al-Ameri M, Hård J, Mold JE, et al. (2019). Unique transcriptional and protein-expression signature in human lung tissue-resident NK cells. Nat. Commun 10, 3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, and Soerens AG (2019). Tissue-Resident T Cells and Other Resident Leukocytes. Annu. Rev. Immunol 37, 521–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melsen JE, Lugthart G, Vervat C, Kielbasa SM, van der Zeeuw SAJ, Buermans HPJ, van Ostaijen-Ten Dam MM, Lankester AC, and Schilham MW (2018). Human Bone Marrow-Resident Natural Killer Cells Have a Unique Transcriptional Profile and Resemble Resident Memory CD8+ T Cells. Front. Immunol 9, 1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron M, Kumar BV, Meng W, Granot T, Carpenter DJ, Senda T, Chen D, Rosenfeld AM, Zhang B, Lerner H, et al. (2018). Human Lymph Nodes Maintain TCF-1hi Memory T Cells with High Functional Potential and Clonal Diversity throughout Life. J. Immunol 201, 2132–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaldo E, Vacca P, Chiossone L, Croxatto D, Loiacono F, Martini S, Ferrero S, Walzer T, Moretta L, and Mingari MC (2016). Unique Eomes(+) NK Cell Subsets Are Present in Uterus and Decidua During Early Pregnancy. Front. Immunol 6, 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan MG, and Lanier LL (2016). NK cells and cancer: you can teach innate cells new tricks. Nat. Rev. Cancer 16, 7–19. [DOI] [PubMed] [Google Scholar]
- Muruganandah V, Sathkumara HD, Navarro S, and Kupz A (2018). A Systematic Review: The Role of Resident Memory T Cells in Infectious Diseases and Their Relevance for Vaccine Development. Front. Immunol 9, 1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagler A, Lanier LL, Cwirla S, and Phillips JH (1989). Comparative studies of human FcRIII-positive and negative natural killer cells. J. Immunol 143, 3183–3191. [PubMed] [Google Scholar]
- Nikolich-Žugich J (2018). The twilight of immunity: emerging concepts in aging of the immune system. Nat. Immunol 19, 10–19. [DOI] [PubMed] [Google Scholar]
- O’Leary JG, Goodarzi M, Drayton DL, and von Andrian UH (2006). T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol 7, 507–516. [DOI] [PubMed] [Google Scholar]
- O’Sullivan TE, Sun JC, and Lanier LL (2015). Natural Killer Cell Memory. Immunity 43, 634–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange JS (2006). Human natural killer cell deficiencies. Curr. Opin. Allergy Clin. Immunol 6, 399–409. [DOI] [PubMed] [Google Scholar]
- Pawelec G, and Derhovanessian E (2011). Role of CMV in immune senescence. Virus Res 157, 175–179. [DOI] [PubMed] [Google Scholar]
- Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, and Kershaw MH (2011). Activating and inhibitory receptors of natural killer cells. Immunol. Cell Biol 89, 216–224. [DOI] [PubMed] [Google Scholar]
- Peng H, and Tian Z (2017). Diversity of tissue-resident NK cells. Semin. Immunol 31, 3–10. [DOI] [PubMed] [Google Scholar]
- Poggi A, Benelli R, Venè R, Costa D, Ferrari N, Tosetti F, and Zocchi MR (2019). Human Gut-Associated Natural Killer Cells in Health and Disease. Front. Immunol 10, 961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu P, Simonds EF, Bendall SC, Gibbs KD Jr., Bruggner RV, Linderman MD, Sachs K, Nolan GP, and Plevritis SK (2011). Extracting a cellular hierarchy from high-dimensional cytometry data with SPADE. Nat. Biotechnol 29, 886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MJ, and Ritz J (1990). Biology and clinical relevance of human natural killer cells. Blood 76, 2421–2438. [PubMed] [Google Scholar]
- Scalzo AA, Corbett AJ, Rawlinson WD, Scott GM, and Degli-Esposti MA (2007). The interplay between host and viral factors in shaping the outcome of cytomegalovirus infection. Immunol. Cell Biol 85, 46–54. [DOI] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, and Masopust D (2014). Cutting edge: resident memory CD8 T cells occupy frontline niches in secondary lymphoid organs. J. Immunol 192, 2961–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD, Han H, Chiang SC, Foley B, Mattsson K, et al. (2015). Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 42, 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville SD, Freud AG, and Caligiuri MA (2017). Modeling Human Natural Killer Cell Development in the Era of Innate Lymphoid Cells. Front. Immunol 8, 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senda T, Dogra P, Granot T, Furuhashi K, Snyder ME, Carpenter DJ, Szabo PA, Thapa P, Miron M, and Farber DL (2019). Microanatomical dissection of human intestinal T-cell immunity reveals site-specific changes in gut-associated lymphoid tissues over life. Mucosal Immunol 12, 378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi FD, Ljunggren HG, La Cava A, and Van Kaer L (2011). Organ-specific features of natural killer cells. Nat. Rev. Immunol 11, 658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sojka DK, Plougastel-Douglas B, Yang L, Pak-Wittel MA, Artyomov MN, Ivanova Y, Zhong C, Chase JM, Rothman PB, Yu J, et al. (2014). Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife 3, e01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Beilke JN, and Lanier LL (2009). Adaptive immune features of natural killer cells. Nature 457, 557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Beilke JN, and Lanier LL (2010). Immune memory redefined: characterizing the longevity of natural killer cells. Immunol. Rev 236, 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, and Lanier LL (2012). Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J. Exp. Med 209, 947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo PA, Miron M, and Farber DL (2019). Location, location, location: Tissue resident memory T cells in mice and humans. Sci. Immunol 4, eaas9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrançois L, and Farber DL (2011). Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol 187, 5510–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome JJ, Yudanin N, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, Kato T, Lerner H, Shen Y, and Farber DL (2014). Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell 159, 814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victorino F, Sojka DK, Brodsky KS, McNamee EN, Masterson JC, Homann D, Yokoyama WM, Eltzschig HK, and Clambey ET (2015). Tissue-Resident NK Cells Mediate Ischemic Kidney Injury and Are Not Depleted by Anti-Asialo-GM1 Antibody. J. Immunol 195, 4973–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E, Ugolini S, Blaise D, Chabannon C, and Brossay L (2012). Targeting natural killer cells and natural killer T cells in cancer. Nat. Rev. Immunol 12, 239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, et al. (2018). Innate Lymphoid Cells: 10 Years On. Cell 174, 1054–1066. [DOI] [PubMed] [Google Scholar]
- Vosshenrich CA, García-Ojeda ME, Samson-Villéger SI, Pasqualetto V, Enault L, Richard-Le Goff O, Corcuff E, Guy-Grand D, Rocha B, Cumano A, et al. (2006). A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat. Immunol 7, 1217–1224. [DOI] [PubMed] [Google Scholar]
- Wang W, Erbe AK, Hank JA, Morris ZS, and Sondel PM (2015). NK Cell-Mediated Antibody-Dependent Cellular Cytotoxicity in Cancer Immunotherapy. Front. Immunol 6, 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Vasaikar S, Shi Z, Greer M, and Zhang B (2017). WebGestalt 2017: a more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res 45 (W1), W130–W137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H (2016). ggplot2: Elegant Graphics for Data Analysis (Springer-Verlag; ). [Google Scholar]
- Yu J, Freud AG, and Caligiuri MA (2013). Location and cellular stages of natural killer cell development. Trends Immunol 34, 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudanin NA, Schmitz F, Flamar AL, Thome JJC, Tait Wojno E, Moeller JB, Schirmer M, Latorre IJ, Xavier RJ, Farber DL, et al. (2019). Spatial and Temporal Mapping of Human Innate Lymphoid Cells Reveals Elements of Tissue Specificity. Immunity 50, 505–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Scott JM, Hwang I, and Kim S (2013). Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRγ deficiency. J. Immunol 190, 1402–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP, and Xue HH (2010). Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity 33, 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwirner NW, and Ziblat A (2017). Regulation of NK Cell Activation and Effector Functions by the IL-12 Family of Cytokines: The Case of IL-27. Front. Immunol 8, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The processed data and transcriptome dataset generated during this study is available on NCBI GEO with the accession number GEO: GSE133383.