Highlights
-
•
Novel coronavirus pneumonia patients are frequently encountered in the sixth and seventh decades of life
-
•
Significantly decreased white blood cells, alkaline phosphatase and d-dimer were observed in novel coronavirus pneumonia patients compared with influenza patients
-
•
Lactate dehydrogenase, erythrocyte sedimentation rate and fibrinogen were significantly increased in novel coronavirus pneumonia patients compared with those in influenza patients
-
•
An optimal diagnostic model with moderate value was established based on the combination of 18 biomarkers from routine laboratory tests to discriminate novel coronavirus pneumonia patients from influenza patients
Keywords: SARS-CoV-2, Influenza virus, Novel coronavirus pneumonia patients, Influenza patients, Laboratory tests
Abstract
Background
The differential diagnosis between novel coronavirus pneumonia patients (NCPP) and influenza patients (IP) remains a challenge in clinical practice.
Methods
Between January 2018 and March 2020, 1,027 NCPP and 1,140 IP were recruited from Tongji hospital. Routine blood examination, biochemical indicators and coagulation function analysis were simultaneously performed in all participants.
Results
There was no sex predominance in NCPP. The NCPP were frequently encountered in the sixth and seventh decades of life. The mean age of NCPP (56 ± 16 years) was higher than IP (47 ± 17 years), but without statistical difference. Although most results of routine laboratory tests between NCPP and IP had no significant differences, some laboratory tests showed an obvious change in NCPP. It was observed that NCPP had significantly decreased white blood cells, alkaline phosphatase and d-dimer compared with IP. However, the results of lactate dehydrogenase, erythrocyte sedimentation rate and fibrinogen were significantly increased in NCPP compared with IP. The diagnostic model based on a combination of 18 routine laboratory indicators showed an area under the curve of 0.796 (95% CI, 0.777–0.814), with a sensitivity of 46.93% and specificity of 90.09% when using a cut-off value of 0.598.
Conclusions
Some routine laboratory results had statistical difference between NCPP and IP. A diagnostic model based on a combination of routine laboratory results provided an adjunct approach in the differential diagnosis between NCPP and IP.
1. Introduction
Recently, a novel coronavirus (SARS-CoV-2) has caused a severe outbreak in many regions of the world. It has been a cause of severe respiratory infection in humans between December 2019 and March 2020 (Chan et al., 2020, Gralinski and Menachery, 2020, The Lancet, 2020). More than 87,000 individuals have been confirmed as infected with the virus in China as of 1 March 2020 and most cases were reported in Wuhan city (World Health Organization, 2020). SARS-CoV-2 most commonly manifests as an acute or subacute illness such as fever, cough, myalgia and fatigue. Other symptoms, including sputum production, headache, hemoptysis and diarrhea, have also been observed in patients with severe illness (Huang et al., 2020).
Meanwhile, influenza closely mimics a novel coronavirus pneumonia (NCP) and usually causes similar respiratory symptoms (Paules and Subbarao, 2017). Although most influenza patients (IP) are children, there is a certain percentage of adults who can be infected with influenza, especially in winter and spring (Uyeki, 2017). It is sometimes difficult to differentiate IP from NCP patients (NCPP) due to their non-specific and indistinguishable symptoms. While prompt diagnosis and patient isolation are the hallmarks for initial control of the new epidemic, the more specific and classified laboratory characteristics of NCPP still require further investigation.
This study systematically investigated the laboratory characteristics of adult patients who were confirmed to have SARS-CoV-2 infection and those with influenza virus infection. Furthermore, it successfully established a combined-biomarker model that had potential utility in distinguishing NCPP from IP.
2. Methods
2.1. Study participants
This study was carried out from January 2018 to March 2020 at Tongji Hospital (the largest hospital in Hubei province, China). Patients with laboratory confirmed SARS-CoV-2 infection were recruited between January 2020 and March 2020, and patients infected with influenza virus were enrolled from January 2018 to June 2019. NCPP were diagnosed if patients had positive SARS-CoV-2 reverse transcription-polymerase chain reaction (RT-PCR) results and negative influenza virus real-time PCR results as well as typical computed tomography (CT) features. IP were diagnosed if they had positive influenza virus real-time PCR results and CT manifestations of viral pneumonia or if patients had positive influenza virus-specific IgM antibody and typical influenza clinical symptoms as well as CT manifestations of viral pneumonia. Patients aged < 18 years were excluded. All patients had routine laboratory tests, including simultaneous routine blood examination, biochemistry and coagulation function. This study was approved by the Ethical committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, China (TJ-C20200128).
2.2. Routine blood examination
Ethylenediaminetetraacetic acid-anticoagulated peripheral blood samples were collected from participants, and routine blood examination was performed using XN-9000 Sysmex (Sysmex Co., Kobe, Japan) according to the manufacturer's instructions. The obtained indicators were as follows: white blood cell count (WBC#), neutrophil percentage (NEUT%), neutrophil count (NEUT#), lymphocyte percentage (LYMPH%), lymphocyte count (LYMPH#), monocyte percentage (MONO%), monocyte count (MONO#), eosinophil percentage (EO%), eosinophil count (EO#), basophil percentage (BASO%), basophil count (BASO#), red blood cell count (RBC#), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), coefficient variation of red blood cell volume distribution width (RDW-CV), standard deviation in red cell distribution width (RDW-SD), platelet count (PLT#), platelet distribution width (PDW), mean platelet volume (MPV), platelet larger cell ratio (PLCR), and thrombocytocrit (THR).
2.3. Biochemical analysis
Heparin anti-coagulating peripheral blood samples were collected from patients and biochemical indicators were measured using ROCHE COBAS 8000 (Mannheim, Germany) according to the manufacturer's instructions. The obtained indexes were as follows: alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein (TP), albumin (ALB), globulin (GLB), total bilirubin (TBILI), direct bilirubin (DBILI), direct bilirubin (IBILI), creatinine (CREA), urea (UR), uric acid (UA), γ-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), calcium (Ca), magnesium (Mg), natrium (Na), phosphate (P), chlorine (Cl), kali (K), bicarbonate ion (HCO3), hypersensitive C-reactive protein (hsCRP), erythrocyte sedimentation rate (ESR), and procalcitonin (PCT).
2.4. Coagulation function analysis
Sodium citrate anti-coagulated peripheral blood samples were collected from patients and coagulation functions were detected using STA-R coagulation analyzers (Diagnostic Stago, France) according to the manufacturer's instructions. The obtained parameters were as follows: prothrombin time (PT), activated partial thromboplastic time (APTT), thrombin time (TT), international normalized ratio (INR), prothrombin activity (PTA), fibrinogen (FIB), and d-dimer (DD).
2.5. Statistical analysis
Continuous variables were compared with the Mann-Whitney U test and categorical variables were compared by χ2 test. A two-sided α of < 0.05 was considered statistically significant. To build the diagnostic model for differentiating NCPP from IP, all variables with statistical significance were taken as candidates for further multivariable logistic regression analyses; and then the regression equation (diagnostic model) was obtained and a score for each individual was calculated. Receiver operating characteristic (ROC) analysis was performed to determine the performance of the diagnostic model in distinguishing NCPP from IP. Area under the curve (AUC), sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), negative likelihood ratio (NLR), and accuracy, together with their 95% confidence intervals (CI), were calculated. Statistical analyses were performed using SPSS 25.0 (SPSS, Chicago, IL, USA) and GraphPad Prism version 6 (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Participants
In total, 1,027 NCPP and 1,140 IP were recruited from Tongji hospital between January 2018 and March 2020. The demographic information is summarized in Table 1 . There was no sex predominance in NCPP. The NCPP were frequently encountered in the sixth and seventh decades of life. The mean ages of NCPP and IP were 56 ± 16 and 47 ± 17 years, respectively. The mean age of NCPP patients was higher than IP patients, but without statistical difference.
Table 1.
Demographic characteristics of the study participants.
| NCPP (n = 1,027) |
IP (n = 1,140) |
|||||
|---|---|---|---|---|---|---|
| Age, years | Total | Male (n = 517) | Female (n = 510) | Total | Male (n = 505) | Female (n = 635) |
| 18–29 | 52 | 35 | 17 | 198 | 78 | 120 |
| 30–39 | 131 | 72 | 59 | 249 | 94 | 155 |
| 40–49 | 171 | 94 | 77 | 157 | 68 | 89 |
| 50–59 | 205 | 91 | 114 | 219 | 98 | 121 |
| 60–69 | 264 | 116 | 148 | 204 | 105 | 99 |
| 70–79 | 142 | 74 | 68 | 81 | 44 | 37 |
| 80–95 | 62 | 35 | 27 | 32 | 18 | 14 |
NCPP, novel coronavirus pneumonia patients; IP, influenza patients
3.2. Results of various indicators in NCPP and IP
For routine blood examination, the results showed that WBC#, NEUT#, MONO#, EO%, EO#, BASO%, BASO#, MCV, RDW-CV, RDW-SD, and PDW in NCPP were significantly lower than in IP. In contrast, LYMPH%, RBC#, HGB, HCT, MCH, and MCHC in NCPP were significantly higher than in IP (Table 2 ). The other markers including LYMPH#, MONO%, NEUT%, PLT#, THR, MPV, and PLCR had no significant difference between NCPP and IP.
Table 2.
The results of routine blood examination in study participants.
| Variables | NCPP (n = 1,027) | IP (n = 1,140) | P* |
|---|---|---|---|
| WBC# (×109/L) | 5.45 (4.46–7.17) | 6.14 (4.66–8.24) | < 0.001 |
| NEUT% (%) | 67.5 (59.3–76.6) | 68.9 (59.4–77.7) | 0.197 |
| NEUT# (×109/L) | 3.68 (2.68–5.16) | 4.09 (2.85–6.11) | < 0.001 |
| LYMPH% (%) | 22.0 (14.6–29.4) | 20.5 (13.3–28.6) | 0.009 |
| LYMPH# (×109/L) | 1.15 (0.83–1.51) | 1.17 (0.83–1.56) | 0.279 |
| MONO% (%) | 8.5 (6.3–11.0) | 8.5 (6.5–11.1) | 0.544 |
| MONO# (×109/L) | 0.47 (0.34–0.61) | 0.52 (0.37–0.69) | < 0.001 |
| EO% (%) | 0.2 (0.0–2.9) | 0.3 (0.0–1.2) | < 0.001 |
| EO# (×109/L) | 0.01 (0.00–0.05) | 0.02 (0.00–0.07) | < 0.001 |
| BASO% (%) | 0.2 (0.0–0.3) | 0.2 (0.1–0.3) | < 0.001 |
| BASO# (×109/L) | 0.01 (0.00–0.02) | 0.01 (0.01–0.02) | < 0.001 |
| RBC# (×1012/L) | 4.43 (4.00–4.84) | 4.37 (3.96–4.78) | 0.012 |
| HGB (g/L) | 134 (122–146) | 131 (119–143) | < 0.001 |
| HCT (%) | 39.7 (36.2–43.1) | 39.1 (35.5–42.4) | 0.002 |
| MCV (fL) | 89.1 (86.4–91.7) | 89.6 (86.7–92.4) | 0.003 |
| MCH (pg) | 30.6 (29.5–31.6) | 30.4 (29.3–31.3) | 0.002 |
| MCHC (g/L) | 343 (335–351) | 337 (329–346) | < 0.001 |
| RDW-CV | 12.2 (11.9–12.8) | 12.5 (12.0–13.2) | < 0.001 |
| RDW-SD (fL) | 39.5 (37.8–41.8) | 40.9 (38.8–43.2) | < 0.001 |
| PLT# (×109/L) | 195 (152–248) | 198 (156–248) | 0.421 |
| PDW (fL) | 12.0 (10.8–13.6) | 12.3 (11.0–13.9) | 0.021 |
| MPV (fL) | 10.6 (10.0–11.3) | 10.6 (10.0–11.4) | 0.452 |
| PLCR (%) | 29.5 (24.5–35.1) | 29.6 (24.9–36.0) | 0.254 |
| THR (%) | 0.21 (0.17–0.26) | 0.21 (0.17–0.26) | 0.328 |
NCPP, novel coronavirus pneumonia patients; IP, Influenza patients; WBC#, white blood cell count; NEUT%, neutrophil percentage; NEUT#, neutrophil count; LYMPH%, lymphocyte percentage; LYMPH#, lymphocyte count; MONO%, monocyte percentage; MONO#, monocyte count; EO%, eosinophil percentage; EO#, eosinophil count; BASO%, basophil percentage; BASO#, basophil count; RBC#, red blood cell count; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW-CV, coefficient variation of red blood cell volume distribution width; RDW-SD, standard deviation in red cell distribution width; PLT#, platelet count; PDW, platelet distribution width; MPV, mean platelet volume; PLCR, platelet larger cell ratio; THR, thrombocytocrit
Data were presented as medians (25th–75th percentages)
Comparisons were performed between NCPP and IP groups using Mann-Whitney U test
For biochemistry, GGT, ALP, UA, Ca, Cl, and P in NCPP were significantly lower than in IP. Conversely, ALT, AST, TP, GLB, IBILI, LDH, TG, HDL-C, LDL-C, CREA, UR, Mg, K, Na, HCO3, hsCRP, and ESR in NCPP were significantly higher than in IP (Table 3 ). The other marker, including ALB, TBILI, DBILI, TC, and PCT, had no significant difference between NCPP and IP.
Table 3.
The results of biochemical indicators in study participants.
| Variables | NCPP (n = 1,027) | IP (n = 1,140) | P* |
|---|---|---|---|
| ALT (U/L) | 25 (18–38) | 24 (16–36) | 0.019 |
| AST (U/L) | 27 (21–36) | 25 (19–35) | < 0.001 |
| TP (g/L) | 69.3 ± 5.6 | 68.5 ± 6.4 | 0.003 |
| ALB (g/L) | 36.5 ± 4.9 | 36.6 ± 5.0 | 0.329 |
| GLB (g/L) | 32.8 ± 4.4 | 31.8 ± 4.8 | < 0.001 |
| TBILI (μmol/L) | 9.5 (7.4–12.6) | 9.1 (6.9–12.9) | 0.125 |
| DBILI (μmol/L) | 3.9 (3.1–5.4) | 4.0 (3.1–6.0) | 0.145 |
| IBILI (μmol/L) | 5.5 (4.2–7.3) | 4.9 (3.8–6.9) | < 0.001 |
| GGT (U/L) | 30 (21–48) | 35 (21–54) | 0.003 |
| ALP (U/L) | 65 (56–78) | 75 (63–96) | < 0.001 |
| LDH (U/L) | 260 (217–327) | 235 (196–298) | < 0.001 |
| TC (mmol/L) | 3.91 ± 0.75 | 3.97 ± 0.99 | 0.998 |
| TG (mmol/L) | 1.75 ± 0.88 | 1.63 ± 0.84 | < 0.001 |
| HDL-C (mmol/L) | 0.99 ± 0.19 | 0.97 ± 0.22 | 0.002 |
| LDL-C (mmol/L) | 2.45 ± 0.55 | 2.41 ± 0.68 | 0.004 |
| CREA (μmol/L) | 72 (61–87) | 69 (59–82) | < 0.001 |
| UR (mmol/L) | 5.89 ± 3.84 | 5.54 ± 3.41 | 0.001 |
| UA (μmol/L) | 253 (206–313) | 260 (219–304) | 0.031 |
| Ca (mmol/L) | 2.14 ± 0.11 | 2.17 ± 0.11 | < 0.001 |
| Mg (mmol/L) | 0.87 ± 0.07 | 0.86 ± 0.09 | 0.001 |
| Cl (mmol/L) | 100.4 ± 4.2 | 101.4 ± 3.7 | < 0.001 |
| K (mmol/L) | 4.21 ± 0.42 | 4.15 ± 0.40 | < 0.001 |
| Na (mmol/L) | 139.7 ± 3.9 | 139.1 ± 3.4 | < 0.001 |
| P (mmol/L) | 1.04 ± 0.26 | 1.05 ± 0.20 | 0.002 |
| HCO3 (mmol/L) | 24.5 ± 2.9 | 24.0 ± 3.1 | < 0.001 |
| hsCRP (mg/L) | 20.0 (5.8–45.8) | 15.7 (4.8–40.1) | 0.024 |
| ESR (mm/h) | 35 (24–47) | 27 (17–40) | < 0.001 |
| PCT (ng/mL) | 0.08 (0.05–0.16) | 0.07 (0.04–0.19) | 0.193 |
NCPP, novel coronavirus pneumonia patients; IP, Influenza patients; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TP, total protein; ALB, albumin; GLB, globulin; TBILI, total bilirubin; DBILI, direct bilirubin; IBILI, indirect bilirubin; CREA, creatinine; UR, urea; UA, uric acid; GGT, γ-glutamyl transpeptidase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Ca, calcium; Mg, magnesium; Na, natrium; P, phosphate; Cl, chlorine; K, kali; HCO3, bicarbonate ion; hsCRP, hypersensitive C-reactive protein; ESR, erythrocyte sedimentation rate; PCT, procalcitonin
Data were presented as means ± SD or medians (25th–75th percentages).
Comparisons were performed between NCPP and IP groups using Mann-Whitney U test
For coagulation function, PT, PTA and DD in NCPP were significantly lower than in IP. In contrast, APTT, TT and FIB in NCPP were significantly higher than in IP (Table 4 ). However, the INR had no statistical difference between NCPP and IP.
Table 4.
The results of coagulation function indexes in study participants.
| Variables | NCPP (n = 1,027) | IP (n = 1,140) | P* |
|---|---|---|---|
| PT (s) | 14.06 ± 1.09 | 14.09 ± 1.83 | < 0.001 |
| APTT (s) | 39.9 ± 4.5 | 39.6 ± 5.0 | 0.020 |
| TT (s) | 16.9 ± 1.4 | 16.6 ± 2.0 | < 0.001 |
| INR | 1.08 ± 0.11 | 1.10 ± 0.20 | 0.091 |
| PTA (%) | 91 ± 11 | 92 ± 14 | < 0.001 |
| FIB (g/L) | 4.71 ± 1.08 | 4.27 ± 1.18 | < 0.001 |
| DD (mg/L) | 1.24 (0.65–2.75) | 1.72 (0.85–3.30) | < 0.001 |
NCPP, novel coronavirus pneumonia patients; IP, influenza patients; PT, prothrombin time; APTT, activated partial thromboplastic time; TT, thrombin time; INR, international normalized ratio; PTA, prothrombin activity; FIB, fibrinogen; DD, d-dimer
Data were presented as means ± SD or medians (25th–75th percentages)
Comparisons were performed between NCPP and IP groups using Mann-Whitney U test
3.3. Using a diagnostic model based on a combination of various indicators to distinguish NCPP from IP
To establish a diagnostic model based on a combination of various indicators for distinguishing NCPP from IP, all variables with statistical significance were used for multivariable logistic regression analysis. A diagnostic model was built as follows:
P = 1/[1 + e-(-0.049*WBC#-0.571*MONO#-11.562*BASO#+0.005*HGB-0.055*RDW-SD+0.021*TP-0.023*ALP+0.003*LDH+0.245*TG+0.92*HDL-C-2.594*Ca+0.597*K-0.054*Cl+0.079*HCO3+0.019*ESR+0.118*TT-0.038*DD+0.262*FIB+3.149)]
P, predictive value; e, natural logarithm
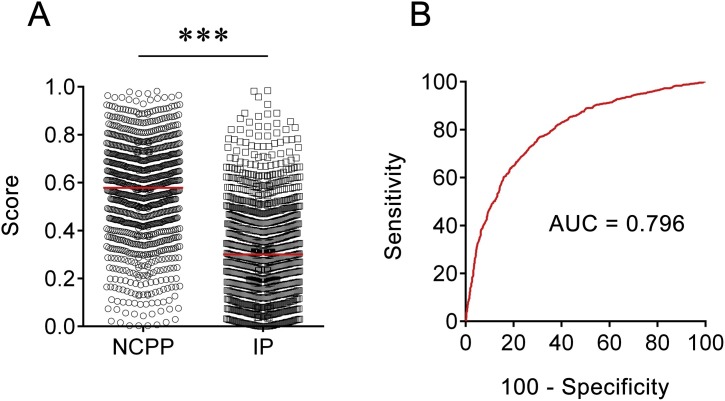
ROC analysis showed that the AUC of the diagnostic model was 0.796 (95% CI, 0.777–0.814) (Fig. 1 ). When the cut-off value was set at 0.598, the following diagnostic parameters of the diagnostic model were obtained: sensitivity, 46.93% (95% CI, 43.85–50.04%); specificity, 90.09% (95% CI, 88.17–91.73%); PPV, 81.01% (95% CI, 77.57–84.04%); NPV, 65.33% (95% CI, 62.91–67.67%); PLR, 4.73 (95% CI, 3.93–5.71); NLR, 0.59 (95% CI, 0.56–0.62); and accuracy, 69.64%. These data suggest that the established model based on a combination of 18 indicator biosignatures had moderate performance in differentiation between NCPP and IP.
Fig. 1.
The performance of the diagnostic model in distinguishing patients with SARS-CoV-2 infection from those with influenza virus infection. (A) Scatter plots showing the score of the diagnostic model in NCPP (n = 1027) and IP (n = 1140). Horizontal lines indicate the median. ***p < 0.001 (Mann-Whitney U test) (B) ROC analysis showing the value of the diagnostic model in distinguishing NCPP from IP. NCPP, novel coronavirus pneumonia patients; IP, Influenza patients; AUC, area under the curve.
4. Discussion
The differentiation between NCPP and IP is still a challenge in clinical practice, especially during the outbreak of COVID-19. Fever, cough and shortness of breath are the most common symptoms in NCPP, but these symptoms are also reported in IP. Using symptoms has very limited value in distinguishing NCPP from IP. Bilateral pneumonia and ground-glass opacity are the typical imaging findings of NCPP, but they are also common in IP (Shi et al., 2020, Zhou et al., 2020). At present, differentiation between NCPP and IP is mainly according to specific nucleic acid detection for SARS-CoV-2 and influenza virus; however, false negative results could occur in clinical practice. Thus, further investigation is needed into which method can best be used to differentiate between NCCP and IP.
The comparison of laboratory test results between patients infected with SARS-CoV-2 and those infected with influenza virus has rarely been investigated. It is believed that the current study is the first large-scale clinical evaluation to compare routine laboratory test results between NCPP and IP. Based on the combination of 18 biomarkers, an optimal diagnostic model was successfully built, which has potential value in distinguishing NCPP from IP in a large cohort of participants. Besides, this model is the first to be constructed based on a combination of routine laboratory tests. It is undeniable that the diagnostic model shows moderate value in differential diagnosis between NCPP and IP. However, it is believed that this model could serve as a complement to other approaches, including CT and molecular tests, especially in some resource-limited areas.
There were some implications when the routine laboratory tests were compared between NCPP and IP. In general, the difference in laboratory indexes between adult patients with SARS-CoV-2 infection and those with influenza virus infection is not very obvious, which means that there may be some common reaction mechanism in the body's response to these two kinds of viruses. However, some indicators were found that were quite different between NCPP and IP. Patients with SARS-CoV-2 infection had a greater reduction in the number of white blood cells, especially in the number of neutrophils, compared with patients with influenza virus infection. This is inconsistent with previous studies showing that a decrease in lymphocytes is one of typical characteristics of laboratory results in NCPP (Huang et al., 2020, Chen et al., 2020). Actually, the number of lymphocytes was also found to be decreased in IP (most values were below the lower limit of the normal range). Thus, based on the decrease of lymphocytes, patients maybe misdiagnosed as NCP during the outbreak of COVID-19 when they actually have influenza virus infection. The current results suggest that patients who have decreased numbers of lymphocytes and neutrophils may be more likely to be infected with SARS-CoV-2.
On the other hand, the results showed that the inflammatory indicators, including ESR and hsCRP, were significantly increased in NCPP compared with IP, which indicated that the inflammatory and immune responses in NCPP may be more active. This suggests that more attention must be paid to the immune status of the NCPP and that immune status monitoring may be helpful for differential diagnosis between patients with SARS-CoV-2 infection and those with influenza virus infection. Surprisingly, higher levels of RBC and TP were observed in NCPP compared with IP, which indicates that the nutritional status of NCPP may be better than IP. However, higher levels of ALT, AST, IBILI, LDH, CREA and UR were also observed in NCPP patients compared with IP, which indicate that the impairment of liver and kidney function may be more common in NCPP. It was also observed that NCPP had higher levels of Mg, K, Na and HCO3 than IP, which indicate that electrolyte and acid-base balance disorders are more common in NCPP. Furthermore, higher levels of APTT and FIB in NCPP may indicate that NCPP has a higher risk of developing blood clots than IP, suggesting that more attention should be paid to coagulation in the treatment and care of NCPP. In all, these data suggest that internal environment disturbance is one of the important characteristics of NCPP. Further study into other areas such as imaging and histopathology is necessary, which could help a clearer distinction to be made between SARS-CoV-2 and influenza virus infection in clinical practice.
Several limitations should be noted. First, this study did not include children, and improved differential diagnosis between NCPP and IP in children is also urgently needed. Second, the performance of the diagnostic model in distinguishing NCPP from IP was preliminary. Further validation in multiple centers with a large sample size is required.
In conclusion, a diagnostic model was identified by incorporating routine blood examination, biochemical indicators and coagulation function, which is proven to have moderate performance in discriminating NCPP from IP. This model might serve as a useful adjunct to radiological and molecular methods in distinguishing NCPP from IP in clinical practice. Furthermore, these observations are relevant to guide further development of strategies for treatment and prognosis of both NCPP and IP.
Declaration of interests
All authors declare no competing interests.
Author contributions
YL, FW, and ZS designed the study and had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. YL, XY, YX, LM, QL, GT, and HS contributed to experiments. YL, WL and HH contributed to the statistical analysis. All authors contributed to data interpretation, and reviewed and approved the final manuscript.
5. Funding
This work was funded by grants from the National Mega Project on Major Infectious Disease Prevention of China (2017ZX10103005-007) and the National Natural Science Foundation (81401639).
Acknowledgements
We acknowledge the patients for cooperating with our investigation. We thank all healthcare workers involved in the diagnosis and treatment of patients in Wuhan.
References
- Chan J.F., Kok K.H., Zhu Z., Chu H., To K.K., Yuan S. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan. China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralinski L.E., Menachery V.D. Return of the Coronavirus: 2019-nCoV. Viruses. 2020;12(2) doi: 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paules C., Subbarao K. Influenza. Lancet. 2017;390(10095):697–708. doi: 10.1016/S0140-6736(17)30129-0. [DOI] [PubMed] [Google Scholar]
- The Lancet Emerging understandings of 2019-nCoV. Lancet. 2020;395(10221):311. doi: 10.1016/S0140-6736(20)30186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan. China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeki T.M. Influenza. Annals of Internal Medicine. 2017;167(5):ITC33–ITC48. doi: 10.7326/AITC201709050. [DOI] [PubMed] [Google Scholar]
- World Health Organization, Novel coronavirus (2019-ncov) situation report 41. Available online:https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/(accessed on 2 March 2020).
- Zhou S., Wang Y., Zhu T., Xia L. CT Features of Coronavirus Disease 2019 (COVID-19) Pneumonia in 62 Patients in Wuhan. China. AJR Am J Roentgenol. 2020:1–8. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]