Abstract
The rapid emergence of the COVID-19 pandemic is unprecedented and poses an unparalleled obstacle in the sixty-five year history of organ transplantation. Worldwide, the delivery of transplant care is severely challenged by matters concerning - but not limited to - organ procurement, risk of SARS-CoV-2 transmission, screening strategies of donors and recipients, decisions to postpone or proceed with transplantation, the attributable risk of immunosuppression for COVID-19 and entrenched health care resources and capacity. The transplant community is faced with choosing a lesser of two evils: initiating immunosuppression and potentially accepting detrimental outcome when transplant recipients develop COVID-19 versus postponing transplantation and accepting associated waitlist mortality. Notably, prioritization of health care services for COVID-19 care raises concerns about allocation of resources to deliver care for transplant patients who might otherwise have excellent 1-year and 10-year survival rates. Children and young adults with end-stage organ disease in particular seem more disadvantaged by withholding transplantation because of capacity issues than from medical consequences of SARS-CoV-2. This report details the nationwide response of the Dutch transplant community to these issues and the immediate consequences for transplant activity. Worrisome, there was a significant decrease in organ donation numbers affecting all organ transplant services. In addition, there was a detrimental effect on transplantation numbers in children with end-organ failure. Ongoing efforts focus on mitigation of not only primary but also secondary harm of the pandemic and to find right definitions and momentum to restore the transplant programs.
Keywords: COVID-19, SARS-CoV-2, Outbreak, Transplantation, Transplant programs
Abbreviations: COVID-19, Coronavirus Disease 2019; DTF/NTS, Dutch Transplant Foundation/Nederlandse Transplantatie Stichting; DTS/NTV, Dutch Transplant Society/Nederlandse Transplantatie Vereniging; ET, Eurotransplant; HCW, Healthcare Workers; ICU, Intensive Care Unit; MELD, Model for End-stage Liver Disease; PCR, Polymerase Chain Reaction; PPE, Personal Protection Equipment; RIVM, RijksInstituut voor Volksgezondheid en Milieu (National Institute for Public Health and the Environment); SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus-2; WHO, World Health Organization
1. Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic is affecting societies worldwide [1,2]. The scope of this disease is unprecedented and the global delivery of healthcare is under severe stress [3]. The first documented case of COVID-19 in the Netherlands, which counts 17.2 million inhabitants, was on February 27, 2020. In less than 60 days, despite increasingly stringent measures of the Dutch government to halt the spread of the infection, 28,153 individuals have tested positive for Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), 9127 patients have been admitted to hospitals across the country (of which 2508 in the Intensive Care Units (ICU) [4]) and 3134 have died, according to RIVM (National Institute for Public health and the Environment, April 15, 2020) [5]. Because of limited testing capacity many more, untested individuals are likely infected with SARS-CoV-2 in the Netherlands. With increasing numbers of COVID-19 patients being admitted to hospitals, the ability to provide acute, non-COVID-19 care, is at risk. In this context, especially maintaining organ transplantation care is complex, as numerous aspects related to donor and recipient management, need to be taken into account [[6], [7], [8]]. Main questions that need to be addressed include the following: Under which circumstances can organ donation from deceased donors take place? Is it safe to perform transplants from live donors? What are additional risks for transplant recipients in terms of immunosuppression and COVID-19? How should potential recipients be managed, both pre- and post-transplantation? Which treatments should be considered for transplant recipients with COVID-19? What are logistic implications and, most importantly, what is the impact of the outbreak on donation and transplant volumes and waitlist outcomes? The transplant community in the Netherlands, organized through the Dutch Transplant Society (DTS/NTV) and the Dutch Transplant Foundation (DTF/NTS, competent authority), rapidly aligned to reach national agreement on circumstances under which organ donation and transplant services can be safeguarded in the face of the COVID-19 pandemic. This manuscript details decisions that our transplant community made collectively in the first period in absence of solid scientific and clinical evidence while the pandemic was unfolding. In addition, it demonstrates the immediate devastating effects on donation and transplant volumes and mortality on the waiting list and preliminary efforts to safely restore the programs.
2. National and international collaboration
The DTF maintains the national list for patients awaiting organ transplantation and is responsible for organ donation policy, the allocation of organs through the international organ exchange organization Eurotransplant (ET), and coordination of the Dutch live donor kidney paired exchange program. DTF closely collaborates with national organ advisory committees of the DTS, whose members are content experts and represent all transplant centers. On March 9th, before the World Health Organization (WHO) declared the pandemic, the advisory committees increased their meeting frequency from once every 3 to 6 months in person to ± three times per week through videoconferencing. DTS, DTF and chairs of the advisory committees similarly met to share the most recent information and policies concerning COVID-19. Guidelines were made public through internet communications and newsletters from DTF for both patients and professionals [9]. DTF participated in bi-weekly meetings with representatives of all ET members states to learn about developments and issues concerning national restrictions, donor screening, transport logistics and transplantation activities related to COVID-19. Recommendations published by RIVM, The Transplant Society [10] and European Centre for Disease Prevention and Control [11] were swiftly adopted. The existing framework of collaboration allowed for rapid exchange, first focusing on how to navigate through the pandemic, followed by tracking down the consequences for donation and transplant volumes and finding definitions how to restore transplant programs.
3. Developments in hospital capacity
In regard to donation and transplant activity, ICU bed capacity per million inhabitants is of major importance. Under normal conditions, the Netherlands has approximately 1150 ICU beds, which is 6.7 ICU beds per 100.000 people. In comparison: the USA has about 34.7 beds per 100.000, Germany 29.2, Italy 12.5, France 11.6 and the UK 6.6. In the current pandemic, hospital capacity and critical care facilities are severely stressed by the number of COVID-19 patients. In order to provide optimal care for these patients, a centrally coordinated national effort was made to double ICU capacity to a total of 2400 in early April 2020. Regular ICU- and medium care, including transplant care was scaled down to meet this demand. Regarding our concerns for donation and transplant rates we therefore raised national awareness through (social) media and by direct contact with the network of intensive care physicians involved in organ donation and organ tissue transplant coordinators. In addition, a letter was sent to relevant professional organizations and the national coordination center for capacity. Currently approximately 500 ICU beds are reserved to allow for (semi-)urgent non-COVID-19 care, including donation and transplantation.
4. Organ donation from deceased donors
Health care workers (HCW) involved in organ donation are at increased risk of acquiring and spreading SARS-CoV-2, since they work closely together and travel to various hospitals and visit virus hotspots. To preserve a safe organ donation chain, recommendations to reduce the risk of infection of HCW and transplant recipients were made recognizing limitations of available scientific evidence. First, SARS-CoV-2 testing of all deceased donors was implemented, since asymptomatic carriers and transmission through organ transplantation were deemed possible [12]. SARS-CoV-2 viral load testing was performed preferably on endotracheal sputum rather than a swab for increased sensitivity (Table 1A). When testing is not possible, the donor has been tested positive or the test result is unknown, organ donation should not proceed. As viral load tests tend to have long turn-around times, until now three donor families have retracted their permission for organ donation during work up at the ICU, because they could not bear the extended waiting times. So far, no donor has been tested positive for SARS-CoV-2; in one donor the retrieval procedure was cancelled because of a high suspicion of COVID-19 based on chest CT, but with a negative PCR. This was followed by discussions about necessity of CT scan for donor screening [13]. A CT scan provides rapid information on COVID-19 in patients with moderate to severe symptoms and is of additional value in patients with a single false negative nasopharyngeal swab for SARS-CoV-2 [14]. To date, there is no data on sensitivity and specificity in asymptomatic patients and therefore not recommended in international guidelines [15]. The risk of losing additional organ donors because of false negative COVID-19 associated abnormalities on CT-scan such as ground-glass opacities and consolidation in e.g. neurogenic oedema is deemed high. In this light, chest CT-scan is now only used in potential organ donors with negative nasopharyngeal swab but an inconclusive history for excluding COVID-19. To maintain social distancing during organ procurement, additional vehicles for transport to donor hospitals are used, and chauffeurs and HCW were advised to wear facial personal protection equipment (PPE). So far, no donation professionals were infected during procedures. To facilitate extra time needed for recipient test results to become available, allocation for liver, heart and lung transplantation is initiated before donor SARS-CoV-2 screening is known (Table 1A). Additionally, the second recipient on the allocation list is allocated to minimize risk of donor loss related to prolonged cold ischemia times. Despite these efforts, donation volumes markedly decreased in the first month as the pandemic unfolded (15 March 2020–15 April 2020) compared to the months before (Fig. 1A). The reasons for this are not completely clear. Traffic has decreased due to increased working from home, leading to less traffic accidents and trauma patients becoming organ donors. Also, there have been signs that people have become more reluctant to call for medical help, fearing to be a burden for medical professionals or to be infected with COVID-19 at a health care facility. This could also be the case when urgent medical assistance is needed, as in acute coronary syndromes, subarachnoidal bleedings et cetera. Finally, donor awareness among emergency physicians and intensivists could have decreased due to the strain put on them by the COVID-19 epidemic. Currently, we are investigating these issues. Additionally, transport logistics have been a main challenge in international organ exchange, because of different policies and restrictions in different countries (e.g. closed national/regional borders; significant drop in air traffic).
Table 1.
Donor and recipient screening and approach to transplant activity.
| A. Summary of donor and recipient screening | |||
|---|---|---|---|
| Deceased donor screening | Living donor screening | Pre transplant screening | Approach to waiting list patient after COVID infection |
| Universal NAT (NP or BALa) | Universal NAT (NP) | Clinical, NAT where testing available For liver, heart and lung: To facilitate the extra time needed for recipient test results to become available, allocation for was initiated before donor SARS-CoV-2 screening result is known |
Kidney/pancreas: COVID-19 positive patients could return on the active organ waiting list 2 weeks after resolution of clinical symptoms and negative COVID-19 NP swab PCR Liver: COVID-19 positive patients could return on the active organ waiting list after resolution of clinical symptoms and negative COVID-19 NP swab PCR Heart/Lung: COVID-19 positive patients could return on the active organ waiting list after resolution of clinical symptoms and negative COVID-19 NP swab PCR |
| B. Step-wise approach to transplant activity | |||
|---|---|---|---|
| Kidney/Pancreas/Islet transplantation | Liver transplantation | Heart Lung Transplantation | |
|
|
|
|
Abbreviations: NAT, nucleic acid testing; BAL, bronchoalveolar lavage; NP, nasopharyngeal; PCR, Polymerase Chain Reaction.
BAL was preferred.
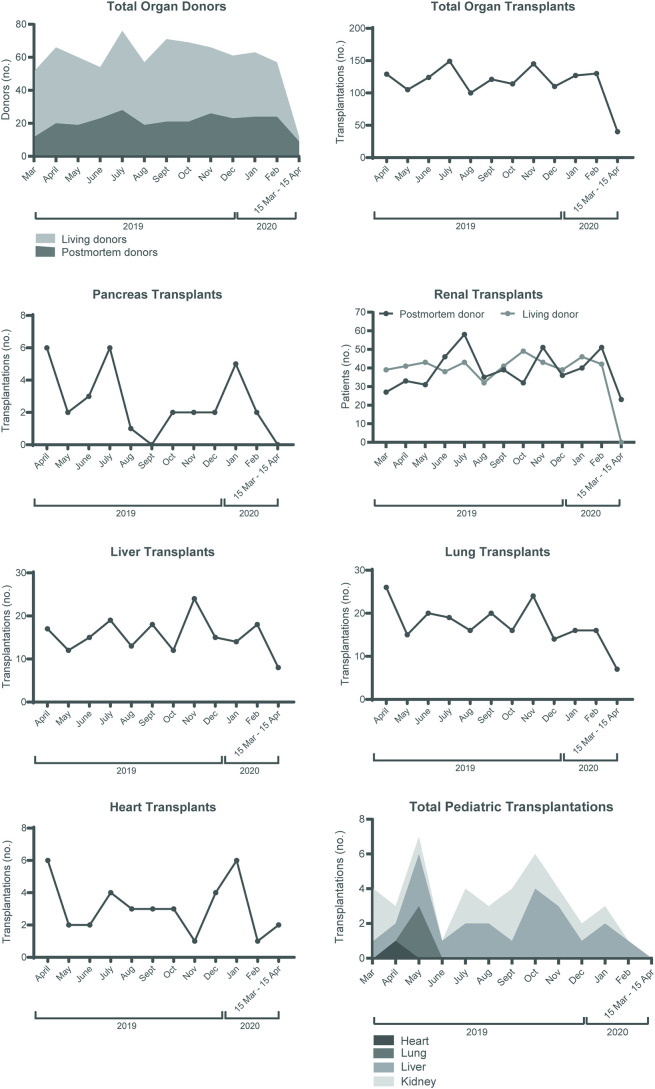
Fig. 1.
Impact of the COVID-19 outbreak on organ donation and transplant activity in the Netherlands.
Depicted is the number of donors and transplants performed over the last year per month in the Netherlands. The month when the pandemic was unfolding includes the dates 15 March 2020–15 April 2020. A) Living donors are shown in light grey and post-mortem donors in dark grey. B) the number of total organ transplants, C) pancreas transplants D) renal transplants from living (grey) and post-mortem donors (black), E) liver transplants, F) lung transplants G) heart transplants and H) the total number of pediatric transplantations (age 0–16 years).
5. Organ donation from live donors
In line with international policies, the Dutch transplant community decided to suspend the majority of living donor transplant programs. It was considered generally unethical and unsafe to expose healthy individuals to an operation that would potentially increase their risk of acquiring SARS-CoV-2 in hospital or developing more significant COVID-19 postoperatively because of weakened immunity. As a result, all living-donor kidney transplant programs, a yearly volume of about 500 per year, have halted resulting in approximately 65 cancelled transplants in the 6 weeks since COVID-19 was first observed in the Netherlands. A national committee, consisting of relevant stakeholders including surgeons, nephrologists, ethicists and DTS, DTF and patient representatives, has been installed to determine under which circumstances living-donor kidney transplants can be restored (step-wise) in a safe and responsible manner. Living-donor pediatric liver transplantation, with a volume of 15 per year (50% of all pediatric liver transplants), however, continues. The decision to continue this program was taken after careful consideration of donor risks in view of the lifesaving nature of the procedure and absence of liver-replacement therapy. In anticipation of a prolonged period of low numbers of deceased donor organs, living donation is promoted where possible, and it is decided to transplant across the ABO blood group barrier in small children.
6. Risks and benefits to transplant recipients
As has been discussed in several other reports, the attributable risk of immunosuppression on the development and severity of COVID-19 is unknown. Current knowledge is mainly based on recently published case reports [[16], [17], [18], [19], [20]]. Previous reports on transplant recipient outcome for other respiratory viruses and Acute Respiratory Distress Syndrome are contradictory [21,22] and several immunosuppressive agents are even hypothesized to have antiviral properties [23,24]. It is however evident that frail individuals, including patients with multiple comorbidities, are at increased risk of COVID-19 [5]., One could therefore argue that transplantation may in fact, in the longer run, ameliorate that risk. In light of limited and contradictory evidence, and in part driven by stretched and overwhelmed hospital capacity, the Dutch transplant community was an early adopter of the recommendations made by Kumar et al. (Table 1B) [6]. In general, this led to prudence in performing kidney transplantation until the impact of induction and maintenance immunosuppression on COVID-19 is more understood. Deceased-donor kidney transplantation is considered case-by-case in a shared decision process with the patient, carefully weighing the urgency and benefit of transplantation against risks. This approach allowed for regional differences among centers while sharing a similar policy. More specifically, kidney and pancreas transplantations for which T- and/or B-cell depleting induction therapy is deemed necessary (ABO and Human Leukocyte Antigen-incompatible and other high-immunological risk transplantations) are halted. Even though current evidence suggest that children are generally less impacted by COVID-19, pediatric kidney transplant programs are restricted similarly to adult programs. Given the high waiting list mortality and suboptimal or absent alternative treatment options for end-stage disease, liver-, heart- and lung-transplantation should continue in principle. For liver it is agreed that transplantation should continue to be focused on the patients with the highest need, i.e. high-urgent cases, those with Model for End-stage Liver Disease (MELD) >20, or with otherwise life-threatening disease such as hepatocellular carcinoma approaching borders of Milan criteria (Table 1B). As discussed before, with progression of the COVID-19 pandemic, ICU capacity across the country is limited. As a result, per March 25, all heart and lung transplant-centers have limed their capacity for now to urgent cases with low life expectancy if not transplanted (Table 1B). It is expected that the need for urgent lung transplantation may rise in the upcoming weeks for patients with severe ARDS [25]. As community acquired risk for COVID-19 is high, it is agreed that COVID-19 positive patients could return on the active organ waiting list after resolution of clinical symptoms and negative COVID-19 nasopharyngeal swab or sputum PCR (Table 1B). At this point, there are no specific criteria for these patients in the ET region, but mono-organ failure with preserved rehabilitation capacity is generally recommended.
Regarding post-transplant care, all elective post-transplant outpatient care is replaced by telephone and e-consultations when possible. In addition, centers shared local treatment protocols for transplant recipients. Protocols were based on RIVM recommendations for therapy and included recommendations on triage (admission versus home monitoring), adjustment of immunosuppression and drug-drug interactions [26] and international guidelines [27,28]. COVID registries were started and linked to existing national and international (i.e., European ERACODA) databases [29].
7. Impact of the pandemic on transplant activity
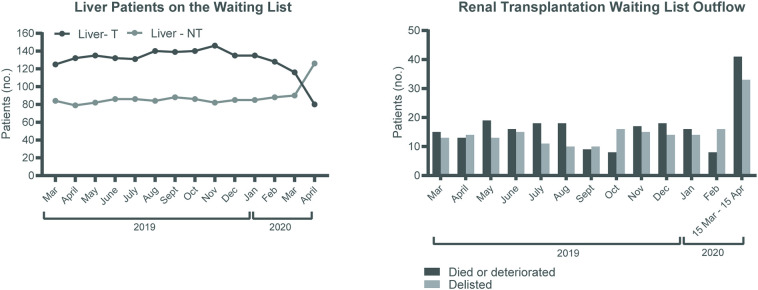
The total amount of all organ transplants markedly decreased from 100 to 150 to 40 transplants per month in the first COVID-19 month, a decrease of 67% (Fig. 1B). This is in contrast to the COVID-19 outbreak in Italy, were the activity remained stable for each type of solid organ [30]. However, here the first 4 weeks of the outbreak was taken into account, while we have taken the period 27th of February till the 15th of April. Pancreas transplants decreased to 0 and renal transplants from 80 to 100 to 20 per month (Fig. 1C-D). The number of liver and lung transplants markedly declined from a mean of 16 to 8 per month and from 18 to 7 per month, respectively, a decrease of approximately 50% (Fig. 1E-G). There was no pediatric transplant activity in the first COVID-19 month (Fig. 1H). Related to the focus on patients with the highest need there was a change in transplantable and non-transplantable status on the liver transplantation waiting list as shown in Fig. 2A. Another alarming and slightly unexpected finding was the higher number of renal patients removed from the waiting list because of mortality or deterioration in the first 6 weeks since the first COVID-19 case in the Netherlands was observed (Fig. 2B). It is too early on in the pandemic to determine cause and effect, and similar trends have not been observed for other organs yet, but this should be monitored closely. Effects will become clear in the coming months as centers have limited capacity for transplantation and screening of new patients. Efforts focus on the right definitions and step-wise momentum to fully restore the transplant programs.
Fig. 2.
Impact of the COVID-19 outbreak on the liver transplant waiting list and kidney waiting list outflow in the Netherlands.
A) the number transplantable (T) and non-transplantable (NT) patients on the waiting list for a liver transplantation in the Netherlands over the last year. B) the number of end stage renal disease patients taken of the renal transplant waiting list due to death or deterioration of the disease (black bars) or due to other reasons (grey bars) in the Netherlands over the last year. Other reasons include on hold (5), medical contra indication (3), too good for transplantation (19), moved abroad (1), patient doesn't want to be transplanted anymore (3), no reason mentioned (2).
8. Summary
The COVID-19 pandemic affects the whole chain of organ donation and transplantation. In the Netherlands the transplant community quickly aligned. This resulted in the development of new national guidelines regarding organ donation in the ICU, test requirements for potential donors and organ recipients and national rules regarding the acceptance of organs by transplant centers. Worrisome, donation and transplant volumes markedly decreased and mortality on the waiting list of renal transplantation increased. The pandemic will have ongoing effects in the long term. Waiting lists will increase, resulting in increased waiting list mortality and worse pre-operative conditions. It is therefore of great importance to find the right momentum and definitions under which to fully restore transplant programs.
Funding
The authors declare that no funding was received for this work.
Declaration of Competing Interest
None.
Acknowledgments
The authors would like to thank the remaining members of the Dutch Transplant Society (NTV): M.J. Hoogduijn, A. van der Meer, J.H. Annema and H.G.D. Leuvenink; the National Consultation Kidney Transplantation (LONT): S.P. Berger, M.D. Stenhuijs, F.J. Bemelman, M. Idu, M.H.L. Christiaans, G.W. Schurink, J. van de Wetering, D. Kimenai, V.A.L. Huurman, L.B. Hilbrands, P. Poyck, J.S. Sanders, R. Pol, A. van Zuilen, R. Toorop, A. Nurmohamed, A. Hoksbergen and H. de Jong; National Consultation Transplantation Thoracic Organs (LOTTO): D.S.B.M. Mol-Schreurs, M.E. Erasmus, E.A.M. Verschuuren, K. Damman, N. de Jonge, H.D. Luijk, O.C. Manintveld and J.A. Bekkers, Landelijk Overleg Uitname Teams (LORUT): K.M. de Vries, M. van der Poll, M. van der Jagt, R.A. Pol, M.E. Erasmus, J.P.C. Sonneveld, J.A.M. Hagenaar, W. Hordijk and M. Danhof; National Consultation on liver transplantation (LOL): R.J. Porte, R. Scheenstra, J. Dubbeld, B. van Hoek, W. Polak and H.J. Metselaar for their contributions in the organ committees; and Y.H. Hoogerwerf for contributions to overall communication.
References
- 1.Fauci A.S., Lane H.C., Redfield R.R. Covid-19 - navigating the uncharted. N. Engl. J. Med. 2020;382(13):1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emanuel E.J., Persad G., Upshur R., Thome B., Parker M., Glickman A., Zhang C., Boyle C., Smith M., Phillips J.P. Fair allocation of scarce medical resources in the time of Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMsb2005114. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.National Intensive Care Evaluation - COVID-19 Infecties op de IC'’s. https://stichting-nice.nl/. Accessed April 15, 2020.
- 5.Rijksinstituut voor Volksgezondheid en Milieu. https://www.rivm.nl/coronavirus/covid-19. Accessed April 15, 2020.
- 6.Kumar D., Manuel O., Natori Y., Egawa H., Grossi P., Han S.H., Fernandez-Ruiz M., Humar A. COVID-19: a global transplant perspective on successfully navigating a pandemic. Am. J. Transplant. 2020 doi: 10.1111/ajt.15876. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martino F., Plebani M., Ronco C. Lancet Respir Med Published Online April 16. 2020. Kidney transplant programmes during the COVID-19 pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Global Transplantation Covid Report 2020. https://tts.org/index.php?option=com_content&view=article&id=696&Itemid=115 [DOI] [PMC free article] [PubMed]
- 9.Transplantatie stichting. https://www.transplantatiestichting.nl/publicaties-en-naslag/nieuws/het-coronavirus-en-adviezen-voor-transplantatiepatienten. Accessed 28 March 2020.
- 10.The Transplantation Society https://tts.org. Accessed 28 March 2020.
- 11.European Centre for Disease Prevention and Control. https://www.ecdc.europa.eu/en. Accessed 28 March 2020.
- 12.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leidraad voor Opname van Patiënten met een ‘Verdenking op’ COVID-19 in het Ziekenhuis. Federatie Medisch Specialisten; 2020. March 27. [Google Scholar]
- 14.Xie C., Jiang L., Huang G., Pu H., Gong B., Lin H., Ma S., Chen X., Long B., Si G., Yu H., Jiang L., Yang X., Shi Y., Yang Z. Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int. J. Infect. Dis. 2020;93:264–267. doi: 10.1016/j.ijid.2020.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin G.D., Ryerson C.J., Haramati L.B., Sverzellati N., Kanne J.P., Raoof S., Schluger N.W., Volpi A., Yim J.J., Martin I.B.K., Anderson D.J., Kong C., Altes T., Bush A., Desai S.R., Goldin J., Goo J.M., Humbert M., Inoue Y., Kauczor H.U., Luo F., Mazzone P.J., Prokop M., Remy-Jardin M., Richeldi L., Schaefer-Prokop C.M., Tomiyama N., Wells A.U., Leung A.N. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner society. Radiology. 2020:201365. doi: 10.1148/radiol.2020201365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J., Lin H., Wu Y., Fang Y., Kumar R., Chen G., Lin S. COVID-19 in posttransplant patients-report of 2 cases. Am. J. Transplant. 2020 doi: 10.1111/ajt.15896. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li F., Cai J., Dong N. First cases of COVID-19 in heart transplantation from China. J. Heart Lung Transplant. 2020 doi: 10.1016/j.healun.2020.03.006. March 17 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu B., Wang Y., Zhao Y., Shi H., Zeng F., Chen Z. Successful treatment of severe COVID-19 pneumonia in a liver transplant recipient. Am. J. Transplant. 2020 doi: 10.1111/ajt.15901. Apr 3 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aigner C., Dittmer U., Kamler M., Collaud S., Taube C. COVID-19 in a lung transplant recipient. J. Heart Lung Transplant. 2020 doi: 10.1016/j.healun.2020.04.004. Apr 13 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Columbia University Kidney Transplant Program Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J. Am. Soc. Nephrol. 2020;31(4) doi: 10.1681/ASN.2020030375. April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim Y.J., Boeckh M., Englund J.A. Community respiratory virus infections in immunocompromised patients: hematopoietic stem cell and solid organ transplant recipients, and individuals with human immunodeficiency virus infection. Semin Respir. Crit. Care Med. 2007;28(2):222–242. doi: 10.1055/s-2007-976494. [DOI] [PubMed] [Google Scholar]
- 22.Al Ghamdi M., Alghamdi K.M., Ghandoora Y., Alzahrani A., Salah F., Alsulami A., Bawayan M.F., Vaidya D., Perl T.M., Sood G. Treatment outcomes for patients with middle eastern respiratory syndrome coronavirus (MERS CoV) infection at a coronavirus referral center in the Kingdom of Saudi Arabia. BMC Infect. Dis. 2016;16:174. doi: 10.1186/s12879-016-1492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic. Liver Transpl. 2020 doi: 10.1002/lt.25756. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.de Wilde A.H., Zevenhoven-Dobbe J.C., van der Meer Y., Thiel V., Narayanan K., Makino S., Snijder E.J., van Hemert M.J. Cyclosporin a inhibits the replication of diverse coronaviruses. J. Gen. Virol. 2011;92(Pt 11):2542–2548. doi: 10.1099/vir.0.034983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han W., Zhu M., Chen J., Zhang J., Zhu S., Li T., Cai H., Fang Q., Wei G., Liang T. Lung transplantation for elderly patients with end-stage COVID-19 pneumonia. Ann. Surg. 2020 doi: 10.1097/SLA.0000000000003955. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meziyerh S., Zwart T.C., van Etten R.W., Janson J.A., van Gelder T., Alwayn I.P.J., de Fijter J.W., Reinders M.E.J., Moes D.J.A.R., de Vries A.P.J. Severe COVID-19 in a renal transplant recipient; a focus on pharmacokinetics. Am. J. Transplant. 2020 doi: 10.1111/ajt.15943. Published online April, 2020 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medicamenteuze Behandelopties bij Patiënten met COVID-19 (Infecties met SARS-CoV-2). https://swab.nl/nl/covid-19. Accessed March 28, 2020.
- 28.ISHLT Guidance for Cardiothoracic Transplant and VAD Centers (Updated March 22). https://ishlt.org/covid-19-information. Accessed March 28, 2020.
- 29.Kieneker L., Pena M., de Vries H. 2020. ERACODA: The ERA-EDTA COVID-19 Database for Patients on Kidney Replacement Therapy. April 8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angelico R., Trapani S., Tommaso M.M., Lombardini L., Tisone G., Cardillo M. The COVID-19 outbreak in Italy: initial implications for organ transplantation programs. Am. J. Transpl. 2020 doi: 10.1111/ajt.15904. April 03. [DOI] [PMC free article] [PubMed] [Google Scholar]