Dear Editor.
The correlation between SARS-CoV-2 infection and rheumatic disease, such as rheumatoid arthritis (RA) and Systemic Lupus Erythematosus (SLE), have been received attention of rheumatologists [1]. In this topic, there are two issues should be concerned: 1) Whether the SARS-CoV-2 infection trigger autoimmune disorder in patients with COVID-19? 2) How is the SARS-CoV-2 susceptibility in rheumatic disease patients? This paper provides an overview on these topics.
Could SARS-CoV-2 increase the risk of autoimmune disease
Indeed, It has been established that SARS-CoV-2 infection enhances the release of multiple cytokines, such as IL-1b, IFN-γ, TNF-α, IL-4, IL-10, IL-8, IL-9, IL-6, etc. [2,3]. Among these increased cytokines, there are both pro-inflammatory cytokines and anti-inflammatory cytokines, which suggested that the balance of immune response was undergoing a great test in patients with COVID-19.
As we know, autoimmune tolerance is an important part of immune homeostasis, which could be destroyed by multiple reasons, including pathogen infections. The production of multiple auto-antibodies is an important marker of autoimmune tolerance deficiency which is one of a trigger for autoimmune disorder diseases. Kerr JR have showed that parvovirus B19 infection could give rise to production of a variety of auto-antibodies (such as anti-nuclear antibody, anti-dsDNA antibody, etc), and be a trigger for development of a diverse array of autoimmune diseases [4].
A recent study by Zhou Y and colleagues showed that autoimmune phenomena exist in some patients with COVID-19 [5]. Among 20 COVID-19 subjects, the prevalence of anti-52 kDa SSA/Ro antibody, anti-60 kDa SSA/Ro antibody and antinuclear antibody were 20%, 25% and 50% respectively, while multiple antibodies (i.e. Anti-Scl-70, Anti-Jo-1 antibody, Anticentromere B antibody, Anti-SmD1 antibody, Anti-SSB antibody, Anti-double-stranded DNA antibody, Anti-Streptolysin O antibody) were negative. We also test the rheumatoid factor (RF), anti-cyclic peptide containing citrulline antibodies (anti-CCP antibody) and Anti-Neutrophil Cytoplasmic Antibodies (ANCA), which are the indexes of rheumatic immune disease, in serum of ten COVID-19 patients. Our results showed that anti-CCP antibody were increased in two patients, while the RF and ANCA were negative in all subjects.
Of note, Caso F et al. pointed that radiological aspects of lung involvement in COVID-19, resemble characterizing pneumonia of autoimmune diseases, such as rheumatoid arthritis (RA), systemic sclerosis and eosinophilic granulomatosis with polyangiitis [6].
Taken together, the existence of auto-antibodies hint the increased risk of autoimmune disorder diseases in some patients with COVID-19. Further studies in larger cohorts of patients are advocated to clarify this opinion.
Evidences for SARS-CoV-2 susceptibility in patients with rheumatic disease
In our opinion, rheumatic disease patients are not immune to SARS-CoV-2 infection, which is coincide with Sawalha AH and Goyal M et al.'s views [7,8]. This opinion has been reinforced by the data from The COVID-19 Global Rheumatology Alliance Global Registry [9], which showed that 600 patients with rheumatic disease who have been diagnosed with COVID-19, including 144 RA patients and 72 SLE patients.
According to the WHO situation report (29 Apr 2020, 2:00 CEST), COVID-19 has been confirmed in 2,959,929 patients (including 202,733 deaths). In the context of COVID-19 epidemic, due to the social isolation management measures are widely adopted and people's awareness of self-protection were improvement, the current data of rheumatic disease patients infected by SARS-CoV-2 might not reflect the real susceptibility of patients. However, the potential susceptibility of SARS-CoV-2 in SLE patients would be deduced from the following indirect evidences.
First, in theory, due to the impairment of immune system and immunosuppressive related therapy, SLE patients are vulnerable to infection, including bacterial, viral and fungal. Danza A have pointed that immuneosuppressive drug, such as prednisone, methylprednisolone and cyclophosphamide increase the risk of infection [10]. A retrospective cohort study base on 24,504 SLE patients and 98,016 matched controls by Li TH et al. showed that SLE patients are at higher risk of severe herpes simplex virus (Incident rate ratio [IRR] 3.93, 95%CI: 3.1–5.0, p < .001) [11].
Second, Sawalha AH et al.'s paper have shown a significant overexpression ACE2 in SLE CD4+ T cells [12]. As ACE2 is a functional receptor for the viral spike glycoprotein that allows the entry of SARS-CoV-2 into cells, the result hints the increasing SARS-CoV-2 susceptibility in SLE patients.
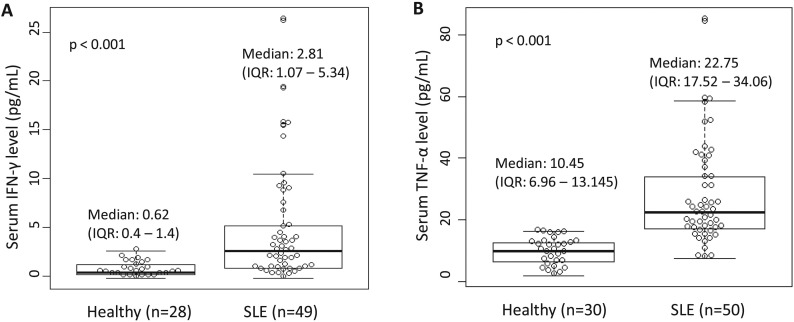
Third, Wang PH have showed that IFN-γ could stimulate the expression of ACE2 in human primary keratinocytes [13]. Of Note, multiple inflammatory cytokines including IFN-γ and TNF-α, have been found to be increased in patients with COVID-19 [2]. These results suggested that SARS-CoV-2 can up-regulate its receptor ACE2 in human host to facilitate their infection and spread. We have detected the serum IFN-γ and TNF-α levels in SLE patients and healthy subjects by using Luminex multiplex assays. Compared with healthy controls, the serum IFN-γ levels were significant higher in SLE patients (Fig. 1A). Combine with the evidence that up-regulation effect of IFN-γ on ACE2 expression, the increased levels of IFN-γ provide another evidence that SLE patients might be inherently more susceptible to SARS-CoV-2 infections.
Fig. 1.
Serum IFN-γ and TNF-α levels in SLE patients and healthy subjects. (A) IFN-γ and (B) TNF-α are significant increased in SLE patients.
Forth, Haga S and colleagues have found that TNF-α-converting enzyme (TACE) facilitates SARS-Cov viral entry into cell, via inducing ACE2 ectodomain shedding, and the process is tightly coupled with TNF-α production [14]. Thus, TNF-α might be a marker for efficient viral entry. Of note, we have found the increased of serum TNF-α level in SLE patients (Fig. 1B), which hint the TACE was active in SLE, and which hint the potential convenience for SARS-Cov-2 infection.
Finally, The other issue is that whether hydroxychloroquine reduce the risk of SARS-CoV-2 infection in SLE patients. Up to date, there is no conclusive evidence of hydroxychloroquine can prevent SARS-CoV-2 infection [15]. And moreover, the therapeutic effect of hydroxychloroquine on COVID-19 remains in doubt. Although there was study have showed that hydroxychloroquine induces viral clearance after 6 days of treatment (70% of patients treated with hydroxychloroquine alone determined a viral clearance compared with 12.5% of patients who did not receive hydroxychloroquine) [16]. However, recently, Magagnoli J's study showed that hydroxychloroquine didn't reduce the risk of mechanical ventilation in patients hospitalized with COVID-19 [17]. And moreover, the toxicity of hydroxychloroquine should not be ignored.
Taken together, SLE patients might be more susceptibility to SARS-CoV-2. Thus, management of COVID-19 infection prevention in SLE patients should follow the prevention guidance from WHO. Hydroxychloroquine do not be used as an Preventive therapy for SARS-CoV-2 infection in SLE patients.
Declaration of Competing Interest
The authors declared that there are no conflicts of interests.
References
- 1.Favalli E.G., Ingegnoli F., De Lucia O., Cincinelli G., Cimaz R., Caporali R. COVID-19 infection and rheumatoid arthritis: faraway, so close! Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with COVID-19 in Wuhan. China Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerr J.R. The role of parvovirus B19 in the pathogenesis of autoimmunity and autoimmune disease. J. Clin. Pathol. 2016;69(4):279–291. doi: 10.1136/jclinpath-2015-203455. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y., Han T., Chen J., Hou C., Hua L., He S. Clinical and Autoimmune Characteristics of Severe and Critical Cases with COVID-19. Clin Transl Sci. 2020 doi: 10.1111/cts.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caso F., Costa L., Ruscitti P., Navarini L., Del Puente A., Giacomelli R. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun. Rev. 2020;19(5):102524. doi: 10.1016/j.autrev.2020.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawalha A.H. Patients with lupus are not protected from COVID-19. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-217656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goyal M. SLE patients are not immune to covid-19: importance of sending the right message across. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-217658. [DOI] [PubMed] [Google Scholar]
- 9.COVID-19 Global Rheumatology Alliance https://rheum-covid.org/updates/combined-data.html [Accessed 29 Apr 2020]
- 10.Danza A., Ruiz-Irastorza G. Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus. 2013;22(12):1286–1294. doi: 10.1177/0961203313493032. [DOI] [PubMed] [Google Scholar]
- 11.Li T.H., Lai C.C., Wang W.H. Risk of severe herpes simplex virus infection in systemic lupus erythematosus: analysis of epidemiology and risk factors analysis in Taiwan. Ann. Rheum. Dis. 2019;78(7):941–946. doi: 10.1136/annrheumdis-2018-214844. [DOI] [PubMed] [Google Scholar]
- 12.Sawalha A.H., Zhao M., Coit P., Lu Q. Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. Clin. Immunol. 2020;215:108410. doi: 10.1016/j.clim.2020.108410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang P.H., Cheng Y. Increasing host cellular receptor—angiotensin-converting enzyme 2 (ACE2) expression by coronavirus may facilitate 2019-nCoV infection. bioRxiv. 2020 doi: 10.1101/2020.02.24.963348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haga S., Yamamoto N., Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc. Natl. Acad. Sci. U. S. A. 2008;105(22):7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spinelli F.R., Ceccarelli F., Di Franco M., Conti F. To consider or not antimalarials as a prophylactic intervention in the SARS-CoV-2 (Covid-19) pandemic. Ann. Rheum. Dis. 2020;79(5):666–667. doi: 10.1136/annrheumdis-2020-217367. [DOI] [PubMed] [Google Scholar]
- 16.Gautret P., Lagier J.-C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;105949 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Magagnoli J., Narendran S., Pereira F., Cummings T., Hardin J.W., Sutton S.S. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv. 2020 doi: 10.1101/2020.04.16.20065920. [DOI] [PMC free article] [PubMed] [Google Scholar]