Repurposing chloroquine and hydroxychloroquine against COVID-19
Therapeutic options in response to the COVID-19 pandemic are urgently needed [1]. A keyword in these worldwide efforts is ‘repurposing’ – the development of approved antiviral drugs as candidates for COVID-19. Chloroquine (CQ) and its hydroxyl analog hydroxychloroquine (HCQ) are showing preliminary inhibitory effects against COVID-19 and apparent efficacy in clinical studies 2, 3. We propose a variant of the repurposing strategy: developing single enantiomers of these old racemic drugs. We call for urgently pursuing the chiral switches of HCQ and/or CQ for the treatment of COVID-19.
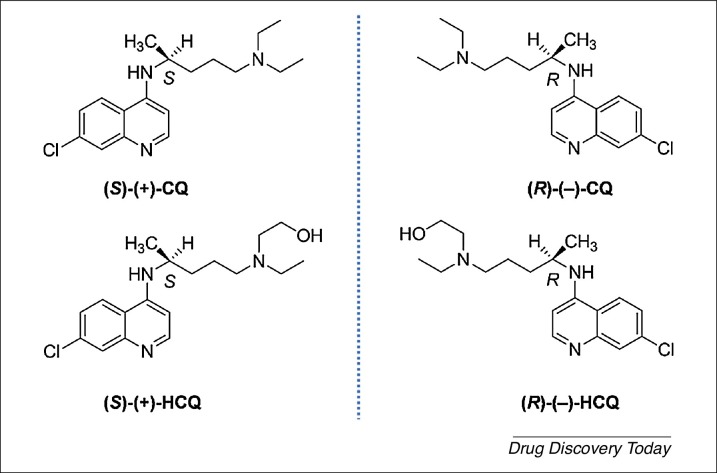
Chloroquine phosphate (CQ phosphate), IUPAC name: N 4-(7-chloro-4-quinolinyl)-N 1,N 1-diethylpentane-1,4-diamine diphosphate, and hydroxychloroquine sulfate (HCQ sulfate), IUPAC name: 2-[[4-[(7-chloro-4-quinolinyl)amino]pentyl]ethylamino]ethanol sulfate, are old antimalarial drugs approved by the FDA in 1949 and 1955, respectively (Figure 1 ) (Drugs@FDA; https://www.accessdata.fda.gov/scripts/cder/daf/). HCQ sulfate has also been approved for rheumatoid arthritis (RA), lupus erythematosus (LE) and other autoimmune diseases.
Figure 1.
Chemical structures of the paired (S)-(+) and (R)-(–) enantiomers of chloroquine (CQ) and hydroxychloroquine (HCQ). CAS Numbers: (S)-(+)-CQ: [58175-86-3]; (R)-(–)-CQ: [58175-87-4]; (S)-(+)-HCQ: [137433-24-0]; (R)-(–)-HCQ: [137433-23-9].
The activity as immune modulators exerted by CQ and/or HCQ suggested their potential use in the treatment of human infections (namely, bacterial, fungal and viral), which are often associated with inflammation and/or immune activation. In vitro activity of CQ and HCQ has been reported for a wide range of viruses, including HIV, where CQ showed broad-spectrum anti-HIV-1 and anti-HIV-2 in vivo activity achieved by inhibition of viral particle glycosylation and synergism with protease inhibitors. Several studies have also shown the capability of CQ phosphate to inhibit replication of coronaviruses – a large family of RNA viruses that usually cause mild-to-moderate upper-respiratory-tract diseases, among which there is the severe acute respiratory syndrome (SARS)-associated coronavirus. In these studies, CQ proved to inhibit SARS-CoV replication on Vero E6 cells with EC50 values in the low-micromolar interval, ranging from 4.1 to 8.8 μM [4]. Notably, CQ was found to be effective in preventing replication also of SARS-CoV-2 at an EC50 value of 1.13 μM [3]. HCQ has just been reported to efficiently inhibit COVID-19 infection in vitro [5].
In anticipation of increased demand for CQ phosphate and HCQ sulfate, the FDA is taking steps to ensure that adequate supply of these drug products is available by publishing product-specific guidances (PSGs) to support generic drug development for these drugs (Docket No. FDA-2007-D-0369).
CQ and HCQ through the looking glass: the importance of chirality
CQ and HCQ are chiral drugs administered as racemates [i.e., each as a 1:1 mixture of two paired enantiomers, namely (S)-(+) and (R)-(–)] (Figure 1). The chirality of drugs has become a major theme in the design, discovery, development, patenting and marketing of new drugs [6]. For many years, including at the time of the approvals of CQ and HCQ, the pharmacopoeias were dominated by racemates. This trend was inverted in the mid-1990s: most of the chiral new molecular entity (NME) drugs were developed and marketed as single enantiomers [6]. The strategy of chiral switches has emerged – the development of a single enantiomer from a chiral drug that has previously been developed (and often approved and marketed) as a racemate or as a mixture of diastereomers. Nevertheless, the development and approval of racemic drugs has continued to be viable and the continuing approval of racemic NMEs could have implications for the persistence of the chiral-switch strategy [7].
According to the EMA guidelines for the development of a new single enantiomer from an approved racemate [8], suitable ‘bridging studies’ should be carried out to link the complete racemate data to the incomplete data on the selected enantiomer. The extent of bridging studies should be defined on a case-by-case basis.
The chiral switch of HCQ was initiated in the early 1990s – with method-of-use patents US 5,314,894 (priority date 15-09-1992, assignee Sterling Winthrop, New York) and EP 0588430B1 claiming the enantiomer (S)-(+)-hydroxychloroquine [(S)-(+)-HCQ] for treatments of malaria, RA and LE. However, these initiatives and earlier and subsequent studies on HCQ and CQ enantiomers have not led to regulatory single-enantiomer drug approval(s) for any indication. The Sterling Winthrop portfolios of CQ and HCQ, including the pharmacological studies of the two enantiomers, were probably transferred to Sanofi (proprietor of EP 0588430B1) in June 1994.
Developing (S)-(+)-HCQ: the more-promising enantiomer
We aim preferentially at (S)-(+)-hydroxychloroquine [(S)-(+)-HCQ], the more-promising enantiomer (patents: US 5,314,894 and EP 0588430B1, proprietor Sanofi, priority date 15-09-1992, now expired), followed by (S)-(+)-chloroquine [(S)-(+)-CQ]. The rationale on which our call is based is driven by the following considerations. COVID-19 is a pandemic without any approved drug or vaccine. CQ and HCQ could potentially display therapeutic efficacy for the treatment of COVID-19 2, 3, 9. The toxicity profiles of CQ and HCQ have been well known for many years; their administration is safe, although both can have serious side effects, especially at high doses or when combined with other medicines. Advantages of (S)-(+)-HCQ (the eutomer) vis-à-vis (R)-(–)-HCQ and the racemate (R,S)-(±)-HCQ have been recorded in the above-mentioned patents, especially with regard to retinopathy, a severe side effect of HCQ which is caused by an enantioselective accumulation of the (R)-(–)-HCQ enantiomer in the ocular tissue, R/S ratio = 1.58 ± 0.24 (in rabbits, EP 0588430B1). Furthermore, studies of enantioselectivity in the pharmacokinetics of HCQ reported that there was no (S)-(+)-HCQ (R)-(–)-HCQ interconversion between the enantiomers [10].
The pointed clinical implications of using the (S)-(+)-HCQ ‘substantially free’ of the (R)-(–)-HCQ as the active ingredient were lower adverse effects and the possibility of higher dose levels and/or longer periods of administration. Various syntheses of the enantiomers of HCQ and CQ have been reported, including a simple method of synthesis for large-scale production of the CQ enantiomers (patent CN 105693605B, priority date 09-03-2016). Urgent guidance for navigating and circumventing the QTc interval prolongation and torsadogenic potential side effects of HCQ and CQ potential therapies for COVID-19 are noted [11].
According to the EMA guidelines [8] (vide supra), it would be productive in the present case [namely, (S)-(+)-HCQ] to use data on the corresponding racemate (i.e., HCQ) as far as is applicable to the enantiomer. Under the highly demanding, urgent circumstances, relaxations of the regulations are needed. The FDA has just created the Coronavirus Treatment Acceleration Program (CTAP) to speed up coronavirus therapies and move new treatments to patients as quickly as possible. The EMA indicated that it will be flexible and pragmatic during the assessment of affected clinical trial data submitted to the agency as part of marketing authorization applications. Hopefully, the bridging studies (vide supra) will be reduced, in consultation between the sponsor and the regulator, to shorten the development and approval periods.
On 17 March 2020, the Italian Medicines Agency (AIFA) expressed a favorable opinion on including the off-label use of CQ and HCQ for the treatment of COVID-19. On 28 March 2020, the FDA issued an Emergency Use Authorization (EUA) to allow HCQ sulfate and CQ phosphate products donated pro bono publicoby leading pharmaceutical companies to the US Strategic National Stockpile (SNS) to be distributed and used for certain hospitalized patients with COVID-19.
Emergency drug approvals of (S)-(+)-HCQ and/or (S)-(+)-CQ should be considered. Government agencies in major jurisdictions could also take up the challenge. It has not escaped our minds that the incentives of regulatory and secondary patent exclusivities could be diminished in the current crisis. However, a successful chiral switch of HCQ could be rewarded. Sanofi, the owner of the portfolios of CQ and HCQ, is in a preferred position to pursue the chiral switch. Philanthropic foundations might also be recruited for the cause of overcoming the COVID-19 pandemic.
Concluding remarks
Our call for repurposing HCQ and/or CQ by urgently developing the chiral switches of these racemates to their (S)-(+)-enantiomers for the treatment of COVID-19 is based on the expectations of safer pharmacological profiles of the selected enantiomers, favorable risk:benefit profiles and shortened development and approval processes. Demand for HCQ has grown dramatically in recent weeks as a result of the attention raised by the CTAP program. The further step that we propose here, taking into account the necessary vigilance and risk management, is the switch to (S)-(+)-HCQ – the more-promising single enantiomer of a known drug that proved safe and well tolerated in most patients.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 2.Gao J., et al. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 3.Wang M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colson P., et al. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents. 2020;55:105932. doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J., et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16–19. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calcaterra A., D’Acquarica I. The market of chiral drugs: chiral switches versus de novo enantiomerically pure compounds. J. Pharm. Biomed. Anal. 2018;147:323–340. doi: 10.1016/j.jpba.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Agranat I., et al. The predicated demise of racemic new molecular entities is an exaggeration. Nat. Rev. Drug Discov. 2012;11:972–973. doi: 10.1038/nrd3657-c1. [DOI] [PubMed] [Google Scholar]
- 8.Investigation of Chiral Active Substances 3CC29a, previously EU III/3501/91. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002816.pdf.
- 9.Colson P., et al. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int. J. Antimicrob. Agents. 2020;55:105923. doi: 10.1016/j.ijantimicag.2020.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ducharme J., et al. Enantioselective disposition of hydroxychloroquine after a single oral dose of the racemate to healthy subjects. Br. J. Clin. Pharmacol. 1995;40:127–133. doi: 10.1111/j.1365-2125.1995.tb05768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giudicessi J.R., et al. Urgent guidance for navigating and circumventing the QTc prolonging and torsadogenic potential of possible pharmacotherapies for COVID-19. Mayo Clin. Proc. 2020;95 doi: 10.1016/j.mayocp.2020.03.024. Article in press. [DOI] [PMC free article] [PubMed] [Google Scholar]