Abstract
Chronic obstructive pulmonary disease (COPD) is a lung inflammatory disease characterized by progressive airflow limitation, chronic respiratory symptoms and frequent exacerbations. There is an unmet need to identify novel therapeutic alternatives beside bronchodilators that prevent disease progression. Levels of both Nitric Oxide (NO) and IL-6 were significantly increased in the plasma of patients in the exacerbation phase (ECOPD, n = 13) when compared to patients in the stable phase (SCOPD, n = 38). Levels of both NO and IL-6 were also found to inversely correlate with impaired lung function (%FEV1 predicted). In addition, there was a strong positive correlation between levels of IL-6 and NO found in the plasma of patients and those spontaneously produced by their peripheral blood mononuclear cells (PBMCs), identifying these cells as a major source of these key inflammatory mediators in COPD. GTS-21, an agonist for the alpha 7 nicotinic receptors (α7nAChR), was found to exert immune-modulatory actions in PBMCs of COPD patients by suppressing the production of IL-6 and NO. This study provides the first evidence supporting the therapeutic potential of α7nAChR agonists in COPD due to their ability to suppress the production of key inflammatory markers associated with disease severity.
Keywords: Systemic inflammation, COPD pathogenesis, IL-6, Nitric oxide, Nicotinic receptors
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a lung disease associated with inflammatory damages of the distal airways (bronchitis) and alveoli (emphysema) (Barnes, 2017). COPD is a major smoking-associated lung disease characterized by chronic respiratory symptoms, progressive airflow limitation and frequent exacerbations (Barnes, 2016). The diagnosis of COPD is based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy assessing the presence of airflow limitation using spirometry (post-bronchodilator forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC) ratio of <0.7 without significant reversibility). The management of COPD mostly relies on the use of bronchodilators and corticosteroids (Johns, et al., 2014; Ruppel, et al., 2018).
There is still an urgent need to identify the key inflammatory players acting in the lungs that are involved in pathogenesis and/or progression of COPD (Cazzola, et al., 2007). Increased production of oxidants and inflammatory cytokines produced by various inflammatory cells such as neutrophils, macrophages, CD4+ and CD8 + T cells contribute to the COPD pathogenesis. Recent evidence also suggests the presence of eosinophilic inflammation in COPD patients experiencing exacerbations (Singh, et al., 2018; Selvarajah, et al., 2016). The presence of systemic inflammation evidenced by the presence of several circulating inflammatory markers has been associated with extra-pulmonary manifestations of the disease (Tzortzaki, et al., 2006; Shaw, et al., 2014). Interestingly, accumulating evidence suggests that the IL-6/nitric oxide (NO) axis may play a pivotal role in the regulation of both local and systemic inflammatory mechanisms, thus representing potential therapeutic targets for COPD management/treatment (Cazzola, et al., 2007; Wei, et al., 2015; Rosenberg and Kalhan, 2012).
Various sources in the lung such as epithelial cells, interstitial fibroblasts, macrophages and other inflammatory cells can produce IL-6 in response to a variety of stimuli including allergens, respiratory viruses, environmental particles, and inhaled toxic particles (Rincon and Irvin, 2012). IL-6 has been implicated in the regulation of CD4+ T cell-mediated response including secretion of cytokines, production of inflammatory proteins (CRP, fibrinogen) and iNOS expression (Del Giudice and Gangestad, 2018; Agusti, et al., 1999; Tanaka, et al., 2014). These mediators, in turn, have been associated with lung damage through their modulatory effects on mucus production, matrix deposition, and proteases release from granulocytes (Rincon and Irvin, 2012), all of which have been linked to the physiopathology and severity of COPD. Some studies indicated that patients with stable COPD had higher serum IL-6 concentrations than healthy controls while the association between IL-6 concentrations and the severity of pulmonary function impairment is still controversial (Wei, et al., 2015).
Nitric oxide (NO) is an important intracellular messenger in cardiovascular, immune, and neural systems. NO can impact lung function via multiple mechanisms including its ability to regulate bronchodilation and/or vasodilation (Stefanska, et al., 2012; Seimetz, et al., 2011; Lundberg, et al., 2008). NO released from cells rapidly auto-oxidizes to yield NO2− and NO3− (nitrates and nitrites respectively, termed NOx), and NOx concentration in the blood has been used in patients as an indicator of endogenous formation of NO and to assess its relationship with disease status (Ignarro, et al., 1999; Rhodes, et al., 1995). The increased production of NO seen in COPD patients was tested by looking at the expression of neuronal and inducible isoforms of NO synthases (nNOS and iNOS). iNOS was increased in the peripheral lung tissues of COPD patients and negatively correlated with airflow obstruction (Brindicci, et al., 2010). In addition, both endothelial NOS (eNOS) and iNOS were shown to be significantly up-regulated in the bronchial submucosa of COPD patients (Ricciardolo, et al., 2005). The pathological role of NO resides in its ability to impair many cellular processes and/or enhance inflammatory reactions via the nitration of protein tyrosine residues consequently leading to damage to DNA, lipids, and carbohydrates (Barnes, 2009).
In the last decade, the non-neuronal cholinergic system (NNCS) has been described as a major player in the pathogenesis of several human conditions affecting different organ systems including the lungs (Wessler, et al., 2003; Zoheir, et al., 2012; Kolahian and Gosens, 2012). The nicotinic receptors were found to be expressed on different lung tissues including the epithelial cells (Gwilt, et al., 2007; Lam, et al., 2016), lung fibroblasts (Vicary, et al., 2017), as well as various immune cells involved in COPD such as neutrophils (Safronova, et al., 2016) and alveolar macrophages (Wang, et al., 2019). This non-neuronal ACh has the potential to contribute to COPD pathogenesis (Paleari, et al., 2008) as several reports now suggest that activators of the nicotinic acetylcholine receptors (nAchR) exert immunomodulatory action in immune cells. Nicotine, for example, has been shown to inhibit the production of pro-inflammatory cytokines from macrophages and lymphocytes cells via the activation of the α7nAChR (Wang, et al., 2003). GTS-21, a selective agonist of the α7nAChR, has also demonstrated immunomodulatory and anti-inflammatory effects in RAW 264.7 cells (Yue, et al., 2015). Further animal studies using GTS-21 and other selective receptor agonists (PNU-282,987), or performed in receptor knockout animals have confirmed the anti-inflammatory role of α7nAChR in disease states such as LPS-induced animal septic model (Wang, et al., 2019) or lung injury induced by acid or sepsis (Su, et al., 2007).
In this report, we provide further evidence that GTS-21 may represent a therapeutic option in COPD by inhibiting the production of key inflammatory mediators such as IL-6 and NO from immune cells. We show that levels of both mediators were increased in the serum of COPD patients and correlated with impaired lung function. In addition, production of IL-6 and NO by peripheral blood mononuclear cells (PBMCs) was significantly increased in COPD patients when compared healthy controls. GTS-21 strongly suppressed the production of both IL-6 and NO in PBMCs. We suggest that activation of α7nAChR on PBMCs can suppress the production of circulating inflammatory markers known to be associated with COPD pathogenesis.
2. Subjects, materials, and methods
2.1. Study participants
A total of 66 subjects were enrolled in this study and recruited from the department of lung health at the University Hospital of Rouiba (Algiers). The demographics of the recruited COPD patients and healthy subjects are presented in Table 1 . The enrolled subjects were divided into two groups defined as healthy subjects (HS, n = 15) and COPD patients, which was organized into stable group (SCOPD, n = 38), and exacerbation group (ECOPD, defined as patients with more than two hospitalizations per year, n = 13). COPD was defined according to the GOLD criteria. COPD was diagnosed when the post-bronchodilator FEV1/FVC (forced vital capacity) ratio was less than 70 % (GOLD stages). The GOLD expert panel classified COPD into four stages, ranging from A to D according to lung obstruction and other clinical symptoms (0: at risk; A: mild; B: Mild; C: moderate; D: severe). The healthy subjects group had a normal lung function and no prior history of respiratory conditions (asthma or tuberculosis). All subjects who were receiving systemic corticosteroids (oral or intravenous injection therapy) within the four weeks before the study for the exacerbation patients or three months for the stable one were excluded. The study was approved by the local ethical committee (Beni Messous University Hospital) and written informed consent was obtained from all patients and controls before enrolment.
Table 1.
General characteristics of the enrolled subjects.
| Controls | COPD | |
|---|---|---|
| N | 15 | 51 |
| Gender | All male | All male |
| Age (years)* | 61.2 ± 3.45 | 66.96 ± 1.58 |
| FEV1% predicted* | 89.51 ± 1.90 | 55.05 ± 2.23** |
| BMI (Kg/m2)* | 23.5 ± 0.79 | 22.93 ± 0.7 |
| Smoking status (N) | ||
| Current smokers | 6 | 14 |
| Ex-smokers | 7 | 33 |
| Never smokers | 2 | 4 |
Notes: BMI= Body mass index, FEV1%= forced expiratory volume in one second predicted; N = number; **P < 0.01 compared to the healthy subjects. *Data are shown as Mean ± SEM.
2.2. Blood collection and PBMC isolation
Two blood samples were obtained from each participant in heparinized vacutainer tubes (BD Biosciences). Collected serum was immediately shielded from light and stored in aliquots at −20 °C until used for measurement of C-reactive protein high sensitivity (CRPhs), nitric oxide (NO), and interleukin 6 (IL-6). PBMCs were isolated by Ficoll gradient centrifugation according to the instructions of the manufacturer (Sigma Aldrich, St. Louis, MO) from n = 21 subjects. Briefly, after centrifugation (2800 rpm, 15 min), PBMCs were collected from the interface and washed twice with sterile phosphate-buffered saline (PBS). Cells were suspended in RPMI-1640 medium supplemented with 10 % fetal calf serum (FCS), 100 U/mL penicillin, and 100 g/mL streptomycin (Sigma-Aldrich, St. Louis, MO). Immediately after PBMCs isolation, the PBMCs viability was confirmed using Trypan blue dye exclusion test (>95 % viability). The number of cells was adjusted to 106 cells per well (200 μL) and seeded in duplicate in 96-well flat-bottom plates (Greiner, Alphen a/d Rijn, The Netherlands) and incubated for 24 h at 37 °C, 95 % O2, 5% CO2 with LPS (100 ng/mL) or GTS-21 dihydrochloride (10 μmol/l prepared from 10 mM stock in deionized water). GTS-21 was purchased from Sigma Aldrich (St. Louis, MO). In another set of experiments, PBMCs were LPS pre-treated for 4 h before being incubated with GTS-21, at the same previous concentration. Untreated cultures were used to assess resting levels. The supernatants were harvested by centrifugation and frozen at −80 °C until use, and the collected cells were tested for viability.
2.3. Detection of CRP and IL-6 levels in patients’ plasma
CRPhs and IL-6 serum levels were determined with an electrochemiluminescence (ECL) method on the Cobas® e411 system (Roche Diagnostics, Basel, Switzerland) according to the instructions of the manufacturer. The reference range for CRPhs in healthy subjects without any inflammatory symptom was <3 mg/L, and the sensitivity of the IL-6 test is >1.5 pg/mL.
2.4. Detection of NO levels in patients’ plasma and cell culture supernatants
NO levels in culture supernatants and plasma samples were assessed by measuring levels of nitrites by the modified Griess’s method, as previously described by (Touil-Boukoffa and al.,1998). Briefly, a 100 μL sample was mixed with 50 μL of 5% sulfanilamide, 0.5 % naphthyl ethylenediamine dihydrochloride, plus 20 % HCl. The preparations were then incubated for 20 min, and optical density was measured using a spectrophotometer at 543 nm. The nitrite concentration was determined using a standard curve generated using sodium nitrite.
2.5. Detection of IL-6 in cell culture supernatants by enzyme-linked immunosorbent assay (ELISA)
IL-6 levels in the cell culture supernatants were measured using a human cytokine immunoassay quantikine ELISA as recommended by the manufacturer (R&D Systems®, Minneapolis, MN, USA).
2.6. Data analysis
Analyses were performed using GraphPad® Prism 5.01 software (GraphPad® Inc., La Jolla, Calif., USA). Positively skewed data were log10 transformed for normality. Statistical analyses were performed using t-test to conduct pairwise comparisons, Pearson's correlations to assess correlations between factors and one-way analysis of variance with Bonferroni post-test to compare multiple groups. P < 0.05 was considered statistically significant.
3. Results
3.1. Participants
Subject demographics, lung function, and consumption tobacco status data of the healthy control and COPD groups are reported in Table 1. The mean age and BMI were matched between the COPD patients and healthy subjects (66.96 ± 1.58 vs 61.2 ± 3.45, respectively, for age and 22.93 ± 0.7 vs 23.5 ± 0.76, respectively, for BMI). At baseline, the COPD group exhibited a decrease in FEV1% predicted in comparison to control subjects (55.05 ± 2.23 vs 89.51 ± 1.9, respectively, **p < 0.01), confirming an impaired lung function in COPD patients. 46.66 % of COPD patients and 64.7 % of healthy subjects were former smokers while the never smokers represented 13.33 % and 7.84 % of the COPD patients and healthy controls, respectively.
3.2. General characteristics of SCOPD and ECOPD
Table 2 describes the demographics of COPD patients in stable (SCOPD) and exacerbation (ECOPD) states. A significant difference in plasma levels of IL-6, NO and CRP was observed between ECOPD and SCOPD groups. The plasma levels of IL-6, NO and CRP were significantly increased by 3.8, 2.16 and 6.29-fold respectively in ECOPD compared to SCOPD (**P < 0.01). No significant difference in lung function (FEV1% predicted values) was noted between the SCOPD and ECOPD. According to the ABCD grading system, 70 % of SCOPD patients were in the A/B categories, while in ECOPD group there was an equally distribution of patients among A/B vs C/D categories. Regarding the smoking history, the proportion of current and ex-smokers was somewhat higher in the SCOPD compared to the ECOPD group (95 % vs 85 %, respectively), although additional studies with a larger sample size are clearly needed to further confirm this observation.
Table 2.
Baseline characteristics of COPD patients in exacerbation (ECOPD) and stable (SCOPD) phases.
| SCOPD | ECOPD | |
|---|---|---|
| (N = 38) | (N = 13) | |
| Age | 65.66 ± 1.94 | 70.08 ± 4.96 |
| (Mean ± SEM) | ||
| FEV1% predicted (Mean ± SEM) | 54.83 ± 2.58 | 53.9 ± 4.96 |
| CRP (mg/L) | ||
| Median | 3.64 | 22.93** |
| (25 %–75 % Percentile) | (1.83−10.41) | (14.35−37.8) |
| IL-6 (pg/mL) | ||
| Median | 51.68 | 194** |
| (25 %–75 % Percentile) | (22.83−91.29) | (129.5–244) |
| NO (μM) | ||
| Median | 34.23 | 73.99** |
| (25 %–75 % Percentile) | (21.01−44.65) | (39.31–95) |
| Smoking status (n) | ||
| Active smokers | 9 | 5 |
| Ex-smokers | 27 | 6 |
| Never smokers | 2 | 2 |
| GOLD | ||
| A | 13 | 4 |
| B | 14 | 3 |
| C | 6 | 3 |
| D | 4 | 3 |
Abbreviations: CRP (mg/L): C reactive protein (milligram/litre); ECOPD: frequent exacerbation chronic obstructive pulmonary disease; FEV1% predicted = forced expiratory volume in one second predicted; GOLD= Global Initiative on Obstructive Lung Disease. (A: Low risk and less symptom, B: Low risk and more symptom, C: High risk and less symptom, D: High risk and more symptom); IL-6 (pg/mL): interleukin 6 (pictograms/ millilitres); NO (μM); nitric oxide (micromolar); SCOPD: stable chronic obstructive pulmonary disease; **P < 0.01 compared to SCOPD.
3.3. Correlation studies
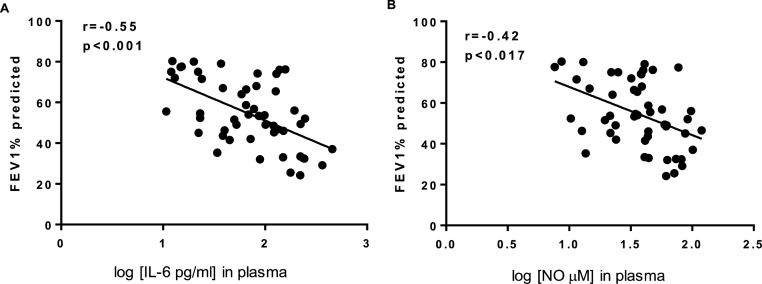
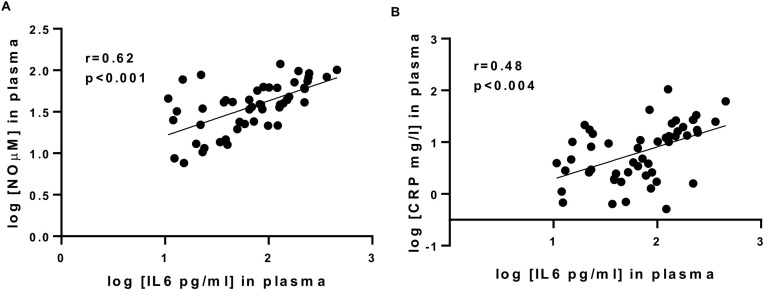
We next performed correlation studies to determine the possible association between levels of inflammatory mediators found in the plasma of COPD patients and lung function. Fig. 1 shows that lung function (using FEV1% predicted values) negatively correlated with plasma levels of IL-6 (r=-0.5655, P < 0.001, Fig.1A) and NO (r=-0.42, P < 0.018, Fig.1B). These observations further support the role of both IL-6 and NO in COPD pathogenesis. Fig. 2 demonstrates that plasma levels of IL-6 positively correlated with plasma levels of NO (r = 0.62 and P < 0.001, Fig.2A) and CRP (r = 0.48 and P < 0.004, Fig.2 .B), identifying IL-6 as a possible factor driving CRP and NO in COPD.
Fig. 1.
Relationship between lung function and plasma concentrations of IL-6 (A) and NO (B) in COPD patients.
Fig. 2.
Relationship between IL-6 plasma concentrations and levels of nitrites (A) or CRP (B) in COPD patients.
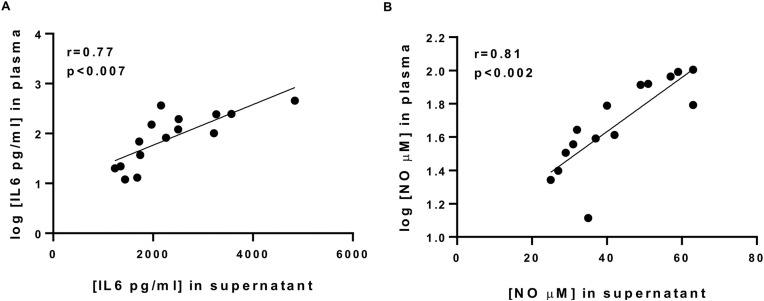
Fig. 3 shows the correlation analyses between levels of IL-6 and the nitrites found in the plasma and those in the supernatants of resting PBMCs in the same patients. The results showed a strong correlation between levels of NO and IL-6 found in the plasma and those found in PBMC supernatants (r = 0.81, P < 0.002; Fig.3 A and r = 0.77., p < 0.001; Fig.3B, respectively). These studies have identified PBMCs as major cellular sources of systemic inflammatory mediators in COPD.
Fig. 3.
Relationship between plasma concentrations of IL-6 (A) or NO (B) of COPD patients and the spontaneous production of IL-6 (A) and nitrites (B) by their cultured PBMCs (n = 15).
3.4. GTS-21 suppressed the production of IL-6 and NO in PBMCs under basal and LPS stimulated conditions
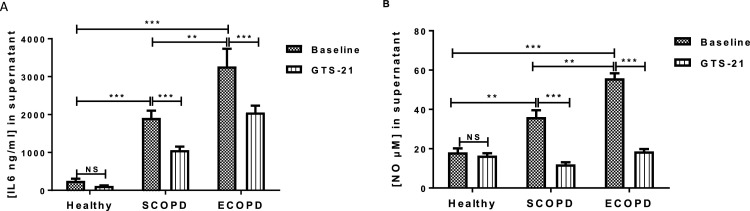
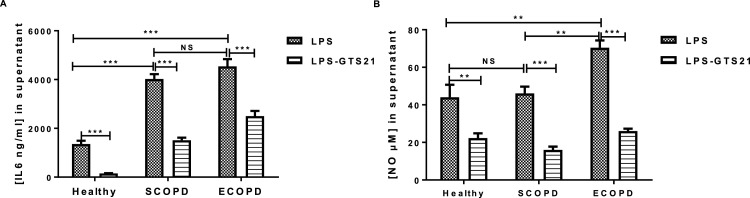
We next determine whether the production of IL-6 and NO by PBMCs was affected by GTS-21, a selective α7 nicotinic acetylcholine receptor (α7nAChR) with promising anti-inflammatory action (Nizri and Brenner, 2013). Fig. 4 compared the basal levels of IL-6 and NO produced by PBMCs from SCOPD, ECOPD, and healthy subjects (HS). These data first demonstrated that basal levels of both IL-6 and NO were significantly higher in COPD patients when compared to that seen in HS subjects. IL-6 levels increased from 250.70 ± 56.12 ng/mL in healthy subjects to 1911 ± 191.5 and 3268 ± 467 ng/mL in SCOPD and ECOPD, respectively (***P < 0.001, Fig.4A). Nitrite levels increased from 18.17 ± 1.97 μM in healthy subjects to 36.10 ± 3.45 and 55.8 ± 2.57 μM in SCOPD and ECOPD, respectively (**P < 0.01, ***P < 0.001, Fig.4B). The presence of GTS-21 (10 μM) did reduce basal levels of IL-6 by 44 % and 0.47 % in SCOPD and ECOPD (***P < 0.001, Fig.4A). Interestingly, GTS-21 had a greater inhibitory effect on NO at basal levels in the PBMCs of either SCOPD or ECOPD which were completely abrogated by the α7nAChR agonist (***P < 0.001, Fig.4B). Fig. 5 shows the impact of GTS-21 on the levels of IL-6 and NO induced by LPS. LPS was found to induce greater levels of IL-6 and NO in both SCOPD and ECOPD when compared to that seen in healthy controls. LPS-induced IL-6 levels increased from 1356 ± 135.7 ng/mL in healthy subjects to 4022 ± 200.6 and 4540 ± 297.1 ng/mL in SCOPD and ECOPD, respectively (***P < 0.001, Fig.5A). LPS-induced nitrite levels were quite similar between healthy subjects and SCOPD (44 ± 6.72 vs 46.1 ± 3.63 μM, NS) but were increased to 70.4 ± 3.89 μM in ECOPD (**P < 0.01, Fig.5B). Levels of IL-6 release by LPS were found to be not different between SCOPD and ECOPD (Fig.5B) while production of NO was significantly increased in ECOPD group when compared to SCOPD (**P < 0.01, Fig.5B). Interestingly, GTS-21 strongly suppressed IL-6 and NO production induced by LPS stimulation in healthy subjects and COPD patients irrespective of disease status (SCOPD and ECOPD) (Fig.5 A-B). While production of IL-6 by LPS was completed inhibited by GTS-21 in healthy subjects, GTS-21 did reduced IL-6 responses by 54 % and 48 % in SCOPD and ECOPD, respectively (***P < 0.001, Fig.5B). GTS-21 suppressed the production of nitrites by LPS by 55 %, 62 % and 68 % in healthy subjects, SCOPD and ECOPD, respectively (***P < 0.001, Fig.5B). These findings suggest that GTS-21 exhibits anti-inflammatory actions by suppressing IL-6 and NO production in both basal and LPS-stimulated PBMCs from COPD patients. It was interesting to note that basal levels of both IL-6 and NO in the supernatants of PBMCs were not affected by GTS-21 in healthy subjects.
Fig. 4.
GTS-21 modulates spontaneous release of IL-6 (A) and NO (B) by PBMCs of COPD patients and healthy subjects. PBMCs (106 cells/mL) from COPD patients (n = 15) or healthy subjects (n = 6) were cultured for 24 h with or without GTS-21 (10 μmol/l). The supernatants were harvested and used for IL-6 (A) or nitrites (B) measurement by ELISA and Griess modified method, respectively. Data are expressed as Mean ± SEM (**p < 0.01, ***p < 0.001).
Fig. 5.
GTS-21 modulates the release of IL-6 (A) and NO (B) induced by LPS in PBMCs of COPD patients and healthy subjects. PBMCs (106 cells/mL) from COPD patients (n = 15) or healthy subjects (n = 6) were cultured with LPS (100 ng/mL) for 24 h or 4 h prior to cells challenging by GTS-21 (10 μmol/l) for 20 h more. The supernatants were harvested and used for IL-6 (A) or nitrites (B) measurement by ELISA and Griess modified method, respectively. Data are expressed as Mean ± SEM (**p < 0.01, ***p < 0.001).
4. Discussion
The present report shows that PBMCs act as an important source of pro-inflammatory mediators found to be increased in the plasma of COPD patients such as IL-6 and NO which levels were found to correlate with impaired lung function. In addition, our study demonstrates that GTS-21, a selective alpha-7 nicotinic acetylcholine receptor (nAChR) agonist, has the potential to reduce levels of these mediators by directly acting on PBMCs and inhibiting their production. This is the first report to demonstrate the therapeutic potential of GTS-21 in the treatment of key features associated with COPD.
The first goal of the present study was to determine the profile of key pro-inflammatory markers known to be associated with COPD pathogenesis in an Algerian cohort of male COPD patients. These patients were steroid-naïve at the time of blood sample collection (for at least 1 month in ECOPD and 3 months in SCOPD). A previous systematic review showed that among most biomarkers investigated, CRP was the one consistently increased in ECOPD, while levels of IL-6 or other cytokines such as TNFα were shown to be also increased but with variable and statistical significance (Chen et al., 2016). We here found that plasmatic levels of IL-6 and CRP were markedly increased during exacerbations (ECOPD) when compared to levels seen in patients with stable COPD (SCOPD) (∼3−4 fold for IL-6/CRP). Similar results could be seen in cohort of Chinese ECOPD when compared to patients in remission which reported similar levels of CRP (∼30−32 mg/mL) but quite higher levels of IL-6 detected at μg/mL range (Lin, et al., 2019). Our study also demonstrated that levels of plasmatic NO were higher in COPD patients compared to healthy controls (data not shown) which were further increased by 2.16-fold in the exacerbation group when compared to stable patients. Only few studies have demonstrated that NO metabolites (nitrites and nitrates), when measured in the serum, were increased in COPD patients (Garcia-Rio, et al., 2010) or during COPD exacerbation when compared to levels seen in the stable group (Karadag, et al., 2008). Other studies reported increased levels of exhaled NO in hospitalized patients during an exacerbation of COPD (Agusti, et al., 1999). Nonetheless, controversies still exist regarding the use of NO measurement in the management of COPD since not all studies have reported similar NO changes in COPD patients during exacerbation (Csoma, et al., 2019; Ricciardolo, et al., 2004). Various factors could affect the production of these inflammatory markers in ECOPD such as the cause of exacerbations, the underlying inflammatory profile (presence of eosinophils), corticosteroid therapy, and whether these markers were measured in the blood or in the exhaled breath condensate. Nonetheless, our finding has raised the possibility that measuring these inflammatory markers in our cohort of patients could serve as biomarkers of COPD exacerbations, although a larger sample size would be required to further test this possibility.
We next performed correlation studies to determine the clinical impact of these different inflammatory markers in COPD. We first found positive correlations between levels of IL-6 and that of CRP or NO in agreement with previous studies linking IL-6 to other inflammatory mediators in COPD including CRP (Garcia-Rio, et al., 2010; Heinrich, et al., 1990; Kolsum, et al., 2009). The ability of IL-6 to induce NO production and iNOS expression has been recently demonstrated in PBMCs of patients with rheumatoid arthritis (Arroul-Lammali, et al., 2017) and in femoris muscles in COPD patients (Agusti, 2005). We also found negative correlations between FEV1 (% predicted) values and plasmatic levels of NO and IL-6 (r=- 0 =-0.55 and r=-0.427, respectively), reinforcing the concept that systemic inflammation drives airflow obstruction in COPD patients. It was interesting to note that whether patients were in stable or exacerbation states did not affect the correlations between NO or IL-6 and airflow limitation (data not shown). Our observation is in part in agreement with previous reports showing that high IL-6 levels, measured either in serum or sputum samples, were associated with impaired lung function or a rapid lung function decline (Garcia-Rio, et al., 2010; Donaldson, et al., 2005; Yende, et al., 2006). Furthermore, IL-6 levels in the sputum were also reported to be present in frequent COPD exacerbators (Koutsokera, et al., 2013). A systematic review and meta-analysis investigating 33 studies reported higher levels of serum IL-6 in stable COPD vs healthy control but failed to see any association with pulmonary lung function. However, the authors did acknowledge that failing to adjust for a number of confounding factors could have influenced the results (Wei, et al., 2015).
The strong correlations observed between levels of nitrites and IL-6 found in the plasma of patients and the supernatants of their cultured PBMCs (r = 0.81, P < 0.0001 and r = 0.77, P < 0.0004, respectively), strongly suggests that PBMCs act as the primary source of such inflammatory mediators in the circulation of COPD patients. Targeting the pro-inflammatory pathways in PBMCs may represent a promising therapeutic option for the treatment of COPD considering the limited clinical efficacy of corticosteroids and their strong side effects (Ernst, et al., 2015). We here provide the first evidence that GTS-21, a selective α7 nicotinic acetylcholine receptor (α7nAChR) expressed in neurons and immune cells (Wang, et al., 2003), can significantly suppress basal and inducible levels of IL-6 and NO in SCOPD and ECOPD patients with no notable effect on basal levels produced in healthy controls. This observation highly suggests the activation of pro-inflammatory pathways in the immune cells of COPD patients are sensitive to GTS-21. Prior studies also observed that similar concentrations of GTS-21 (μM range) inhibited production of different inflammatory mediators induced by LPS such as IL-6, IL1-ß, and TNFα by RAW264.7 cells (Yue, et al., 2015), TNFα by peritoneal mice macrophages (Khan, et al., 2012; Garg and Loring, 2019), or microglia (Thomsen and Mikkelsen, 2012). Interestingly, GTS-21 did suppress production of TNFα or IL-1ß but did not affect inducible levels of IL-6 in human PBMCs treated with LPS (Kox, et al., 2009), somewhat contrasting with the profound effect of GTS-21 on IL-6 production seen in our healthy controls (Fig.4). Because little information was provided about the demographics of the healthy controls used in their study (Kox, et al., 2009), it was difficult to speculate about the reasons for such discrepancy. Other studies have confirmed that GTS-21 can inhibit the production of inflammatory mediators in response to various stimuli (i.e., ligands for TLR2, TLR3, TLR4, TLR9, and RAGE) in human monocytes (Ro) or in mast cells (Rosas-Ballina, et al., 2009; Guzman-Mejia, et al., 2018), further supporting the therapeutic value of GTS-21 in human inflammatory diseases including COPD. The molecular mechanisms by which GTS-21 exerts its anti-inflammatory actions in immune cells have been attributed to the inhibition of the NF-κB pathway (Yoshikawa, et al., 2006), activation of JAK/STAT pathway (Zoheir, et al., 2012) or increased levels of intracellular cAMP (Andersson and Tracey, 2012). Because GTS-21 (i.e., DMXB-A) appears to be safe in humans and is currently being tested for various neurological conditions (Hilderman, et al., 2015; Freedman, et al., 2008), we believe that GTS-21 and other selective agonists could represent novel anti-inflammatory options to treat COPD. Whether GTS-21 could be used in the treatment of coronavirus disease 2019 (COVID-19) due to its ability to suppress IL-6 production by inflammatory cells remains an interesting hypothesis to be tested considering the central role of IL-6 in the regulation of the cytokine release syndrome (CRS) (Liu, et al., 2020). A number of clinical trials testing IL-6 blockade in COVID-19 patients with severe respiratory problems are currently underway.
In this study, we present further evidence of a potential role of IL-6 and NO in the pathogenesis of COPD based on their association with airflow obstruction and increased levels in the plasma and supernatants of cultured PBMCs when compared to levels found in the plasma and PBMCs of healthy subjects. We also demonstrated the therapeutic potential of GTS-21 in COPD which exerts anti-inflammatory actions on PBMCs by inhibiting the production of IL-6 and NO via mechanisms that remain to be determined.
Author contributions
DS performed all the experiments, generated, and analysed the data. DS also provided the first draft of the article. DR and DF helped with the experiments and data analysis. OM and TB-C were involved in the conception and interpretation of the data. BM and GM coordinated the recruitment of healthy and COPD subjects and in the interpretation of the data. AY supervised the experiments, analysed and interpreted all the data, and wrote the paper.
Declaration of Competing Interest
This letter is to certify that none of the authors have an any financial and/or personal relationship with other people or organizations that could inappropriately influence (bias) their work.
Acknowledgements
This study was supported by the Ministry of Higher Education and Scientific Research (PNE/518, Algeria) and by the National Institute for Health Research Leicester Biomedical Research Centre Respiratory. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR and the Department of Health.
References
- Agusti A.G. Systemic effects of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2005;2:367. doi: 10.1513/pats.200504-026SR. [DOI] [PubMed] [Google Scholar]
- Agusti A.G., Villaverde J.M., Togores B., Bosch M. Serial measurements of exhaled nitric oxide during exacerbations of chronic obstructive pulmonary disease. Eur. Respir. J. 1999;14:523. doi: 10.1034/j.1399-3003.1999.14c08.x. [DOI] [PubMed] [Google Scholar]
- Andersson U., Tracey K.J. Reflex principles of immunological homeostasis. Annu. Rev. Immunol. 2012;30:313. doi: 10.1146/annurev-immunol-020711-075015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroul-Lammali A., Rahal F., Chetouane R., Djeraba Z., Medjeber O., Ladjouze-Rezig A., Touil-Boukoffa C. Ex vivo all-trans retinoic acid modulates NO production and regulates IL-6 effect during rheumatoid arthritis: a study in Algerian patients. Immunopharmacol. Immunotoxicol. 2017;39:87. doi: 10.1080/08923973.2017.1285919. [DOI] [PubMed] [Google Scholar]
- Barnes P.J. Histone deacetylase-2 and airway disease. Ther. Adv. Respir. Dis. 2009;3:235. doi: 10.1177/1753465809348648. [DOI] [PubMed] [Google Scholar]
- Barnes P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016;138:16. doi: 10.1016/j.jaci.2016.05.011. [DOI] [PubMed] [Google Scholar]
- Barnes P.J. Cellular and molecular mechanisms of asthma and COPD. Clin. Sci. 2017;131:1541. doi: 10.1042/CS20160487. [DOI] [PubMed] [Google Scholar]
- Brindicci C., Kharitonov S.A., Ito M., Elliott M.W., Hogg J.C., Barnes P.J., Ito K. Nitric oxide synthase isoenzyme expression and activity in peripheral lung tissue of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010;181:21. doi: 10.1164/rccm.200904-0493OC. [DOI] [PubMed] [Google Scholar]
- Cazzola M., Matera M.G., Rogliani P., Page C. Treating systemic effects of COPD. Trends Pharmacol. Sci. 2007;28:544. doi: 10.1016/j.tips.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Chen Y.W., Leung J.M., Sin D.D. A systematic review of diagnostic biomarkers of COPD exacerbation. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csoma B., Bikov A., Nagy L., Toth B., Tabi T., Szucs G., Komlosi Z.I., Muller V., Losonczy G., Lazar Z. Dysregulation of the endothelial nitric oxide pathway is associated with airway inflammation in COPD. Respir. Res. 2019;20:156. doi: 10.1186/s12931-019-1133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M., Gangestad S.W. Rethinking IL-6 and CRP: why they are more than inflammatory biomarkers, and why it matters. Brain Behav. Immun. 2018;70:61. doi: 10.1016/j.bbi.2018.02.013. [DOI] [PubMed] [Google Scholar]
- Donaldson G.C., Seemungal T.A., Patel I.S., Bhowmik A., Wilkinson T.M., Hurst J.R., Maccallum P.K., Wedzicha J.A. Airway and systemic inflammation and decline in lung function in patients with COPD. Chest. 2005;128:1995. doi: 10.1378/chest.128.4.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst P., Saad N., Suissa S. Inhaled corticosteroids in COPD: the clinical evidence. Eur. Respir. J. 2015;45:525. doi: 10.1183/09031936.00128914. [DOI] [PubMed] [Google Scholar]
- Freedman R., Olincy A., Buchanan R.W., Harris J.G., Gold J.M., Johnson L., Allensworth D., Guzman-Bonilla A., Clement B., Ball M.P., Kutnick J., Pender V., Martin L.F., Stevens K.E., Wagner B.D., Zerbe G.O., Soti F., Kem W.R. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am. J. Psychiatry. 2008;165:1040. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rio F., Miravitlles M., Soriano J.B., Munoz L., Duran-Tauleria E., Sanchez G., Sobradillo V., Ancochea J., Committee E.-S.S. Systemic inflammation in chronic obstructive pulmonary disease: a population-based study. Respir. Res. 2010;11:63. doi: 10.1186/1465-9921-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg B.K., Loring R.H. GTS-21 has cell-specific anti-inflammatory effects independent of alpha7 nicotinic acetylcholine receptors. PLoS One. 2019;14 doi: 10.1371/journal.pone.0214942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Mejia F., Lopez-Rubalcava C., Gonzalez-Espinosa C. Stimulation of nAchRalpha7 receptor inhibits TNF synthesis and secretion in response to LPS treatment of mast cells by targeting ERK1/2 and TACE activation. J. Neuroimmune Pharmacol. 2018;13:39. doi: 10.1007/s11481-017-9760-7. [DOI] [PubMed] [Google Scholar]
- Gwilt C.R., Donnelly L.E., Rogers D.F. The non-neuronal cholinergic system in the airways: an unappreciated regulatory role in pulmonary inflammation? Pharmacol. Ther. 2007;115:208. doi: 10.1016/j.pharmthera.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Heinrich P.C., Castell J.V., Andus T. Interleukin-6 and the acute phase response. Biochem. J. 1990;265:621. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilderman M., Qureshi A.R., Al-Abed Y., Abtahi F., Lindecrantz K., Anderstam B., Bruchfeld A. Cholinergic anti-inflammatory pathway activity in dialysis patients: a role for neuroimmunomodulation? Clin. Kidney J. 2015;8:599. doi: 10.1093/ckj/sfv074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L.J., Cirino G., Casini A., Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J. Cardiovasc. Pharmacol. 1999;34:879. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- Johns D.P., Walters J.A., Walters E.H. Diagnosis and early detection of COPD using spirometry. J. Thorac. Dis. 2014;6:1557. doi: 10.3978/j.issn.2072-1439.2014.08.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadag F., Karul A.B., Cildag O., Yilmaz M., Ozcan H. Biomarkers of systemic inflammation in stable and exacerbation phases of COPD. Lung. 2008;186:403. doi: 10.1007/s00408-008-9106-6. [DOI] [PubMed] [Google Scholar]
- Khan M.A., Farkhondeh M., Crombie J., Jacobson L., Kaneki M., Martyn J.A. Lipopolysaccharide upregulates alpha7 acetylcholine receptors: stimulation with GTS-21 mitigates growth arrest of macrophages and improves survival in burned mice. Shock. 2012;38:213. doi: 10.1097/SHK.0b013e31825d628c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolahian S., Gosens R. Cholinergic regulation of airway inflammation and remodelling. J. Allergy (Cairo) 2012;2012 doi: 10.1155/2012/681258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolsum U., Roy K., Starkey C., Borrill Z., Truman N., Vestbo J., Singh D. The repeatability of interleukin-6, tumor necrosis factor-alpha, and C-reactive protein in COPD patients over one year. Int. J. Chron. Obstruct. Pulmon. Dis. 2009;4:149. doi: 10.2147/copd.s5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsokera A., Kostikas K., Nicod L.P., Fitting J.W. Pulmonary biomarkers in COPD exacerbations: a systematic review. Respir. Res. 2013;14:111. doi: 10.1186/1465-9921-14-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kox M., van Velzen J.F., Pompe J.C., Hoedemaekers C.W., van der Hoeven J.G., Pickkers P. GTS-21 inhibits pro-inflammatory cytokine release independent of the Toll-like receptor stimulated via a transcriptional mechanism involving JAK2 activation. Biochem. Pharmacol. 2009;78:863. doi: 10.1016/j.bcp.2009.06.096. [DOI] [PubMed] [Google Scholar]
- Lam D.C., Luo S.Y., Fu K.H., Lui M.M., Chan K.H., Wistuba I.I., Gao B., Tsao S.W., Ip M.S., Minna J.D. Nicotinic acetylcholine receptor expression in human airway correlates with lung function. Am. J. Physiol. Lung Cell Mol. Physiol. 2016;310:L232. doi: 10.1152/ajplung.00101.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.L., Chen W.W., Ding Z.R., Wei S.C., Huang M.L., Li C.H. Correlations between serum amyloid A, C-reactive protein and clinical indices of patients with acutely exacerbated chronic obstructive pulmonary disease. J. Clin. Lab. Anal. 2019;33 doi: 10.1002/jcla.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J.O., Weitzberg E., Gladwin M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008;7:156. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- Nizri E., Brenner T. Modulation of inflammatory pathways by the immune cholinergic system. Amino Acids. 2013;45:73. doi: 10.1007/s00726-011-1192-8. [DOI] [PubMed] [Google Scholar]
- Paleari L., Grozio A., Cesario A., Russo P. The cholinergic system and cancer. Semin. Cancer Biol. 2008;18:211. doi: 10.1016/j.semcancer.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Rhodes P., Leone A.M., Francis P.L., Struthers A.D., Moncada S., Rhodes P.M. The L-arginine:nitric oxide pathway is the major source of plasma nitrite in fasted humans. Biochem. Biophys. Res. Commun. 1995;209:590. doi: 10.1006/bbrc.1995.1541. [DOI] [PubMed] [Google Scholar]
- Ricciardolo F.L., Sterk P.J., Gaston B., Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol. Rev. 2004;84:731. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- Ricciardolo F.L., Caramori G., Ito K., Capelli A., Brun P., Abatangelo G., Papi A., Chung K.F., Adcock I., Barnes P.J., Donner C.F., Rossi A., Di Stefano A. Nitrosative stress in the bronchial mucosa of severe chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2005;116:1028. doi: 10.1016/j.jaci.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Rincon M., Irvin C.G. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int. J. Biol. Sci. 2012;8:1281. doi: 10.7150/ijbs.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M., Goldstein R.S., Gallowitsch-Puerta M., Yang L., Valdes-Ferrer S.I., Patel N.B., Chavan S., Al-Abed Y., Yang H., Tracey K.J. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med. 2009;15:195. doi: 10.2119/molmed.2009.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S.R., Kalhan R. Biomarkers in chronic obstructive pulmonary disease. Transl. Res. 2012;159:228. doi: 10.1016/j.trsl.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Ruppel G.L., Carlin B.W., Hart M., Doherty D.E. Office spirometry in primary care for the diagnosis and management of COPD: national lung health education program update. Respir. Care. 2018;63:242. doi: 10.4187/respcare.05710. [DOI] [PubMed] [Google Scholar]
- Safronova V.G., Vulfius C.A., Shelukhina I.V., Mal’tseva V.N., Berezhnov A.V., Fedotova E.I., Miftahova R.G., Kryukova E.V., Grinevich A.A., Tsetlin V.I. Nicotinic receptor involvement in regulation of functions of mouse neutrophils from inflammatory site. Immunobiology. 2016;221:761. doi: 10.1016/j.imbio.2016.01.016. [DOI] [PubMed] [Google Scholar]
- Seimetz M., Parajuli N., Pichl A., Veit F., Kwapiszewska G., Weisel F.C., Milger K., Egemnazarov B., Turowska A., Fuchs B., Nikam S., Roth M., Sydykov A., Medebach T., Klepetko W., Jaksch P., Dumitrascu R., Garn H., Voswinckel R., Kostin S., Seeger W., Schermuly R.T., Grimminger F., Ghofrani H.A., Weissmann N. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell. 2011;147:293. doi: 10.1016/j.cell.2011.08.035. [DOI] [PubMed] [Google Scholar]
- Selvarajah S., Todd I., Tighe P.J., John M., Bolton C.E., Harrison T., Fairclough L.C. Multiple circulating cytokines are coelevated in chronic obstructive pulmonary disease. Mediators Inflamm. 2016 doi: 10.1155/2016/3604842. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J.G., Vaughan A., Dent A.G., O’Hare P.E., Goh F., Bowman R.V., Fong K.M., Yang I.A. Biomarkers of progression of chronic obstructive pulmonary disease (COPD) J. Thorac. Dis. 2014;6:1532. doi: 10.3978/j.issn.2072-1439.2014.11.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Verma S.K., Kumar S., Ahmad M.K., Nischal A., Singh S.K., Dixit R.K. Correlation of severity of chronic obstructive pulmonary disease with potential biomarkers. Immunol. Lett. 2018;196:1. doi: 10.1016/j.imlet.2018.01.004. [DOI] [PubMed] [Google Scholar]
- Stefanska J., Sarniak A., Wlodarczyk A., Sokolowska M., Doniec Z., Bialasiewicz P., Nowak D., Pawliczak R. Hydrogen peroxide and nitrite reduction in exhaled breath condensate of COPD patients. Pulm. Pharmacol. Ther. 2012;25:343. doi: 10.1016/j.pupt.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Su X., Lee J.W., Matthay Z.A., Mednick G., Uchida T., Fang X., Gupta N., Matthay M.A. Activation of the alpha7 nAChR reduces acid-induced acute lung injury in mice and rats. Am. J. Respir. Cell Mol. Biol. 2007;37:186. doi: 10.1165/rcmb.2006-0240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014;6 doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M.S., Mikkelsen J.D. The alpha7 nicotinic acetylcholine receptor ligands methyllycaconitine, NS6740 and GTS-21 reduce lipopolysaccharide-induced TNF-alpha release from microglia. J. Neuroimmunol. 2012;251:65. doi: 10.1016/j.jneuroim.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Tzortzaki E.G., Tsoumakidou M., Makris D., Siafakas N.M. Laboratory markers for COPD in “susceptible” smokers. Clin. Chim. Acta. 2006;364:124. doi: 10.1016/j.cca.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Vicary G.W., Ritzenthaler J.D., Panchabhai T.S., Torres-Gonzalez E., Roman J. Nicotine stimulates collagen type I expression in lung via alpha7 nicotinic acetylcholine receptors. Respir. Res. 2017;18:115. doi: 10.1186/s12931-017-0596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Yu M., Ochani M., Amella C.A., Tanovic M., Susarla S., Li J.H., Wang H., Yang H., Ulloa L., Al-Abed Y., Czura C.J., Tracey K.J. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Wang J., Li R., Peng Z., Zhou W., Hu B., Rao X., Yang X., Li J. GTS-21 reduces inflammation in acute lung injury by regulating M1 polarization and function of alveolar macrophages. Shock. 2019;51:389. doi: 10.1097/SHK.0000000000001144. [DOI] [PubMed] [Google Scholar]
- Wei J., Xiong X.F., Lin Y.H., Zheng B.X., Cheng D.Y. Association between serum interleukin-6 concentrations and chronic obstructive pulmonary disease: a systematic review and meta-analysis. PeerJ. 2015;3:e1199. doi: 10.7717/peerj.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler I., Kilbinger H., Bittinger F., Unger R., Kirkpatrick C.J. The non-neuronal cholinergic system in humans: expression, function and pathophysiology. Life Sci. 2003;72:2055. doi: 10.1016/s0024-3205(03)00083-3. [DOI] [PubMed] [Google Scholar]
- Yende S., Waterer G.W., Tolley E.A., Newman A.B., Bauer D.C., Taaffe D.R., Jensen R., Crapo R., Rubin S., Nevitt M., Simonsick E.M., Satterfield S., Harris T., Kritchevsky S.B. Inflammatory markers are associated with ventilatory limitation and muscle dysfunction in obstructive lung disease in well functioning elderly subjects. Thorax. 2006;61:10. doi: 10.1136/thx.2004.034181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H., Kurokawa M., Ozaki N., Nara K., Atou K., Takada E., Kamochi H., Suzuki N. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-kappaB phosphorylation and nuclear factor-kappaB transcriptional activity through nicotinic acetylcholine receptor alpha7. Clin. Exp. Immunol. 2006;146:116. doi: 10.1111/j.1365-2249.2006.03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y., Liu R., Cheng W., Hu Y., Li J., Pan X., Peng J., Zhang P. GTS-21 attenuates lipopolysaccharide-induced inflammatory cytokine production in vitro by modulating the Akt and NF-kappaB signaling pathway through the alpha7 nicotinic acetylcholine receptor. Int. Immunopharmacol. 2015;29:504. doi: 10.1016/j.intimp.2015.10.005. [DOI] [PubMed] [Google Scholar]
- Zoheir N., Lappin D.F., Nile C.J. Acetylcholine and the alpha 7 nicotinic receptor: a potential therapeutic target for the treatment of periodontal disease? Inflamm. Res. 2012;61:915. doi: 10.1007/s00011-012-0513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]