Abstract
The coronavirus disease 2019 (COVID-19) pandemic has consumed our healthcare system, with immediate resource focus on the management of high numbers of critically ill patients. Those that fare poorly with COVID-19 infection more commonly have cardiovascular disease (CVD), hypertension and diabetes. There are also several other conditions that raise concern for the welfare of patients with and at high risk for CVD during this pandemic. Traditional ambulatory care is disrupted and many patients are delaying or deferring necessary care, including preventive care. New impediments to medication access and adherence have arisen. Social distancing measures can increase social isolation and alter physical activity and nutrition patterns. Virtually all facility based cardiac rehabilitation programs have temporarily closed. If not promptly addressed, these changes may result in delayed waves of vulnerable patients presenting for urgent and preventable CVD events.
Here, we provide several recommendations to mitigate the adverse effects of these disruptions in outpatient care. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers should be continued in patients already taking these medications. Where possible, it is strongly preferred to continue visits via telehealth, and patients should be counselled about promptly reporting new symptoms. Barriers to medication access should be reviewed with patients at every contact, with implementation of strategies to ensure ongoing provision of medications. Team-based care should be leveraged to enhance the continuity of care and adherence to lifestyle recommendations. Patient encounters should include discussion of safe physical activity options and access to healthy food choices. Implementation of adaptive strategies for cardiac rehabilitation is recommended, including home based cardiac rehab, to ensure continuity of this essential service. While the practical implementation of these strategies will vary by local situation, there are a broad range of strategies available to ensure ongoing continuity of care and health preservation for those at higher risk of CVD during the COVID-19 pandemic.
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has affected every facet of life and has consumed our international healthcare focus. The immediate objective of managing the critical respiratory and cardiovascular manifestations requiring intensive care of the hospitalized patient is paramount, as are public health efforts to flatten the curve of COVID-19 cases to prevent overwhelming health care system resources. However, in this time, there is increasing concern for the welfare of patients with and at high risk for cardiovascular disease (CVD).
Individuals with CVD and risk factors such as hypertension and diabetes appear to be at greater risk of COVID-19 related morbidity and mortality. There are currently 23 million Americans with coronary heart disease, heart failure or stroke, and 118 million with hypertension [1]. Further, there are over 72 million office visits per year with CVD issues as the primary diagnosis [2]. Disruption in access to care and preventive interventions for these higher risk individuals and delay or deferral of these visits can have significant consequences. Further, the alterations to lifestyle habits with social distancing, as well as the cumulative consequences of increased societal stress and anxiety from fear of COVID-19 infection may adversely affect those with CVD. Deaths from COVID-19 in the United States are anticipated to be ~60,000 [3]. By context, approximately 900,000 individuals succumb to CVD in the U.S. annually, and this number could increase due to disruptions in care caused by COVID-19 [1].
The purpose of this scientific statement from the American Society for Preventive Cardiology (ASPC) is to highlight the ramifications of the COVID-19 pandemic for outpatient care practices and risk factor modification in patients with and at high risk for CVD. We also provide a series of recommendations to mitigate disruptions of care and enhance the cardiovascular health for these individuals during this pandemic.
2. COVID-19 and its relation to cardiovascular disease and associated comorbidities
Since the beginning of the COVID-19 pandemic, it was recognized that persons with pre-existing comorbidities fared worse. In particular, CVD, hypertension, and diabetes are among the most common cardiovascular comorbidities in persons with COVID-19 (Table 1A) and with severe COVID-19 (Table 1B) across multiple studies. There are also emerging reports about the relationship between obesity and adverse outcomes in COVID-19 infected patients [4,5]. It is clear that there are important consequences for the cardiovascular system stemming from COVID-19 infection.
Table 1A.
Prevalence of cardiovascular disease and risk factors in COVID-19 patients from two large cohorts.
Table 1B.
Prevalence of cardiovascular disease and risk factors among those with and without severe outcome∗ in a Chinese COVID-19 cohort.
| Comorbidity | Presence of critical illness∗ or death | |
| Yes | No | |
| Coronary heart disease | 9.0% | 2.0% |
| Diabetes | 26.9% | 6.1% |
| Hypertension | 35.8% | 13.7% |
Data from Reference [6].
Defined as admission to an intensive care unit or the use of mechanical ventilation.
The Centers for Disease Control (CDC) reported that, as of March 28, 2020, patients infected with COVID-19 with at least one underlying health condition or other risk factor had poorer outcomes compared to those without these comorbidities [6]. The most common preexisting conditions in infected patients were diabetes mellitus (10.9%), chronic lung disease (9.2%), and CVD (9.0%). While data on mortality rates according to the presence of different comorbidities are not available yet among U.S. patients infected with COVID-19, a report among 72,314 cases of COVID-19 from the Chinese Center for Disease Control and Prevention notes an overall crude estimated mortality of 3–4%. This was significantly higher among those with CVD (10.5%), diabetes (7.3%), or hypertension (6.0%) [7]. Similarly, another report of 1099 patients from China early in the pandemic demonstrated that the prevalence of coronary artery disease (CAD) in severe vs. non-severe cases was 5.8 vs. 1.8%, respectively [8]. COVID-19 patients with poor outcomes, including intensive care unit admission, mechanical ventilation, or death, more commonly had underlying CAD than those with a more benign course (9.0 vs. 2.0%).
2.1. Cardiovascular disease: symptoms and mechanisms
Published studies including at least 100 COVID-19 patients, all from Hubei Province in China, demonstrate a wide variation in the prevalence of established CVD prior to infection, ranging from 4% to 35% [9,10]. However, the largest study of over 1000 patients from 30 provinces in China demonstrated a prevalence of CAD of only 2.5% [8]. Prevalence estimates were significantly higher for CVD, diabetes, and hypertension in a cohort of COVID-19 infected patients from Italy (Table 1A) [11]. Importantly, the case fatality rate was higher in the Italian cohort than the Chinese cohort, reflecting not only an older age group, but possibly also increased prevalence of CVD, hypertension, and diabetes, as well as obesity [12].
There are important symptoms and consequences of COVID-19 infection related to the cardiovascular system [13]. Elevations in high-sensitivity cardiac troponin were reported in 10–20% of patients, and more than 10% of those who died from COVID-19 had substantial myocardial injury with either elevated troponin levels or cardiac arrest during hospitalization, even in the absence of pre-existing CVD [14,15].
In a study that included 138 patients infected with COVID-19 in Wuhan, China (the epicenter of the global pandemic), 7.2% sustained acute myocardial injury, 8.7% developed shock, and 16.7% experienced new onset cardiac arrhythmias [16]. COVID-19 patients are at increased risk of atherosclerotic plaque rupture possibly resulting from dramatic heightening of systemic inflammatory tone. There is also significant inflammation-induced hypercoagulability in patients infected with COVID-19 [17]. Patients can develop or either new onset or decompensated heart failure in the setting of active COVID-19 infection. Heart failure (reduced left ventricular contractility) is likely a manifestation of [1]: respiratory impairment with hypoxia [2]; inflammatory storm induced by a massive elevation in interleukins and cytokines [3]; myocarditis induced by direct viral infection of myocardium; and [4] acute hemodynamic decompensation stemming from severe systemic infection [13].
Guo et al. provide important insights into patients who manifest serum troponin elevations [9]. When comparing mortality rates for patients with and without troponin elevations, hospital mortality was 59.6% vs. 8.9%, respectively. Mortality for patients with established CVD but without troponin elevation was lower but still substantial at 37.5%. There were also linear correlations between serum troponin levels and high sensitivity C-reactive protein and N-terminal pro-brain natriuretic peptide, suggesting associations between myocardial injury, inflammation, and left ventricular stress.
2.2. Diabetes mellitus
The CDC has reported that 10.9% of patients infected with COVID-19 in the U.S. have diabetes [6], a rate slightly higher than those reported in China (7.4%) [7] and Italy (8.9%) [11], likely reflecting the higher prevalence of diabetes in the U.S. general population. A meta-analysis of several Chinese studies found that diabetes was 2.3-fold more common in those with COVID-19 infection and was associated with adverse outcomes [18]. A Chinese study including 174 consecutive patients infected with COVID-19 demonstrated that those with diabetes and no other comorbidities (n = 24) were at higher risk of severe pneumonia, profound increases in inflammation, and hypercoagulability [19]. Biomarkers including IL-6, C-reactive protein, and D-dimer were significantly higher in those with diabetes compared to those without diabetes, suggesting greater levels of inflammation that may lead to rapid deterioration. These initial observations suggest diabetes is associated with poorer outcomes in those with COVID-19 infection.
2.3. Hypertension
It has been proposed that pre-existing hypertension may facilitate the pathogenesis of COVID-19 infection and its sequelae [20]. Estimates of the prevalence of hypertension among Chinese patients infected with COVID-19 range from 9.5% to 34.7% [21,22], with the largest study (1099 patients) reporting a prevalence of 15% [8]. A recent pooled meta-analysis demonstrated that the presence of hypertension was associated with a significant increased risk of severe COVID-19 disease (OR: 2.49 [95%CI: 1.98–3.12]) as well as mortality (OR: 2.42 [95%CI: 1.51–3.90]) [23].
2.4. ACE2 receptor-mediated infection
The spike protein of COVID-19 is a ligand for angiotensin converting enzyme 2 (ACE2), a transmembrane enzyme expressed on a variety of cell types, including alveolar epithelium, intestinal epithelium, vascular endothelium, and cardiac myocytes [24,25]. ACE2 catalyzes the conversion of angiotensin I to Ang(1–7), a potent vasodilator. In the case of COVID-19, ACE2 doubles as a virus binding receptor and promotes its cellular internalization. ACE2 expression is increased in the setting of diabetes and myocardial infarction, both of which are associated with a poorer prognosis in those with COVID-19 infection [26]. Given the fact that many patients with hypertension, diabetes, or CVD take angiotensin converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), which upregulate ACE2, some have speculated that the use of these medications may facilitate infection with COVID-19 [27]. However, there are also data suggesting that ACEIs/ARBs may be beneficial in those with acute respiratory distress syndrome, possibly because of their promotion of anti-inflammatory and antioxidant effects [24]. It is worth noting that only ~25%% of Chinese adults are hypertensive [28], with only 25–30% of those taking ACEs/ARBs, resulting in a small proportion of COVID-19 infected in China on these medications [29].
Given the fact that there are no clinical trial data demonstrating the adverse impact (or lack thereof) of such therapies in COVID-19, all major societal statements thus far recommend continuation of these therapies in patients with COVID-19. However, these same statements do not recommend initiation of ACEIs/ARBs in the absence of other clinical indications (e.g., hypertension, heart failure, or diabetes).
3. Challenges in the continuity of care and outpatient management
3.1. Disruption of ambulatory care
The COVID-19 pandemic has had a profound effect on health care delivery worldwide. Current guidelines from the CDC recommend that all high-risk individuals, including those with traditional cardiovascular risk factors and/or established atherosclerotic cardiovascular disease (ASCVD), stay at home if possible, primarily to limit potential exposure [30]. Individuals who might have been exposed to COVID-19 are further advised to self-quarantine with separation from others for 14 days, self-monitoring for symptoms or signs of infection [31]. Further, many office based practices have discontinued all but the most urgent face to face visits. While these approaches represent key public health strategies to limit the spread of infection, effort is needed to guard against potential unintended consequences.
For example, it remains to be determined whether patients experiencing worrisome cardiac symptoms (e.g., chest pain, shortness of breath, syncope) will contact emergency medical services or go to the emergency department out of fear that they will be exposed to COVID-19. There are already many reports attesting to the fact that admissions for acute coronary syndromes, decompensated heart failure, and stroke have decreased since the pandemic began [32]. In the US, STEMI cath lab activations are 38% lower during the COVID-19 period compared with rates from one year prior [33]. It is unclear whether this observation reflects reluctance amongst those with non-COVID-19 related illness to enter a clinical setting vs. a true decrease in these acute cardiovascular events. One worrisome case series from Hong Kong demonstrated that patients with ST elevation myocardial infarction (STEMI) presenting during the COVID-19 pandemic had a marked delay in the time from symptom onset to first medical contact compared with historical controls (318 vs. 82 min) [34]. For clinicians and public health officials, this represents the challenge in balancing the recommendation to stay at home against the need to seek medical care when appropriate.
Despite efforts to improve our interaction with patients during this challenging time (detailed later in this document), potential limitations exist. For example, many patients may not be amenable or have appropriate technologic ability to participate in telehealth visits. Those seen virtually may be apprehensive to start new medications or titrate existing ones over a concern that side effects may warrant medical attention. In addition, patients may be hesitant, or even embarrassed, to mention less severe problems believing that their care team has more pressing issues related to the pandemic.
Each of these scenarios represents a challenge to cardiovascular clinicians focused on reducing their patient’s near-term and long-term risks. Rising rates of unemployment, loss of health insurance, and concern over the cost of copayments represent additional challenges to be overcome. One estimate suggests that an additional 7.3 million workers (plus their family members) will become uninsured due to loss of healthcare coverage as a result of unemployment during the COVID-19 pandemic [35]. Sadly, underrepresented populations, patients living in rural communities, and those with poorer socioeconomic status are likely to be disproportionately affected. In fact, several recent reports have noted that the COVID-19 pandemic is disproportionately affecting black individuals. As one example, more than 50% of COVID-19 infections and almost 70% of COVID-19 related deaths in Chicago have occurred in black individuals, a group that comprises just 30% of the city’s population [36].
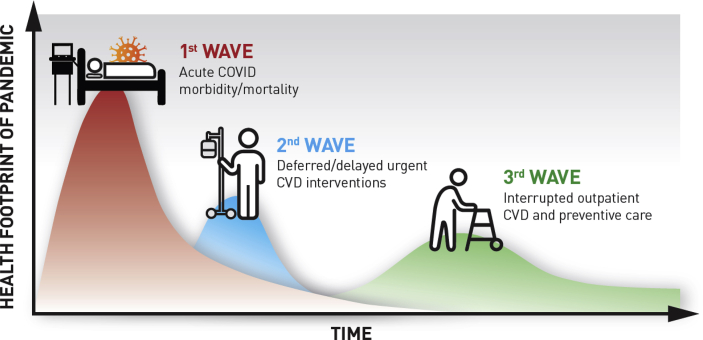
Acknowledging the critical issues currently faced by health care systems (e.g., ventilator shortages, limited personal protective equipment, unprecedented financial challenges), there also exists an ongoing need to address barriers to care delivery in the ambulatory setting. To this end, it is important to provide reassurance to patients that they should not hesitate to reach out to their providers with any questions or concerns. Cognizant of the fears they may have about COVID-19, we must help our patients understand that they should seek immediate medical attention for cardiovascular emergencies such as heart attack or strokes, and remind them of the relevant symptoms associated with these events [37]. Innovative solutions are urgently needed to address financial barriers that are likely to significantly limit optimal delivery of preventive care. Finally, it is important to brace for the next large wave of inpatient and ambulatory care that may be coming as a consequence of the disruption in the traditional care for patients with CVD (Fig. 1). There is marked heterogeneity in terms of the numbers of COVID-19 infected patients and deaths by state and region. Population measures to curb COVID-19 infection may have greater net benefit relative to preventable CV events precipitated by healthcare disruptions in high vs. low COVID-19 prevalence areas.
Fig. 1.
Implications of Delay and Disruption of Care for Patients with and at Risk for Cardiovascular Disease During the COVID-19 Pandemic.
Adapted with permission from Victor Tseng, MD.
∗∗ The chronology, magnitude of impact, and duration of the second and third waves are for illustrative purposes only. At present, there are no publicly available models that can provide specificity regarding estimates.
3.2. Medication access and adherence
Access and adherence to medications can be challenging in the best of times; the COVID-19 pandemic threatens to make matters worse. Today, patients must contend with closed providers’ offices and limited access to ambulatory care services. Those of advanced age are sequestered at home while many younger individuals are newly unemployed. Income streams are down; savings have plummeted; many have lost or will lose health insurance [35]. Baseline temporal and monetary deficits already impede access and adherence to medications. Currently, 1 in every 8 Americans with CVD misses or delays taking medications due to cost [38], and the COVID-19 crisis will only increase these numbers. Compounding this, there are concerns that supply chains may be threatened, including numerous pharmaceutical ingredients that rely on manufacturers in China [39].
Importantly, older individuals who are at highest risk of COVID-19 and associated morbidity are also the same group with greatest use of prescription medications. Early evidence suggests that while most are continuing to make regular trips to the pharmacy for medication refills, they are also increasing their risk of potential exposure and hampering efforts at social distancing [40]. Furthermore, this group is most at risk for exacerbation of their underlying CVD, with worsening of congestive heart failure and angina when missing medications, at a time when fear of COVID-19-related hospitalization contributes to delayed evaluation [34].
Of particular interest are higher tier preventive medications such as PCSK9 inhibitors, and in some cases other lipid lowering and novel anti-diabetic medications. These drugs can have an extensive prior authorization process, have higher copayments, and can require repeat laboratory testing for re-authorization, which may limit renewals. These time consuming processes and higher patient costs result in high abandonment rates even in normal times [41], and present greater challenges during the COVID-19 pandemic.
3.3. Social distancing and lifestyle factors
3.3.1. Social distancing
Social or physical distancing is the act of deliberately increasing the space between people to avoid spreading an illness, and is the most important recommendation from CDC as a community mitigation strategy to limit the spread of COVID-19 [42]. However, social distancing does not mean social isolation. Nonetheless, the impact of this crisis can increase stress, depression, and anxiety in adults and children. In fact, more than half of the respondents to a survey in China reported moderate-to-severe psychologic impact of the COVID-19 outbreak [43]. Additionally, the psychological strain of loneliness can manifest physiologically and exacerbate existing medical conditions. Based on experiences from prior outbreaks [44], those identified to be most susceptible to the stress of this crisis include those at the greatest risk for COVID-19 infection, children and teens, people with mental health conditions, particularly those with prior substance abuse issues, healthcare professionals, and first responders.
Perceived stress has long been associated with increased risk of CVD [45]. Acute stressful triggers like a natural disaster [46] or even a sporting match [47] can potentially trigger a myocardial infarction. Social isolation/loneliness [48] and depression [49] are also associated with increased CVD risk, possibly through dysregulation of the autonomic nervous system, sympathetic system activation with increased heart rate and blood pressure, hypercoagulability, and inflammatory activation which can trigger acute plaque rupture or demand myocardial ischemia. Thus, there is concern that the current crisis may exacerbate pre-existing CVD or precipitate an acute cardiac event. Furthermore, as discussed below, perceived stress can trigger unhealthy behaviors such as smoking, dysregulated eating, poor sleep, and reduced activity. In addition, psychologic stressors can worsen glycemic control in patients with diabetes and worsen body weight through increased energy intake [50,51].
3.3.2. Impact of COVID-19 crisis on lifestyle implementation for CVD prevention
A healthy lifestyle remains the foundation of all CVD prevention efforts. Unfortunately, the current COVID-19 crisis presents challenges to the implementation and optimization of lifestyle efforts including physical activity, nutrition, weight management, and smoking cessation. Nevertheless, aggressive promotion of a healthy lifestyle should continue and there are unique opportunities that can be leveraged for cardiovascular health promotion, even amidst the crisis.
3.3.2.1. Physical activity
One of the many consequences of the current COVID-19 crisis is that physical activity levels have markedly decreased globally as a result of policies and messaging that encourage staying at home, tele-working, and social distancing [52]. These recommendations to stay at home have focused on the benefits in preventing viral infection/transmission but have largely lacked guidance on the importance of maintaining a lifestyle that is as heathy as possible while at home. Many home activities are sedentary and screen time is likely increasing, as adults and children increase the use of computers and smart devices in efforts to stay connected with the outside world and pass the time.
3.3.2.2. Nutrition
There are notable challenges to maintaining healthy nutrition in the era of the COVID-19 pandemic. Increased perceived stress can trigger unhealthy eating patterns such as over-eating, emotional eating, and making unhealthy food choices such as diets with higher intake of saturated fats, simple refined carbohydrates, and sugar-sweetened beverages [[53], [54], [55]]. Since social distancing recommendations advise that trips to grocery stores be limited, there inevitably is decreased access to healthier food items like vegetables, fruits, low fat dairy, and other perishable products. Often foods with longer shelf life are highly processed. In fact, there are early reports of increased alcohol consumption [56] as well as purchase of comfort foods such as soups and snacks [57] during the COVID-19 pandemic.
3.3.2.3. Tobacco cessation
Cigarette smoking still remains the leading cause of preventable disease and death in the United States, as highlighted by the recent Surgeon General’s report on smoking cessation, despite a significant decline in smoking prevalence over the past decades [58,59]. Overcoming nicotine addiction is challenging even in normal times, and negative psychological factors (perceived stress, anxiety, frustration) are smoking triggers [60]. This current anxiety-provoking crisis may create triggers for smoking relapse and continued smoking behaviors, but there are also opportunities to make strides in tobacco cessation.
3.4. Disruption of cardiac rehabilitation
Cardiac rehabilitation (CR) is the cornerstone of secondary prevention in our healthcare system, but there has been little innovation in the delivery of these programs over the last 30 years. This slow pace of CR innovation has become a glaring issue as COVID-19 has disrupted the traditional provider facility-based CR delivery model. Though the in-person, facility-based approach has advantages such as providing social support and helping patients cope with depression after their cardiac event, it has also presented a barrier to many who do not have access to the facility. Despite carrying a Class IA indication, CR is already grossly underutilized; less than 20% of eligible patients participate in CR programs [61].
Virtually all facility-based CR programs have been shut down to prevent spread of COVID-19. This disruption prevents access to the full spectrum of CVD prevention. CR is likely more critical at this time, as its use is associated with reduced hospitalizations for cardiovascular events. Indeed, participants in CR demonstrate a marked reduction in reinfarction (odds ratio 0.53) and death (odds ratio 0.74) [62]. Therefore, continued or increased virtual participation in CR may not only prevent recurrent cardiac events but perhaps free up hospital resources for COVID-19 patients, especially as a second wave of COVID-19 and/or related infectious disease is expected in the late fall/early winter.
4. Strategies to preserve the continuity of care and enhance cardiovascular health during the COVID-19 pandemic
4.1. Implementation and expansion of telehealth visits
To minimize exposure for patients and health care providers, routine maintenance “preventive” cardiology visits can be either rescheduled or held virtually. When possible, there is a strong preference to maintaining visits virtually, rather than delaying or deferring care due to many potential adverse consequences as described above. Further, health system information technology infrastructure can be leveraged for additional targeted virtual visits to those most vulnerable and at highest CVD risk. While virtual visits are commonly referred to as telemedicine or telehealth, herein we will use the term telehealth to align with recent Medicare terminology for coverage changes [63]. Preventive cardiology practices are the ideal setting for telehealth, as visits are less reliant on in office testing and a detailed physical examination but rather more focused on counseling. Further, lifestyle interventions require increased visit frequency, which can be facilitated by shorter, regular telehealth visits. At each visit, patients should be counseled to promptly report any new or concerning cardiac symptoms to their health care team, and to not delay seeking care for any severe symptoms due to fears over COVID-19.
4.1.1. Regulatory considerations
Recognizing that some patients and providers may not have access to HIPAA compliant, secure two-way audio/visual communication tools, the Department of Health and Human Services amended regulations stating that penalties would not be imposed for noncompliance with HIPAA rules in relation to good faith provision of telehealth. Specifically, applications such as FaceTime, Skype, and Zoom, among others can now be used without risk of penalty during this COVID-19 nationwide public health emergency (PHE) [64].
4.1.2. Reimbursement
While coverage varies by plan and payer, the Centers for Medicare and Medicaid Services (CMS) as well as multiple private insurers, including Aetna, Cigna, and Blue Cross BlueShield, made changes to expand telehealth coverage. Previously, Medicare part B reimbursed telehealth visits only for individuals that lived in a rural community with certain geographic restrictions. This restriction was recently removed on a temporary and emergency basis under the 1135 waiver authority and Coronavirus Preparedness and Response Supplemental Appropriations Act [65]. Patient copays and reimbursement for telehealth still vary by state and by payer, though providers can waive copays for patients with Original Medicare. Reimbursement for Medicaid varies by state, though many states have expanded telehealth coverage.
4.1.3. Billing
Medicare allows billing for various types of telehealth services. Virtual check-ins [5–10 min, Healthcare Common Procedure Coding System (HCPCS) code G2012] are the lowest intensity and used to determine if an in-person visit is necessary. This category also includes remote evaluation of recorded video and/or images submitted by an established patient (HCPCS code G2010) (Table 2).
Table 2.
Telehealth and remote monitoring billing codes.
| Category | CPT Code | Details |
|---|---|---|
| CMS Considered “Telehealth” Visits: Previously billed using Place of Service 02-Telehealth; Updated guidance on April 3, 2020 during COVID-19 emergency- use POS “equal to what it would have been had the service been furnished in-person (11 for office). Use Modifier 95 “indicating that the service rendered was actually performed via telehealth. Prior rules required providers to furnish services from a physician office or healthcare facility; current rules allow providers to provide service from their home or “any setting of care” | ||
| Telehealth Consultation (Established patients) | 99211–99215 |
Established patient outpatient visit. Must be combined audio + video. Reimbursement the same as if provided in person. |
| Telehealth Consultation (New patients) | 99201–99205 | New patient outpatient visit∗ Must be combined audio + video. Reimbursement the same as if provided in person. ∗Allowable under COVID19; new patients not previously allowable as televisits. |
| Telephone visit | 99441-99443 (physician) 98966-98968 (non-physician professionals) |
New or Established. Telephone only visits. ∗Allowable under COVID19; previously noncovered service. |
| Remote Monitoring/E-Visits, Not defined by CMS as “Telehealth” | ||
| Remote Patient Monitoring | 99091 | Collection and interpretation of physiologic data (e.g., ECG, blood pressure, glucose monitoring) digitally stored and/or transmitted by the patient and/or caregiver to the physician or other qualified healthcare professional, qualified by education, training, licensure/regulation (when applicable) requiring a minimum of 30 min of time, each 30 days). |
| 99453 | Remote monitoring of physiologic parameter(s) (eg, weight, blood pressure, pulse oximetry, respiratory flow rate), initial; set-up and patient education on use of equipment. (Initial set-up and patient education of monitoring equipment). | |
| 99454 | Device(s) supply with daily recording(s) or programmed alert(s) transmission, each 30 days. (Initial collection, transmission, and report/summary services to the clinician managing the patient). | |
| 99457 | Remote physiologic monitoring treatment management services, 20 min or more of clinical staff/physician/other qualified healthcare professional time in a calendar month requiring interactive communication with the patient/caregiver during the month. (Interpretation of the received data and interaction with patient on a treatment plan by a clinician). | |
| Home Blood Pressure | 99473 | Self-measured blood pressure using a device validated for clinical accuracy; patient education/training and device calibration. |
| 99474 | Separate self-measurements of two readings 1 min apart, twice daily over a 30-day period (minimum of 12 readings), collection of data reported by the patient and/or caregiver to the physician or other qualified health care professional, with report of average systolic and diastolic pressures and subsequent communication of a treatment plan to the patient. | |
| E-visits (Online Digital Evaluation and Management) | 99421–99423 | Online digital evaluation and management service, for an established patient, for up to 7 days cumulative time during the 7 days; 5–10 min (99,421), 11–20 min (99,422), or ≥21 min (99,423). |
| Virtual Check In | HCPCS G2012 | Brief communication technology-based service, e.g. virtual check-in, by a physician or other qualified healthcare professional who can report evaluation and management services, provided to an established patient, not originating from a related E/M service provided within the previous 7 days nor leading to an E/M service or procedure within the next 24 h or soonest available appointment; 5–10 min of medical discussion. Initial inquiry should be initiated by patient; verbal consent to bill required. Documentation required. |
| Remote Evaluation of Pre-Recorded Patient Information | HCPCS G2010 | Remote evaluation of recorded video and/or images submitted by an established patient (e.g., store and forward), including interpretation with follow-up with the patient within 24 business hours, not originating from a related E/M service provided within the previous 7 days nor leading to an E/M service or procedure within the next 24 h or soonest available appointment. |
E-visits are patient-initiated communications through online portals for established patients only. Their reimbursement is purely based on time, and can be conducted with audio only. Current Procedural Terminology (CPT) codes for these “Online Digital Evaluation and Management” encounters (another name for E-visit) include 99421 (5–10 min), 99422 (11–20 min), 99423 (21+ minutes), and reimburse between $13 and $50 per visit [66].
Official telehealth services recognized by CMS require the use of both audio and video, which use traditional office outpatient CPT codes (e.g. 99201–99215), and reimburse at the same rate as in-person visits. Previously, CMS allowed telehealth only for established patients. However, new temporary PHE rules changes allow the use of telemedicine for new patients as well [65]. Prior rules required physicians to be in a health care facility to conduct these visits; this requirement has been removed and CMS has also now updated guidance regarding the Place of Service (POS) modifier which determines additional reimbursement. Previously, telehealth visits used the POS code of 02, signifying they were occurring via telemedicine. Now, providers should use the POS code that corresponds to where the visit would have taken place had it been in-person (i.e., 11, representing in office). Importantly, providers should appropriately document key aspects of these visits, including time spent, patient consent for virtual visit, and confirmation of patient identification (e.g. two identifiers—typically name and date of birth). The level of billing is determined based on medical decision making or on time associated with the encounter.
Finally, the telephone only telehealth visit was recently changed as part of the COVID-19 PHE. These are telephone only evaluation and management services that were previously deemed by Medicare as noncovered services. They include CPT 99441 (5–10 min), 99442 (11–20 min), 99443 (21–30 min) for physicians and 98966–98968 for advanced practice providers and other non-physician professionals. Under the PHE, these services will now be reimbursed comparable to visits incorporating video communication.
Specific telehealth coverage, documentation and coding, and payment level for telehealth services may vary by private insurer. Some, but not all, states have passed executive orders directing private health plans to cover telehealth at rates similar to in-person visits. A listing of state laws related to the COVID-19 PHE can be found here: https://www.cchpca.org/resources/covid-19-related-state-actions.
4.1.4. Technology
Most telehealth vendors have the ability to integrate with the majority of the largest electronic health record (EHR) companies. The American Academy of Family Physicians has maintained a telehealth toolkit, which includes information on various telehealth vendors [67]. However, for many providers, converting in-person to telehealth visits that are conducted via phone only requires relatively little new technology. Visit documentation and billing can be done through their existing EHR. At the simplest levels, providers can call patients using an audio-only connection. There are several non-HIPAA compliant video technologies (e.g., Facetime, WhatsApp) that can be used for patient visits during the PHE. Many vendors including Skype, Zoom, and Google, to name a few, offer HIPAA compliant product communication versions [64].
4.2. Remote monitoring
Remote Patient Monitoring (RPM) is a division of telehealth defined as “a digital health solution that captures and records patient physiologic data outside of a traditional health care environment.” [68] A variety of wired or wireless devices enable collection and remote transmission of clinically relevant data to guide management plans [69]. Patient data of interest includes blood pressure, heart rate, weight, glucose, heart rhythm, and more. Such objective information may inform management plans that keep patients safely at home during the COVID-19 pandemic and beyond.
CMS issued reimbursement codes to incentivize use of RPM in practice and recently added new codes specifically for home blood pressure monitoring (Table 2). The success of RPM hinges on patient engagement with technology. Ideal solutions employ user-centered design and go beyond passive monitoring to empower the patient as an active participant in her or his care. Success of RPM also depends on the accuracy of measurements. When measuring blood pressure, for example, an upper arm cuff is preferred over finger or wrist monitors. In general, app-based cuffless approaches are not recommended [70]. Clinicians can promote measurement accuracy by confirming that patients are using a Food and Drug Administration approved device and by providing education on measurement technique [71].
4.3. Enhancing medication access and adherence
Several practical strategies can be implemented to improve patient access and adherence during the pandemic. Currently, only around 10% of U.S. retail prescriptions are completed through mail order, a number that must be vastly increased [39]. Some local pharmacies perform home delivery which is recommended for older and high risk individuals. In addition, various pharmacy chains are allowing earlier refill options, prior to completion of 30 days since the previous refill. Some states, such as Georgia, have authorized pharmacists to refill prescription drugs, excluding controlled substances, if the prescribing provider cannot be reached [72].
All providers should proactively review with patients the need for extended refills at every contact, inquire about new challenges and barriers for accessing medications, and discuss strategies to obtain medications while minimizing exposures during this health emergency. The full care team should be involved in enhancing medication access and adherence including office staff that can query about refills during scheduling calls and other patient contacts, as well as pharmacists and advanced practice clinicians who can provide telehealth visits to address side effects, barriers, alternatives, and titrations. Any rigid practice rules regarding provision of refills should be liberalized.
Currently, the Food and Drug Administration is carefully monitoring for prescription medication supply chain disruptions [73]. Federal and state authorities should prioritize ensuring availability of agents deemed “essential medicines”, following the paradigm of the World Health Organization for agents that satisfy priority health care needs of a population, including cardiovascular drugs. While certain higher tier medications may not be considered essential medicines from a population perspective, they can serve a critical role for individuals at highest cardiovascular risk. Barriers to appropriate acquisition of these medications should be lowered, including cumbersome prior authorization processes during this emergency.
Finally, the best way to safeguard drug access during times of stress is to guarantee it during times of normalcy. When this crisis has receded, we must immediately adopt lessons learned about improving and facilitating medication access and adherence. We must also be prepared for the onslaught of the newly uninsured and financially strapped that will face extensive challenges to access essential affordable medications.
4.4. Leveraging team-based cardiovascular disease care delivery
Although acute cardiovascular care delivery and subsequent outpatient follow-up is primarily dependent on CVD specialists, chronic long-term delivery of CVD is delivered predominantly by primary care providers. The shortage of physicians in the United States, including those in primary care [74], is even more apparent in the context of the COVID-19 pandemic. Primary care physicians and specialists are being pulled to cover essential inpatient medical services, further straining effective primary care delivery for control of CVD risk factors.
Therefore, leveraging the entire cardiovascular team to deliver effective CVD care has now become even more important. These members of the cardiovascular care team include, but are not limited to, physicians, advanced practice clinicians such as nurse practitioners and physician assistants, pharmacists, dietitians, and patient navigators. This model of leveraging the entire cardiovascular team has been shown to be effective. For example, in a systematic review of clinical trials involving nurses or pharmacists for longitudinal management of hypertension, the odds ratios (95% CI) for blood pressure control were 1.69 (1.48–1.93) for nurses, 2.17 (1.75–2.68) for pharmacists within primary care, and 2.89 (1.83–4.55) for community pharmacists [75]. Similar results were demonstrated in patients with diabetes [76]. The beneficial effects of a collaborative care delivery model for control of cardiovascular risk factors has also been borne out in studies involving large numbers of cardiology practices [77] as well as studies conducted at the level of health care systems [78]. Importantly, these advantages were not associated with an increase in health resource utilization [79].
In the era of COVID-19, there is a mandate to expand telehealth services. To this end, we therefore recommended enhancing and facilitating the roles of all members of the cardiovascular care team to improve delivery of guideline directed cardiovascular care and adherence to lifestyle related recommendations and medications. Some examples include management of chronic diseases like hypertension, diabetes, and hyperlipidemia by advanced practice clinicians using virtual visits; medication reconciliation and counseling on medication adherence by clinical pharmacists using virtual visits; and virtual consultation by dietitians providing practical lifestyle counseling on heart healthy nutrition choices and physical activity recommendations while practicing social distancing.
Expanded use of telehealth in the current environment also allows increased scheduling flexibility and opportunity for various members of the care team (e.g. physicians, advanced practice clinicians, pharmacists and dietitians) to interact with a patient virtually in one visit to provide comprehensive preventive care. Some of the time and effort for the office staff responsible for scheduling patient visits and returning patient calls could be diverted towards coordination between various members of the care team to deliver holistic care, which in turn should also improve patient experience. Which of these combinations work best for a particular practice will depend on the scope of practice laws applicable to the state in which the practice is located. Simultaneous evaluation of the effectiveness of these models will also allow an opportunity to expand use of these team-based care delivery models after the COVID-19 pandemic.
4.5. Maintaining lifestyle habits and coping with stress/anxiety
4.5.1. Stress, anxiety, loneliness, and depression
There are several strategies to reduce stress, anxiety and depression [80]. First, it is important for patients to remain connected with family and friends through phone or other technologies. More severe symptoms of stress and anxiety should be addressed with a health care specialist via telehealth. Further, meditation programs have been shown to reduce multiple negative dimensions of psychological stress and could be helpful [81,82]. Yoga, specifically incorporating mindful breathing may also be beneficial to reduce anxiety, depression and sleep disorders, although many such trials used these techniques for longer periods of times [83]. Physical activity, as discussed below, can play an important role in ameliorating stress and anxiety. While stress and anxiety are often accompanied by increased alcohol consumption, as is already being reported in the COVID-19 pandemic [56], patients should be counseled to avoid this temptation given the adverse effects of excessive alcohol intake on lipids, weight and CVD risk [84]. Finally, despite the urge to follow pandemic trends, those experiencing excess stress or anxiety should consider limiting time spent watching and reading news related to the pandemic, including social media discussions.
4.5.2. Physical activity
Physical activity should continue to be promoted even during this crisis, and there are opportunities for exercise that can be done at home. Many government policies that enforce quarantines/lockdowns still allow for daily outdoor exercise, provided safe physical distancing is upheld. Even if constraints mandate solely exercising indoors, many group exercise classes are available virtually, either with previously recorded videos or real-time live classes that engage with others on-line. Individuals can be encouraged to find a virtual work-out buddy to facilitate a commitment to daily exercise and enhancing social connections. Daily exercise routines and step counts can be logged to track accountability. Smart phones and devices can be used to promote activity through activity-trackers and activity-promoting games. More time at home represents an opportunity to involve the whole family in exercise, educate children about the importance of regular activity, and introduce exercise as part of joyful routines to be maintained once outdoor restrictions have been lifted.
4.5.3. Nutrition
With thoughtful planning, many healthy products can still be purchased. Health care professionals should assess patient access to food items and changes in dietary patterns, and provide information regarding low cost, nutritionally dense foods. Examples include low-sodium canned vegetables and legumes, frozen vegetables, frozen fruits with no added sugars, oatmeal, and other whole grains. Additionally, dry/canned beans and nuts are good sources of plant-based protein and fiber with a longer shelf-life. Encouragement of other healthier coping strategies for stress management, other than stress eating, such as meditation and exercise cannot be overemphasized.
4.5.4. Tobacco cessation
Telehealth preventive visits represent an opportunity to inquire about smoking and the use of electronic nicotine delivery systems (ENDS) products. Moreover, during these telehealth visits, providers should continue to provide counseling on tobacco cessation. Patients can also be directed to several excellent online resources and programs that can be harnessed during this period to assist with tobacco cessation [85,86]. There are five nicotine replacement therapies and two non-nicotine oral medications, and behavioral interventions are available to help with quitting [87]. A combination of both counseling and pharmacotherapy is recommended to maximize the success of quitting. Techniques to help regulate/reappraise emotions during stressful times may help decrease smoking urges [88].
4.6. Innovative cardiac rehabilitation models
There are several viable options to provide CR to individuals remotely. Currently, CR centers are attempting to quickly implement CR via telehealth (i.e., home-based CR or “HBCR”).
HBCR programs are not new but, until now, have not been widely implemented. This lack of widespread adoption is largely due to lack of reimbursement [89]. These programs are as beneficial as facility-based programs in improving exercise capacity and modifiable CVD risk factors such as blood pressure and low-density lipoprotein cholesterol [90]. The recent American Heart Association (AHA)/American College of Cardiology (ACC) consensus statement on HBCR concluded that it is a viable option for low to moderate risk patients [91].
With COVID-19, there is a great opportunity to leverage this momentum for reimbursement of HBCR. COVID-19 has made abundantly clear to all health care stakeholders (payers, patients, providers, etc.) the critical role and effectiveness of telehealth. Without better reimbursement for HBCR (on par with facility-based CR), some HBCR programs are being forced to leverage CPT codes (99,454 and 99,457) that are used for remote physiological monitoring (in which a provider has to spend 20 min or more reviewing and acting on the data). However, this is not a true solution to the HBCR reimbursement issue and professional societies need to lobby for appropriate reimbursement of HBCR services, including at least temporary CMS coverage for CR telehealth services.
Some commercial HBCR vendors include INTERVENT Health [92], Moving Analytics [93], and Chanl Health. Moving Analytics provides Bluetooth-connected devices such as blood pressure cuffs and requires an upfront fee. Others such as Chanl Health are waiving all fees during the COVID-19 pandemic for access to the software without Bluetooth-connected devices. These vendors represent options for delivery of Phase II HBCR for low-to moderate-risk patients. For high-risk patients, such as those with left ventricular assist devices and heart transplant recipients, these programs are not ideal from an exercise standpoint but still provide useful education on nutrition, the importance of medication adherence, and other lifestyle modification strategies. There is also a great opportunity to combine HBCR with innovative digital health and wearable technology such as remote ECG monitors, as well as “smart shirts” (e.g. https://www.chronolife.net) with embedded sensors and electrodes that can transmit real time physiologic data to CR “monitoring centers.” During these unprecedented times, there is a clear need to deploy innovative and value-based strategies such as HBCR and facilitate their integration as part of the standard of care.
5. Conclusions and summary recommendations
The COVID-19 pandemic is a generation-defining event which will have long lasting ramifications for the health of our population and for the health care system, including those involved in the practice of preventive cardiology. We face the immense dual challenge of attending to the onslaught of acute critical illness in COVID-19 patients, while ensuring ongoing continuity of care and health preservation strategies for those at higher risk of CVD. Despite the challenges, ignoring the latter could result in a sizeable increase of preventable CVD events that present in delayed waves for months and years to come.
Summary recommendations for best practices in the outpatient management of patients with and at high-risk for CVD during the COVID-19 pandemic can be found in Table 3. The situation in each city, state and region regarding the COVID-19 crisis is variable in terms of number and severity of cases, strain on and capacity of the health care system, and socio-demographics of the local populations. Furthermore, the choice of how to best adopt and incorporate these recommendations will vary as well. Nonetheless, there are a broad range of considerations, tools, and strategies available to promote and preserve the health of the populations at higher risk of CVD in the outpatient setting during this crisis.
Table 3.
Summary recommendations for outpatient management of patients with and at high risk for cardiovascular disease during the COVID-19 pandemic.
|
|
|
|
|
|
|
|
Funding source
None.
Disclosures
AK- None; TJG-None; MG-None; EDM- None; AMN- Related to this subject matter: none. Funded by NHBLI K01HL133416; PRT- Related to this subject matter: none. Funded by NIDDKRO1DL118278-01, FEMA EMW-2016-FP00788, AHA-SDG#15SDG2233005; PPT-none related to this subject matter; NDW- None; MDS- none.
SJB- Consultant/National Speaker/Scientific Advisory Board: Akcea, Amgen, Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Esperion, GLG Group, Guidepoint Global, Regeneron, Sanofi, Novartis, Novo Nordisk.
SSM-current research support from the American Heart Association, Aetna Foundation, National Institutes of Health, the David and June Trone Family Foundation, and CASCADE FH. He has served as a consultant to Akcea, Amgen, Esperion, Kaneka, Novo Nordisk, Quest Diagnostics, Sanofi, Regeneron, and REGENXBIO. He is a co-inventor on a system to estimate LDL cholesterol levels, patent application pending. He is a founder of and holds equity in Corrie Health, which intends to further develop the platform. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies.
SSV- Grant support: Department of Veterans Affairs, World Heart Federation, Tahir and Jooma Family; Honorarium: American College of Cardiology (Associate Editor for Innovations, acc.org); Steering Committee Member: Patient and Provider Assessment of Lipid Management (PALM) registry [no financial remuneration].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Virani S.S., Alonso A., Benjamin E.J. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Rui P., Okeyode T. National ambulatory medical care survey: 2016 national summary tables. 2016. https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
- 3.https://covid19.healthdata.org/united-states-of-america
- 4.Lighter J., Phillips M., Hochman S. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luzi L., Radaelli M.G. Influenza and obesity: its odd relationship and the lessons for COVID-19 pandemic. Acta Diabetol. 2020 doi: 10.1007/s00592-020-01522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 — United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. Jama. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 8.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 13.Hendren N.S., Drazner M.H., Bozkurt B., Cooper L.T., Jr. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hulot J.-S. Archives of Cardiovascular Diseases; 2020. COVID-19 in patients with cardiovascular diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020 doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiong T.-Y., Redwood S., Prendergast B., Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fadini G.P., Morieri M.L., Longato E., Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest. 2020 doi: 10.1007/s40618-020-01236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo W., Li M., Dong Y. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metabol Res Rev. 2020:e3319. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lippi G., Wong J., Henry B.M. Hypertension and its severity or mortality in Coronavirus Disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020 doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- 21.Liu K., Fang Y.Y., Deng Y. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020 doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan W-j, Ni Z-y, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clerkin K.J., Fried J.A., Raikhelkar J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 25.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bavishi C., Maddox T.M., Messerli F.H. Coronavirus disease 2019 (COVID-19) infection and renin angiotensin system blockers. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1282. [DOI] [PubMed] [Google Scholar]
- 27.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z., Chen Z., Zhang L. Status of hypertension in China: results from the China hypertension survey, 2012-2015. Circulation. 2018;137:2344–2356. doi: 10.1161/CIRCULATIONAHA.117.032380. [DOI] [PubMed] [Google Scholar]
- 29.Lu J., Lu Y., Wang X. Prevalence, awareness, treatment, and control of hypertension in China: data from 1.7 million adults in a population-based screening study (China PEACE Million Persons Project) Lancet. 2017;390:2549–2558. doi: 10.1016/S0140-6736(17)32478-9. [DOI] [PubMed] [Google Scholar]
- 30.https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/what-you-can-do.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fneed-extra-precautions%2Fget-ready.html. Accessed April 5th, 2020. . Centers for Disease Control.
- 31.https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/social-distancing.html. Accessed April 5, 2020. Centers for Disease Control.
- 32.Krumholz H.M. Where have all the heart attacks gone? N Y Times. 2020 [Google Scholar]
- 33.Garcia S., Albaghdadi M.S., Meraj P.W. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.011. [e-publication] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tam C.F., Cheung K.S., Lam S. Impact of coronavirus disease 2019 (COVID-19) outbreak on ST-segment-elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcome. 2020 doi: 10.1161/CIRCOUTCOMES.120.006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woolhandler S., Himmelstein D.U. Intersecting U.S. Epidemics: COVID-19 and lack of health insurance. Ann Intern Med. 2020 doi: 10.7326/M20-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.https://www.chicago.gov/city/en/sites/covid-19/home/latest-data.html
- 37.American College of cardiology urges heart attack, stroke patients to seek medical help. https://www.acc.org/about-acc/press-releases/2020/04/14/10/17/american-college-of-cardiology-urges-heart-attack-stroke-patients-to-seek-medical-help Accessed April 20.
- 38.Khera R., Valero-Elizondo J., Das S.R. Cost-related medication nonadherence in adults with atherosclerotic cardiovascular disease in the United States, 2013 to 2017. Circulation. 2019;140:2067–2075. doi: 10.1161/CIRCULATIONAHA.119.041974. [DOI] [PubMed] [Google Scholar]
- 39.Alexander G.C., Qato D.M. Ensuring access to medications in the US during the COVID-19 pandemic. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.6016. [DOI] [PubMed] [Google Scholar]
- 40.Older Americans are risking coronavirus exposure to get their medications. https://sph.umich.edu/pursuit/2020posts/older-americans-risking-medications.html
- 41.Knowles J.W., Howard W.B., Karayan L. Access to nonstatin lipid-lowering therapies in patients at high risk of atherosclerotic cardiovascular disease. Circulation. 2017;135:2204–2206. doi: 10.1161/CIRCULATIONAHA.117.027705. [DOI] [PubMed] [Google Scholar]
- 42.Fong M.W., Gao H., Wong J.Y. Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings-social distancing measures. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2605.190995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang C., Pan R., Wan X. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int J Environ Res Publ Health. 2020;17 doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawryluck L., Gold W.L., Robinson S., Pogorski S., Galea S., Styra R. SARS control and psychological effects of quarantine, Toronto, Canada. Emerg Infect Dis. 2004;10:1206–1212. doi: 10.3201/eid1007.030703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richardson S., Shaffer J.A., Falzon L., Krupka D., Davidson K.W., Edmondson D. Meta-analysis of perceived stress and its association with incident coronary heart disease. Am J Cardiol. 2012;110:1711–1716. doi: 10.1016/j.amjcard.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Becquart N.A., Naumova E.N., Singh G., Chui K.K.H. Cardiovascular disease hospitalizations in Louisiana parishes’ elderly before, during and after hurricane Katrina. Int J Environ Res Publ Health. 2018;16 doi: 10.3390/ijerph16010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilbert-Lampen U., Leistner D., Greven S. Cardiovascular events during world cup soccer. N Engl J Med. 2008;358:475–483. doi: 10.1056/NEJMoa0707427. [DOI] [PubMed] [Google Scholar]
- 48.Hakulinen C., Pulkki-Råback L., Virtanen M., Jokela M., Kivimäki M., Elovainio M. Social isolation and loneliness as risk factors for myocardial infarction, stroke and mortality: UK Biobank cohort study of 479 054 men and women. Heart. 2018;104:1536–1542. doi: 10.1136/heartjnl-2017-312663. [DOI] [PubMed] [Google Scholar]
- 49.Gan Y., Gong Y., Tong X. Depression and the risk of coronary heart disease: a meta-analysis of prospective cohort studies. BMC Psychiatr. 2014;14:371. doi: 10.1186/s12888-014-0371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faulenbach M., Uthoff H., Schwegler K., Spinas G.A., Schmid C., Wiesli P. Effect of psychological stress on glucose control in patients with Type 2 diabetes. Diabet Med. 2012;29:128–131. doi: 10.1111/j.1464-5491.2011.03431.x. [DOI] [PubMed] [Google Scholar]
- 51.Lemmens S.G., Rutters F., Born J.M., Westerterp-Plantenga M.S. Stress augments food ’wanting’ and energy intake in visceral overweight subjects in the absence of hunger. Physiol Behav. 2011;103:157–163. doi: 10.1016/j.physbeh.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 52.Fitbit. Fitbit News The impact of coronavirus on global activity. https://blog.fitbit.com/covid-19-global-activity/ Available online at:
- 53.Lim S., Tellez M., Ismail A.I. Chronic stress and unhealthy dietary behaviors among low-income African-American female caregivers. Curr Dev Nutr. 2020;4:nzaa029. doi: 10.1093/cdn/nzaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richardson A.S., Arsenault J.E., Cates S.C., Muth M.K. Perceived stress, unhealthy eating behaviors, and severe obesity in low-income women. Nutr J. 2015;14:122. doi: 10.1186/s12937-015-0110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yau Y.H., Potenza M.N. Stress and eating behaviors. Minerva Endocrinol. 2013;38:255–267. [PMC free article] [PubMed] [Google Scholar]
- 56.Nielsen says beverage alcohol retail sales are soaring during the crisis. https://www.forbes.com/sites/thomaspellechia/2020/03/25/nielsen-says-beverage-alcohol-retail-sales-are-soaring-during-the-crises/#15d944512444 [Forbes]
- 57.’I Just Need the Comfort’ Processed foods make a pandemic comeback. https://www.nytimes.com/2020/04/07/business/coronavirus-processed-foods.html [New York Times]
- 58.National Center for Chronic Disease Prevention and Health Promotion (US) Centers for Disease Control and Prevention (US); Atlanta (GA): 2014. Office on smoking and health. The health consequences of smoking—50 Years of progress: a report of the Surgeon general.https://www.ncbi.nlm.nih.gov/books/NBK179276/ Available from: [PubMed] [Google Scholar]
- 59.Cessation Smoking. 2020. A report from the Surgeon general. Atlanta, GA. [Google Scholar]
- 60.Karekla M., Panayiotou G., Collins B.N. Predictors of urge to smoke under stressful conditions: an experimental investigation utilizing the PASAT-C task to induce negative affect in smokers. Psychol Addict Behav. 2017;31:735–743. doi: 10.1037/adb0000309. [DOI] [PubMed] [Google Scholar]
- 61.Mazzini M.J., Stevens G.R., Whalen D., Ozonoff A., Balady G.J. Effect of an American Heart Association Get with the Guidelines program-based clinical pathway on referral and enrollment into cardiac rehabilitation after acute myocardial infarction. Am J Cardiol. 2008;101:1084–1087. doi: 10.1016/j.amjcard.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 62.Lawler P.R., Filion K.B., Eisenberg M.J. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Am Heart J. 2011;162:571–584 e2. doi: 10.1016/j.ahj.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 63.Medicare, Medicaid Programs Policy and regulatory revisions in Response to the COVID-19 public health emergency. https://www.federalregister.gov/documents/2020/04/06/2020-06990/medicare-and-medicaid-programs-policy-and-regulatory-revisions-in-response-to-the-covid-19-public#h-13
- 64.Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html [U.S. Department of Health & Human Services]
- 65.Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet [Centers for Medicare & Medicaid Services]
- 66.Telehealth coding and billing during COVID-19. https://www.acponline.org/practice-resources/covid-19-practice-management-resources/telehealth-coding-and-billing-during-covid-19 [American College of Physicians]
- 67.General provider telehealth and telemedicine tool kit. https://www.aafp.org/dam/AAFP/documents/advocacy/prevention/crisis/CMSGeneralTelemedicineToolkit.pdf [American Association of Family Practice]
- 68.AMERICAN medical ASSOCIATION®DIGITAL health implementation playbook. https://www.ama-assn.org/system/files/2018-12/digital-health-implementation-playbook.pdf [American Medical Association]
- 69.Sana F., Isselbacher E.M., Singh J.P., Heist E.K., Pathik B., Armoundas A.A. Wearable devices for ambulatory cardiac monitoring: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:1582–1592. doi: 10.1016/j.jacc.2020.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Plante T.B., Urrea B., MacFarlane Z.T. Validation of the instant blood pressure smartphone app. JAMA Intern Med. 2016;176:700–702. doi: 10.1001/jamainternmed.2016.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monitoring your blood pressure at home. https://www.heart.org/en/health-topics/high-blood-pressure/understanding-blood-pressure-readings/monitoring-your-blood-pressure-at-home [American Heart Association]
- 72.Gov. Kemp issues new executive orders, provides COVID-19 update. https://gov.georgia.gov/press-releases/2020-03-23/gov-kemp-issues-new-executive-orders-provides-covid-19-update
- 73.Statment F.D.A. Coronavirus (COVID-19) supply chain update. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-supply-chain-update
- 74.New findings confirm predictions on physician shortage. https://www.aamc.org/news-insights/press-releases/new-findings-confirm-predictions-physician-shortage [AAMC]
- 75.Carter B.L., Rogers M., Daly J., Zheng S., James P.A. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009;169:1748–1755. doi: 10.1001/archinternmed.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fazel M.T., Bagalagel A., Lee J.K., Martin J.R., Slack M.K. Impact of diabetes care by pharmacists as part of health care team in ambulatory settings: a systematic review and meta-analysis. Ann Pharmacother. 2017;51:890–907. doi: 10.1177/1060028017711454. [DOI] [PubMed] [Google Scholar]
- 77.Virani S.S., Maddox T.M., Chan P.S. Provider type and quality of outpatient cardiovascular disease care: insights from the NCDR PINNACLE registry. J Am Coll Cardiol. 2015;66:1803–1812. doi: 10.1016/j.jacc.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Virani S.S., Akeroyd J.M., Ramsey D.J. Comparative effectiveness of outpatient cardiovascular disease and diabetes care delivery between advanced practice providers and physician providers in primary care: implications for care under the Affordable Care Act. Am Heart J. 2016;181:74–82. doi: 10.1016/j.ahj.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 79.Virani S.S., Akeroyd J.M., Ramsey D.J. Health care resource utilization for outpatient cardiovascular disease and diabetes care delivery among advanced practice providers and physician providers in primary care. Popul Health Manag. 2018;21:209–216. doi: 10.1089/pop.2017.0090. [DOI] [PubMed] [Google Scholar]
- 80.https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/managing-stress-anxiety.html
- 81.Goyal M., Singh S., Sibinga E.M. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174:357–368. doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levine G.N., Lange R.A., Bairey-Merz C.N. Meditation and cardiovascular risk reduction: a scientific statement from the American heart association. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tellhed U., Daukantaitė D., Maddux R.E., Svensson T., Melander O. Yogic breathing and mindfulness as stress coping mediate positive health outcomes of Yoga. Mindfulness. 2019;10:2703–2715. [Google Scholar]
- 84.Ronksley P.E., Brien S.E., Turner B.J., Mukamal K.J., Ghali W.A. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. Bmj. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.https://www.cardiosmart.org/Healthy-Living/Stop-Smoking/Smoking-and-Heart-Disease
- 86.Barua R.S., Rigotti N.A., Benowitz N.L. ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2018;2018(72):3332–3365. doi: 10.1016/j.jacc.2018.10.027. [DOI] [PubMed] [Google Scholar]
- 87.Arnett D.K., Blumenthal R.S., Albert M.A. ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;2019(140):e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Szasz P.L., Szentagotai A., Hofmann S.G. Effects of emotion regulation strategies on smoking craving, attentional bias, and task persistence. Behav Res Ther. 2012;50:333–340. doi: 10.1016/j.brat.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 89.Sandesara P.B., Lambert C.T., Gordon N.F. Cardiac rehabilitation and risk reduction: time to "rebrand and reinvigorate. J Am Coll Cardiol. 2015;65:389–395. doi: 10.1016/j.jacc.2014.10.059. [DOI] [PubMed] [Google Scholar]
- 90.Rawstorn J.C., Gant N., Direito A., Beckmann C., Maddison R. Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart. 2016;102:1183–1192. doi: 10.1136/heartjnl-2015-308966. [DOI] [PubMed] [Google Scholar]
- 91.Thomas R.J., Beatty A.L., Beckie T.M. Home-based cardiac rehabilitation: a scientific statement from the American association of cardiovascular and pulmonary rehabilitation, the American heart association, and the American College of cardiology. Circulation. 2019;140:e69–e89. doi: 10.1161/CIR.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 92.Gordon N.F., Salmon R.D., Wright B.S., Faircloth G.C., Reid K.S., Gordon T.L. Clinical effectiveness of lifestyle health coaching: case study of an evidence-based program. Am J Lifestyle Med. 2017;11:153–166. doi: 10.1177/1559827615592351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Harzand A., Witbrodt B., Davis-Watts M.L. Feasibility of a smartphone-enabled cardiac rehabilitation program in male Veterans with previous clinical evidence of coronary heart disease. Am J Cardiol. 2018;122:1471–1476. doi: 10.1016/j.amjcard.2018.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]