Abstract
Serum albumin has long been an essential supplement for ex vivo hematopoietic and immune cell cultures. However, serum albumin media supplements represent a major source of biological contamination in cell cultures, which often cause loss of cellular function. As serum albumin displays significant batch-to-batch variability, it has also been blamed for causing major issues in experimental reproducibility. We recently discovered the synthetic polymer polyvinyl alcohol (PVA) as an inexpensive, GMP-compatible, and biologically-inert serum albumin-replacement for ex vivo hematopoietic stem cell cultures. Importantly, PVA is free from the biological contaminants that have plagued serum albumin-based medias. Here, we demonstrate that PVA can replace serum albumin in a range of blood and immune cell cultures including cell lines, primary leukemia samples, and human T lymphocytes. PVA can even replace human serum in the generation and expansion of functional Chimeric Antigen Receptor (CAR) T cells, offering a safer and more cost-efficient approach for this clinical therapy. In summary, PVA represents a chemically-defined, biologically-inert, and inexpensive alternative to serum albumin for range of cell cultures in hematology and immunology.
Keywords: Polyvinyl alcohol, serum albumin, CAR T cell therapy, cytokines, cell culture
Introduction:
Serum albumin has long been an essential supplement for ex vivo cultures of hematopoietic cells and lymphocytes1. However, serum albumin supplements represent a major source of biological contaminants and often display significant batch-to-batch variability, causing issues in experimental reproducibility and time-consuming batch-testing2. Progressive attempts to limit these contaminants have involved moving from the use of serum such as fetal bovine serum (FBS) or human serum to purified serum albumin (e.g. bovine serum albumin fraction V) and/or recombinantly-derived human serum albumin2 (HSA). Additionally, serum albumin supplements represent a major cost in cell culture, particularly where Good Manufacturing Practice (GMP)-grade reagents are required.
We recently identified polyvinyl alcohol (PVA) in combination with insulin-selenium-transferrin-ethanolamine (ITSX) as a serum replacement for ex vivo hematopoietic stem cell (HSC) culture3. In comparison to serum albumin media supplements, PVA represents an inexpensive, chemically-defined, and GMP-compatible alternative. Here, we demonstrate that PVA can also be used as a serum albumin replacement for in vitro culture of leukemia cells and T lymphocytes, including Chimeric Antigen Receptor (CAR) T cells. Adaptive immunotherapies such as CAR T cell therapies have become an exciting new therapeutic approach to treat and cure various cancers4–6. However, the large-scale ex vivo expansion of T lymphocytes7 for these therapies currently rely on expensive pre-screened human serum. Our results suggest that PVA is a chemically-defined alternative to serum albumin for CAT T cell expansion, which may offer advantages in terms of cost, safety, and reproducibility.
Materials and Methods:
Cell line cultures
Mouse 32D/MPL (previously generated by the laboratory8) and human K562 cells (purchased from ATCC) were cultured in RPMI1640 media (Gibco) supplemented with 10% (v/v) fetal bovine serum (FBS; Sigma), 1% insulin-transferrin-selenium-ethanolamine (ITSX; Gibco), 1 mg/ml recombinant human serum albumin (HSA; Albumin Biosciences), and/or 1 mg/ml polyvinyl alcohol (PVA; Sigma) at 37°C with 5% CO2. 32D/MPL cells were supplemented with 10 ng/ml recombinant mouse TPO (R&D Systems or Peprotech). Both cell lines tested negative for mycoplasma using the MycoAlert Plus Mycoplasma Detection Kit (Lonza), following manufacturer instructions. Cell counting was performed using the Chemometec NC-3000 automated cell counter.
Primary AML cell cultures
Primary human AML samples were collected and stored by the Stanford Hematology Tissue Bank, following informed patient consent. according to the Administrative Panel on Human Subjects Research Institutional Review Board (IRB)-approved protocols (Stanford IRB no. 18329, no. 6453, and no. 5637). Primary AML cells were cultured in MEMa (containing 1% Penicillin-Streptomycin-Glutamine; Gibco) supplemented with 10% FBS, 1% ITSX, and/or 1 mg/ml PVA at 37°C with 5% CO2. The following cytokines were added to the culture (as described previously9): human TPO, human SCF, human FLT3L, human IL-3, human IL-6, human GM- CSF, and human G-CSF (all at 20 ng/ml).
Primary T cell cultures
Peripheral blood mononuclear cells (PBMCs) from de-identified healthy donors were purchased from the Stanford Blood Center. CD3+ T cells were purified and stimulation with anti-CD3/CD28 dynabeads, then cultured in RPMI1640 media (Gibco) supplemented with 5% (v/v) human serum (company), 1% insulin-transferrin-selenium-ethanolamine (ITSX; Gibco), and/or 1 mg/ml PVA at 37°C with 5% CO2.
CAR T cell assays
CD3+ T cells were purified and stimulated as above, before lentiviral transduction on day 2 and 3 with anti-CD19-CAR lentivirus (lentivirus plasmid was provided by Dr. Crystal Mackall with slight modification). Lentiviral infection was conducted with Retronecine coating (Clontech) with spinfection (1,000 g for 1 hour at 32 °C) at MOI = 10. After an additional 7-day expansion, in vitro killing activity was determined by bioluminescent-mediated viability measurement10 with firefly luciferase (FLuc)-transduced NALM6 cells.
ELISA assays
Mouse TPO and human IL-15 ELISA Kits were purchased from R&D Systems (MTP00 and D1500) and performed according manufacturer instructions.
Statistical analysis
One-way and two-way ANOVA tests were performed as indicated in the figures using Prism 7 software.
Results and Discussion:
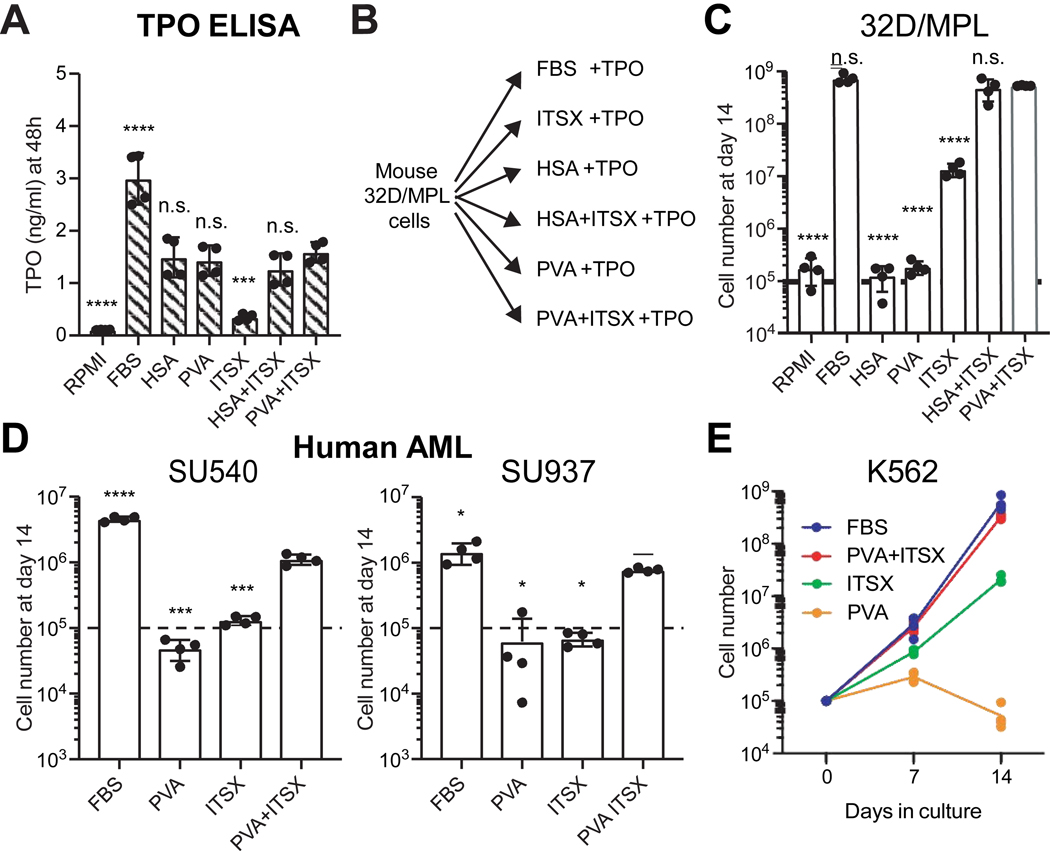
As a direct replacement for recombinant HSA in our HSC culture system, we hypothesized that PVA stabilizes proteins in the media, such as the key HSC cytokine thrombopoietin3 (TPO). Using ELISA assays, we confirmed that PVA stabilized TPO concentrations similar to HSA (Figure 1A), while significantly more TPO was lost from the RPMI-only and ITSX-only medias (only ~5% remained after 24 hours at 37°C for ITSX-only). Consistent with our previous results with HSC media3 and the relative stability of TPO in these different media conditions, the proliferation of the thrombopoietin (TPO)-dependent 32D/MPL mouse cell line8 was highest in PVA+ITSX or HSA+ITSX conditions, comparable to expansion in FBS. By contrast, 32D/MPL cells more slowly proliferated in ITSX-only conditions, and essentially failed to grow in those conditions lacking insulin (Figure 1B,C).
Figure 1: Poly-vinyl alcohol can replace serum albumin in leukemic cell cultures.
(A) ELISA assay for mouse TPO stability in RPMI-based culture media after 24 hours. Mean ± S.D of technical duplicates from biological replicates.
(B) ) Schematic summary of TPO-dependent mouse 32D/MPL cells grown in various RPMI-based medias supplemented 10ng/ml TPO.
(C) Total numbers of 32D/MPL cells after a 14-day culture in the RMPI-based medias described in (B) with TPO at 10 ng/ml. Dotted line indicates starting cell concentration. Mean ± S.D of four independent cultures.
(D) Total numbers of primary human AML cells (two AML samples from the Stanford Hematology Biobank) after a 14-day culture in MEMa-based medias, with indicated supplements and cytokines (TPO, SCF, FLT3L, IL-6, IL-3, GM-CSF, and G-CSF; all 20 ng/ml). Dotted line indicates starting cell concentration. Mean ± S.D of four independent cultures.
(E) Total numbers of human K-562 cells over a 14-day culture in the RMPI-based medias with indicated supplements. Mean of four independent cultures.
In all panels, statistical analysis was performed by one-way ANOVA (analysis measured against the PVA+ITSX condition with significance indicated by: *, p<0.05, **, p<0.01, ***, p>0.001; ****, p>0.0001; n.s, non-significant.
To expand the applications of PVA in hematology research, we next tested the use of PVA in primary human acute myeloid leukemia (AML) cultures. PVA+ITSX supported growth of primary human AML samples cultured in the presence of cytokines, and almost equivalent to FBS containing media (Figure 1D). PVA+ITSX also supported more rapid proliferation of the human erythroleukemia K562 cell line11, without addition of exogenous cytokines (Figure 1E). PVA may therefore have broad application for growing various hematological cell types beyond HSC cultures, although future work will need to determine how each cytokine/growth factor functions in albumin-free conditions. The PVA culture system also provides a useful platform with which dissect the biological components in serum that influence cell growth.
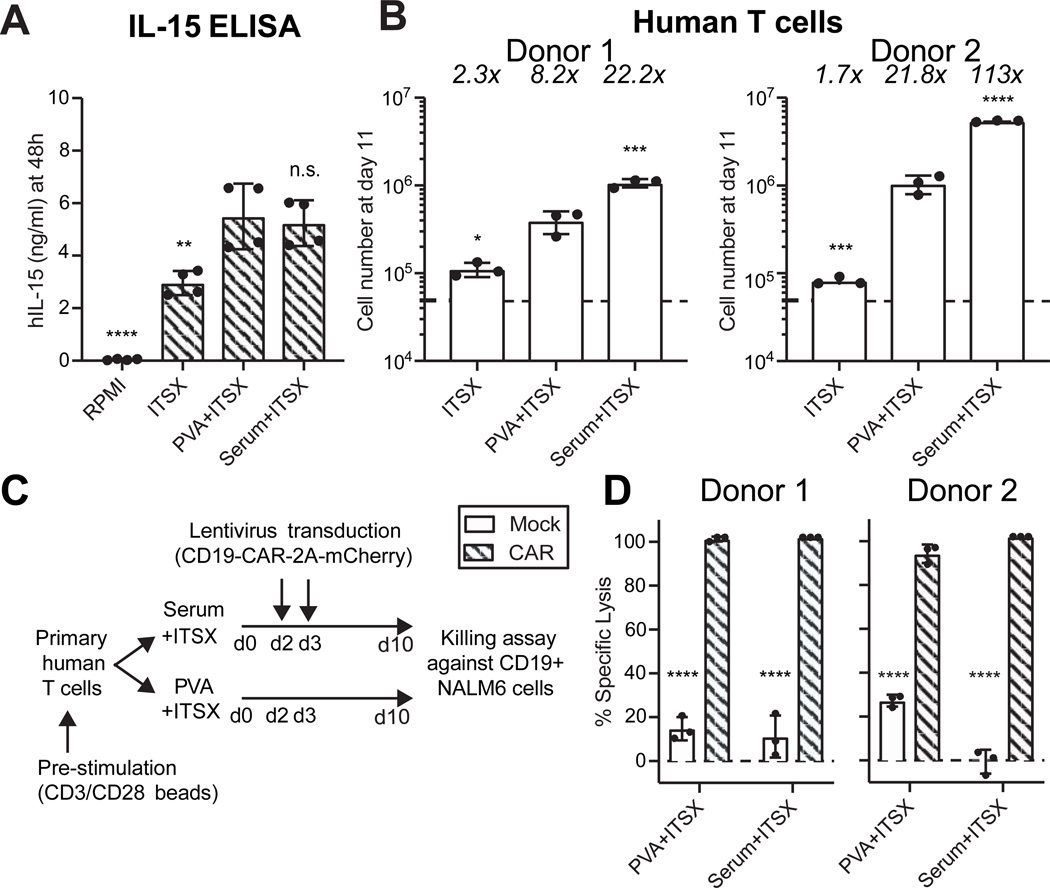
We also investigated the application of PVA to lymphocyte cell culture. We initially confirmed that the key T cell cytokine IL-1512 was similarly stabilized in PVA+ITSX and Serum+ITSX medias (Figure 2A). Primary human T cells expanded in serum albumin-free conditions containing PVA+ITSX by ~8–22-fold over 11 days in culture (Figure 2B). This expansion was slightly lower than in the Serum+ITSX cultures (~22–113-fold), suggesting human serum contains additional factors promoting T cell growth, which remain to be identified. Consistent with the reduced stability of IL-15 in ITSX-only media, this condition supported only ~2-fold expansion of T cells over 11 days (Figure 2B). These results confirm that PVA can be used as a human serum-replacement for expanding primary human T cells and identify an inexpensive and chemically-defined carrier for human T cell culture.
Figure 2: Poly-vinyl alcohol can replace serum albumin in T lymphocyte cell cultures.
(A) ELISA assay for human IL-15 stability in culture media after 24 hours. Mean ± S.D of technical duplicates from biological replicates.
(B) Total number of primary T cells after an 11-day culture in RPMI-based medias with the indicated supplements and cytokines (IL-15 and IL-7; 10 ng/ml each), following with anti-CD3/CD28 dynabeads stimulation. Mean ± S.D of triplicated experiments for two healthy donors.
(C) Schematic of CAR-T cell production by primary T cell collection and stimulation with anti-CD3/CD28 dynabeads at day 0, lentiviral transduction at days 2 and 3, and expansion until day 10, all in RMPI+PVA+ITSX and RPMI+Serum+ITSX. At day 10, transduced mCherry+ anti-CD19-CAR T cells were used in a killing assay using CD19+ NALM6 target cells at a ratio of 5:1 (CAR-T cells:target cells).
(D) T cell killing activity of anti-CD19 CAR T cells targeting CD19-expressing NALM6 cells, described in (C). Mean ± S.D of technical triplicates for two separate donors. Representative of two biological replicates.
Statistical analysis was performed by one-way ANOVA (A-B) or two-way ANOVA (C) analysis measured against the PVA+ITSX condition with significance indicated by: *, p<0.05, **, p<0.01, ***, p>0.001; ****, p>0.0001; n.s, non-significant.
For CAR T therapies, T cells must not only expand ex vivo, but also function to kill target cancer cells. We therefore confirmed that PVA-expanded T cells retained full functionality by assessing the use of PVA for expanding anti-CD19 CAR T cells13 (Figure 2C). PVA+ITSX exerted comparable anti-CD19 killing activity as those expanded in Serum+ITSX (Figure 2D). The replacement of serum albumin with PVA may therefore provide significant improvements in terms of both cost and safety for ex vivo T cell cultures.
In summary, PVA offers inexpensive and chemically-defined culture conditions for a number of cell types in hematology and immunology. We expect that these serum albumin-free media conditions will have important implications for how we culture cells for both basic research and clinical cell therapies.
Highlights:
Polyvinyl alcohol (PVA) stabilizes recombinant cytokines in liquid media
PVA supports albumin-free growth of cytokine-dependent leukemia cells
PVA can replace serum albumin for ex vivo expansion of functional CAR T cells
Acknowledgements:
We thank K. Chan for performing mycoplasma testing. This research was funded by CIRM (LA1_C12–06917; DISC1–10555), the NIH (R01DK116944; R01HL147124), the Leukemia and Lymphoma Society (3385–19), and the Ludwig Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest:
HN is a co-founder and shareholder of Century Therapeutics. RM is a cofounder, consultant, shareholder, and director of Forty Seven Inc.
References:
- 1.Francis GL. Albumin and mammalian cell culture: implications for biotechnology applications. Cytotechnology. 2010;62(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ieyasu A, Ishida R, Kimura T, et al. An All-Recombinant Protein-Based Culture System Specifically Identifies Hematopoietic Stem Cell Maintenance Factors. Stem Cell Reports. 2017;8(3):500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilkinson AC, Ishida R, Kikuchi M, et al. Long-term ex vivo hematopoietic-stem-cell expansion allows nonconditioned transplantation. Nature. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–1365. [DOI] [PubMed] [Google Scholar]
- 5.Ghorashian S, Amrolia P, Veys P. Open access? Widening access to chimeric antigen receptor (CAR) therapy for ALL. Exp Hematol. 2018;66:5–16. [DOI] [PubMed] [Google Scholar]
- 6.Ando M, Nakauchi H. ‘Off-the-shelf’ immunotherapy with iPSC-derived rejuvenated cytotoxic T lymphocytes. Exp Hematol. 2017;47:2–12. [DOI] [PubMed] [Google Scholar]
- 7.Levine BL, Miskin J, Wonnacott K, Keir C. Global Manufacturing of CAR T Cell Therapy. Mol Ther Methods Clin Dev. 2017;4:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seita J, Ema H, Ooehara J, et al. Lnk negatively regulates self-renewal of hematopoietic stem cells by modifying thrombopoietin-mediated signal transduction. Proc Natl Acad Sci U S A. 2007;104(7):2349–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bak RO, Dever DP, Reinisch A, Cruz Hernandez D, Majeti R, Porteus MH. Multiplexed genetic engineering of human hematopoietic stem and progenitor cells using CRISPR/Cas9 and AAV6. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu X, Tao L, Rivera A, et al. A simple and sensitive method for measuring tumor-specific T cell cytotoxicity. PloS one. 2010;5(7):e11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lozzio BB, Lozzio CB, Bamberger EG, Feliu AS. A multipotential leukemia cell line (K-562) of human origin. Proc Soc Exp Biol Med. 1981;166(4):546–550. [DOI] [PubMed] [Google Scholar]
- 12.Klebanoff CA, Finkelstein SE, Surman DR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(7):1969–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17(8):1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]