Abstract
Aims
To evaluate the long-term use of secondary prevention medications [statins, β-blockers, renin–angiotensin–aldosterone system (RAAS) inhibitors, and platelet inhibitors] after coronary artery bypass grafting (CABG) and the association between medication use and mortality.
Methods and results
All patients who underwent isolated CABG in Sweden from 2006 to 2015 and survived at least 6 months after discharge were included (n = 28 812). Individual patient data from SWEDEHEART and other mandatory nationwide registries were merged. Multivariable Cox regression models using time-updated data on dispensed prescriptions were used to assess associations between medication use and long-term mortality. Statins were dispensed to 93.9% of the patients 6 months after discharge and to 77.3% 8 years later. Corresponding figures for β-blockers were 91.0% and 76.4%, for RAAS inhibitors 72.9% and 65.9%, and for platelet inhibitors 93.0% and 79.8%. All medications were dispensed less often to patients ≥75 years. Treatment with statins [hazard ratio (HR) 0.56, 95% confidence interval (95% CI) 0.52–0.60], RAAS inhibitors (HR 0.78, 95% CI 0.73–0.84), and platelet inhibitors (HR 0.74, 95% CI 0.69–0.81) were individually associated with lower mortality risk after adjustment for age, gender, comorbidities, and use of other secondary preventive drugs (all P < 0.001). There was no association between β-blockers and mortality risk (HR 0.97, 95% CI 0.90–1.06; P = 0.54).
Conclusion
The use of secondary prevention medications after CABG was high early after surgery but decreased significantly over time. The results of this observational study, with inherent risk of selection bias, suggest that treatment with statins, RAAS inhibitors, and platelet inhibitors is essential after CABG whereas the routine use of β-blockers may be questioned.
Keywords: Coronary artery bypass grafting, Secondary prevention medication, Survival
See page 1662 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz855)
Introduction
Coronary artery bypass grafting (CABG) is the recommended method for the majority of patients with complex multivessel coronary artery disease who are in need of revascularization.1 Secondary prevention medication after CABG is recommended to maintain the long-term benefits of revascularization, both in terms of morbidity and mortality. Current guidelines recommend that after CABG, statins and antiplatelet agents should be given to all patients with no contraindications, renin–angiotensin–aldosterone system (RAAS) inhibitors (angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers) should be given to patients with reduced left ventricular ejection fraction (LVEF), hypertension, or previous myocardial infarction, and β-blockers should be given to patients with previous myocardial infarction and/or reduced LVEF.2–4
There is limited real-world data available on adherence to the recommendations, but the results of several smaller registry studies and randomized trials have suggested that the use of secondary prevention medications after CABG is surprisingly low5–10 and markedly lower than in coronary artery disease patients treated with percutaneous coronary intervention.7–10 Furthermore, there are few data available on associations between the use of secondary prevention medication over time and mortality, particularly using time-updated information about medications. The objectives of this study were (i) to determine the use of secondary prevention medications over time in CABG patients in relation to age and sex and (ii) to investigate associations between the longitudinal use of secondary prevention medications and long-term mortality in a large, contemporary nationwide cohort of CABG patients.
Methods
Study population
The study population was identified in the Swedish Cardiac Surgery Registry, which is part of the SWEDEHEART registry.11 All patients over 18 years of age who underwent isolated first-time CABG from 1 January 2006 to 31 December 2015 were considered for inclusion. Data on dispensed prescriptions of secondary prevention medications and mortality status were registered until 31 December 2015. Since mortality early after surgery is often related to the procedure itself and less likely to be preventable by secondary prevention medication, patients who were not alive 6 months after discharge or with <6 months of follow-up (i.e. discharged after 30 June 2015 or emigrated <6 months after discharge) were excluded (Supplementary material online, Figure S1).
Data sources
Data on the CABG procedure, including preoperative status and postoperative complications, were retrieved from the Swedish Cardiac Surgery Registry, which contains detailed information on all cardiac surgery procedures in Sweden since 1992 with a coverage of 98–99%.12 Comorbidities, additional to those registered in the Swedish Cardiac Surgery Registry, were collected from the National Patient Register, which has full coverage of diagnoses from all hospital admissions in Sweden, with a validity of 85–95%.13 Hospitalizations are reported according to the International Classification of Disease (ICD) 9 and 10. All comorbidities registered until start of follow-up (i.e. 6 months after hospital discharge after CABG) were registered as baseline data (Supplementary material online, Table S1). The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula was used to calculate estimated glomerular filtration rate (eGFR).14 Mortality data were collected from the national Cause of Death Register, which has information on the date and cause of death of all deceased Swedish citizens (according to ICD).
The Swedish Prescribed Drug Register, which has information on all prescriptions dispensed from Swedish pharmacies from July 2005 (according to the Anatomical Therapeutic Chemicals [ATC] classification), was used to obtain data on dispensed prescriptions for statins, β-blockers, RAAS inhibitors, and platelet inhibitors (Supplementary material online, Table S2). Exposure status was recorded at baseline and updated every third month during the follow-up, based on the typical package size in Sweden, which covers 90–100 daily doses. To allow for minor faults in compliance, a patient was considered to be off treatment if he/she had no prescription dispensed over two consecutive 3-month periods. Patients were allowed to change their exposure status during the follow-up period according to their dispensed prescriptions.
The Swedish Population Register was used for basic demographic information, including the date of emigration (if applicable). Patients were followed up until death, emigration, or until 31 December 2015. The merging of registries was based on the personal identification number that all Swedish residents are given at birth or shortly after immigration.
The manuscript has been composed according to recommendations in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.15 The study was performed in accordance with the 1975 Declaration of Helsinki and was approved by the Regional Research Ethical Committee in Gothenburg (Registration number 139-16). The need for individual patient consent was waived.
Statistical analysis
Continuous variables are presented as mean with standard deviation (SD), median with range, or median with interquartile range (IQR) as appropriate. Categorical variables are presented as frequency with percentage. For comparison between two groups, Fisher’s exact test was used for dichotomous variables, Mantel–Haenszel χ2 test for ordered categorical variables, χ2 test for unordered categorical variables, and Mann–Whitney U test for continuous variables. The dispense of medications over time is reported as crude data with age (<75 or ≥75 years) and sex as subgrouping variables. Differences in drug dispense at baseline and 8 years after baseline were analysed using Fisher’s exact test. The piecewise linear trend for medication reduction (break point at 1 year) was investigated using generalized estimating equations, for repeated measures for a patient, applying binomial distribution and log-link function. The results are expressed as relative risk (RR) per 1-year increase, with 95% confidence interval (95% CI).
Crude event rates per 100 person-years were calculated as the number of events divided by the number of years of follow-up, with 95% CI estimated using exact Poisson limits. The time-updated impact of secondary prevention therapy status on all-cause mortality was investigated with Cox proportional hazard models and described using hazard ratio (HR) and 95% CI. Model 1 was adjusted for age and sex. In addition, model 2 included patient characteristics and comorbidities at baseline that have previously been shown to influence long-term mortality in CABG patients (Supplementary material online, Table S3).16 Model 3, considered as the main effect model, also included the use of other time-updated secondary prevention medications.
In a sensitivity analysis, and to further investigate the effect of secondary prevention therapy in relation to time, the adjusted Cox proportional hazard model described above was applied to consecutive 1-year time periods. Only patients who were at risk at the start of a 1-year period were included and medication status at the same start point was used for evaluation of the effect during the 1-year follow-up. Moreover, the time-updated cumulative effect of total exposure time, as a continuous variable, of each medication was analysed in separate models.
Interaction analyses were performed for the following subgroups: age <75 or ≥75 years, sex, previous myocardial infarction, hypertension, heart failure, atrial fibrillation, LVEF <50% or ≥50%, eGFR ≥60 or <60 mL/min/1.73 m2, and diabetes. The subgroups were chosen based on recommendations in guidelines for specific treatment with RAAS inhibitors and β-blockers. Moreover, interactions between medication and follow-up time were examined as post hoc analyses. There were missing data for body mass index (8.3%), LVEF (0.9%), and renal function (1.6%), and these were handled as a separate category, Unknown, in the adjustments. All tests were two-tailed and were interpreted at the 0.05 significance level, and they were performed using SAS® Software version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Study population
Altogether, 30 952 patients underwent isolated first-time CABG surgery in Sweden in the period 2006–15. Of these, 507 (1.6%) were excluded due to not surviving until hospital discharge, 299 (1.0%) not surviving 6 months after hospital discharge, 10 (0.0%) emigrated within 6 months after hospital discharge, and 1324 (4.3%) having follow-up time shorter than 6 months, leaving 28 812 (93.1%) patients included in the analysis (Supplementary material online, Figure S1). Median follow-up time was 4.9 years (IQR 2.5–7.2 years) and the total follow-up time was 139 127 years. During follow-up, 3787 patients (13.1%) died and 118 patients (0.4%) emigrated (censored at the date of emigration). No patients except these were lost to follow-up. The final cohort consisted of 80.4% men and 19.6% women, and 24.5% of the patients were ≥75 years old. At baseline, women had a significantly higher mean age. A higher proportion of women had diabetes, hypertension, heart failure, chronic respiratory disease, renal failure, hyperlipidaemia, stroke, myocardial infarction, and peripheral vascular disease, and a lower proportion had atrial fibrillation (Table 1). A higher proportion of patients aged ≥75 years had comorbidities, with the exception of diabetes and hyperlipidaemia (Table 1).
Table 1.
Patient characteristics at baseline by sex and age category
| All patients | Men | Women | P-value | <75 years | ≥75 years | P-value | |
|---|---|---|---|---|---|---|---|
| (n = 28 812) | (n = 23 156) | (n = 5656) | (n = 21 753) | (n = 7059) | |||
| Men | 23 156 (80.4%) | 23 156 (100.0%) | 0 (0.0%) | 18 035 (82.9%) | 5121 (72.5%) | ||
| Women | 5656 (19.6%) | 0 (0.0%) | 5656 (100.0%) | <0.001 | 3718 (17.1%) | 1938 (27.5%) | <0.001 |
| Age (years) | 67.4 (9.2) | 67.0 (9.1) | 69.4 (9.3) | <0.001 | 63.8 (7.5) | 78.5 (2.9) | <0.001 |
| 68.0 (23.0; 94.0) | 68.0 (23.0; 93.0) | 71.0 (32.0; 94.0) | 65.0 (23.0; 74.0) | 78.0 (75.0; 94.0) | |||
| Age category | |||||||
| 18–74 years | 21 753 (75.5%) | 18 035 (77.9%) | 5656 (65.7%) | 21 753 (100.0%) | 0 (0.0%) | ||
| ≥75 years | 7059 (24.5%) | 5121 (22.1%) | 1938 (34.3%) | <0.001 | 0 (0.0%) | 7059 (100.0%) | <0.001 |
| Body mass index (kg/m2) | 27.4 (4.1) | 27.4 (3.9) | 27.6 (4.8) | 0.44 | 27.8 (4.1) | 26.4 (3.7) | <0.001 |
| n = 26 426 | n = 21 238 | n = 5188 | n = 19 979 | n = 6447 | |||
| Left ventricular ejection fraction | |||||||
| Normal | 19 930 (69.2%) | 15 900 (68.7%) | 4030 (71.3%) | <0.001 | 15 364 (70.6%) | 4566 (64.7%) | <0.001 |
| <50% | 8637 (30.0%) | 7056 (30.5%) | 1581 (28.0%) | 6190 (28.5%) | 2447 (34.7%) | ||
| Unknown | 245 (0.9%) | 200 (0.9%) | 45 (0.8%) | 199 (0.9%) | 46 (0.7%) | ||
| eGFR category (mL/min/1.73 m2) | |||||||
| ≥90 | 7634 (26.5%) | 6554 (28.3%) | 1080 (19.1%) | <0.001 | 7444 (34.2%) | 190 (2.7%) | <0.001 |
| 60–<90 | 15 619 (54.2%) | 12 664 (54.7%) | 2955 (52.2%) | 11 286 (51.9%) | 4333 (61.4%) | ||
| 30–<60 | 4610 (16.0%) | 3226 (13.9%) | 1384 (24.5%) | 2334 (10.7%) | 2276 (32.2%) | ||
| 15–<30 | 281 (1.0%) | 185 (0.8%) | 96 (1.7%) | 156 (0.7%) | 125 (1.8%) | ||
| <15 | 198 (0.7%) | 150 (0.6%) | 48 (0.8%) | 173 (0.8%) | 25 (0.4%) | ||
| Unknown | 470 (1.6%) | 377 (1.6%) | 93 (1.6%) | 360 (1.7%) | 110 (1.6%) | ||
| Indication for surgery | |||||||
| Stable coronary disease | 11 663 (40.5%) | 9630 (41.6%) | 2033 (35.9%) | <0.001 | 9167 (42.1%) | 2496 (35.4%) | <0.001 |
| Unstable angina | 7939 (27.6%) | 6324 (27.3%) | 1615 (28.6%) | 0.063 | 6009 (27.6%) | 1930 (27.3%) | 0.66 |
| Non-STEMI | 7324 (25.4%) | 5666 (24.5%) | 1658 (29.3%) | <0.001 | 5158 (23.7%) | 2166 (30.7%) | <0.001 |
| STEMI | 1886 (6.5%) | 1536 (6.6%) | 350 (6.2%) | 0.24 | 1419 (6.5%) | 467 (6.6%) | 0.80 |
| Comorbidities at baseline | |||||||
| Myocardial infarction | 15 674 (54.4%) | 12 420 (53.6%) | 3254 (57.5%) | <0.001 | 11 433 (52.6%) | 4241 (60.1%) | <0.001 |
| Diabetes | 8725 (30.3%) | 6741 (29.1%) | 1984 (35.1%) | <0.001 | 6837 (31.4%) | 1888 (26.7%) | <0.001 |
| Hypertension | 20 203 (70.1%) | 15 802 (68.2%) | 4401 (77.8%) | <0.001 | 14 951 (68.7%) | 5252 (74.4%) | <0.001 |
| Heart failure | 6036 (20.9%) | 4713 (20.4%) | 1323 (23.4%) | <0.001 | 4045 (18.6%) | 1991 (28.2%) | <0.001 |
| Atrial fibrillation | 8073 (28.0%) | 6585 (28.4%) | 1488 (26.3%) | 0.001 | 5180 (23.8%) | 2893 (41.0%) | <0.001 |
| Stroke | 2546 (8.8%) | 1988 (8.6%) | 558 (9.9%) | 0.003 | 1664 (7.6%) | 882 (12.5%) | <0.001 |
| Chronic respiratory disease | 2786 (9.7%) | 2040 (8.8%) | 746 (13.2%) | <0.001 | 2024 (9.3%) | 762 (10.8%) | <0.001 |
| Peripheral vascular disease | 2729 (9.5%) | 2049 (8.8%) | 680 (12.0%) | <0.001 | 1843 (8.5%) | 886 (12.6%) | <0.001 |
| History of cancer | 3758 (13.0%) | 3043 (13.1%) | 715 (12.6%) | 0.33 | 2223 (10.2%) | 1535 (21.7%) | <0.001 |
| Hyperlipidaemia | 14 563 (50.5%) | 11 523 (49.8%) | 3040 (53.7%) | <0.001 | 11 442 (52.6%) | 3121 (44.2%) | <0.001 |
| Secondary prevention at baseline | |||||||
| Statins | 27 048 (93.9%) | 21 763 (94.0%) | 5285 (93.4%) | 0.14 | 20 541 (94.4%) | 6507 (92.2%) | <0.001 |
| β-Blockers | 26 210 (91.0%) | 21 007 (90.7%) | 5203 (92.0%) | 0.003 | 19 916 (91.6%) | 6294 (89.2%) | <0.001 |
| RAAS inhibitors | 20 993 (72.9%) | 16 797 (72.5%) | 4196 (74.2%) | 0.013 | 15 826 (72.8%) | 5167 (73.2%) | 0.48 |
| Platelet inhibitors | 26 802 (93.0%) | 21 538 (93.0%) | 5264 (93.1%) | 0.91 | 20 517 (94.3%) | 6285 (89.0%) | <0.001 |
For categorical variables, n (%) is presented. For continuous variables, mean (SD)/median (Min; Max)/n = is presented.
For comparison between groups, Fisher’s Exact test (lowest one-sided P-value multiplied by 2) was used for dichotomous variables; the Mantel–Haenszel χ2 test was used for ordered categorical variables; χ2 test was used for non-ordered categorical variables; and the Mann–Whitney U test was used for continuous variables.
eGFR, estimated glomerular filtration rate; RAAS, renin–angiotensin–aldosterone system; STEMI, ST-segment elevation myocardial infarction.
Dispense of secondary prevention medications
Baseline
From 2006 to 2015, the proportion of patients with dispensed prescriptions of statins at baseline increased from 91.5% to 96.0% (P < 0.001). The corresponding proportion for β-blockers increased from 89.0% to 92.4% (P < 0.001) and that for RAAS inhibitors increased from 64.4% to 78.4% (P < 0.001), whereas that for platelet inhibitors decreased from 93.2% to 92.2% (P = 0.004), Supplementary material online, Figure S2.
Dispensed prescriptions over time
The proportions of patients with dispensed prescriptions of medications belonging to each group of secondary prevention drugs over time are presented in Figure 1. The dispensing of all medications gradually decreased over time after surgery. Statins were dispensed to 93.9% of the patients at baseline and to 77.3% 8 years after surgery. The corresponding figures for β-blockers were 91.0% and 76.4%, for RAAS inhibitors 72.9% and 65.9%, and for platelet inhibitors 93.0% and 79.8%. During the first year, the use of β-blockers decreased most, by 7% (RR 0.93, 95% CI 0.92–0.93; P < 0.001), and RAAS inhibitors least, by 3% (RR 0.97, 95% CI 0.96–0.97; P < 0.001) (Supplementary material online, Table S4).
Figure 1.
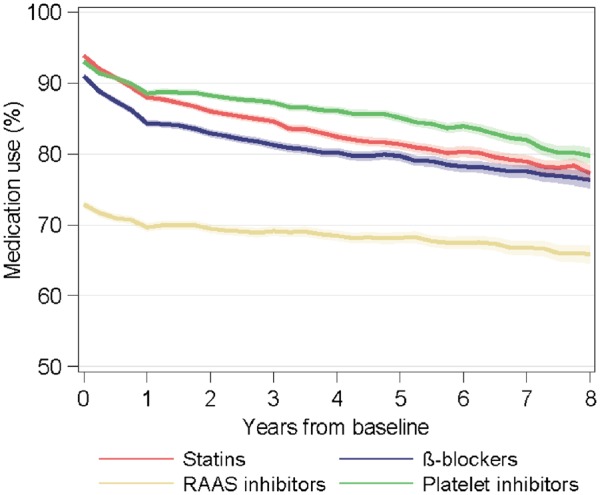
Use of secondary prevention medications in relation to time after surgery. The shaded area represents 95% confidence intervals. RAAS, renin-angiotensin-aldosterone system.
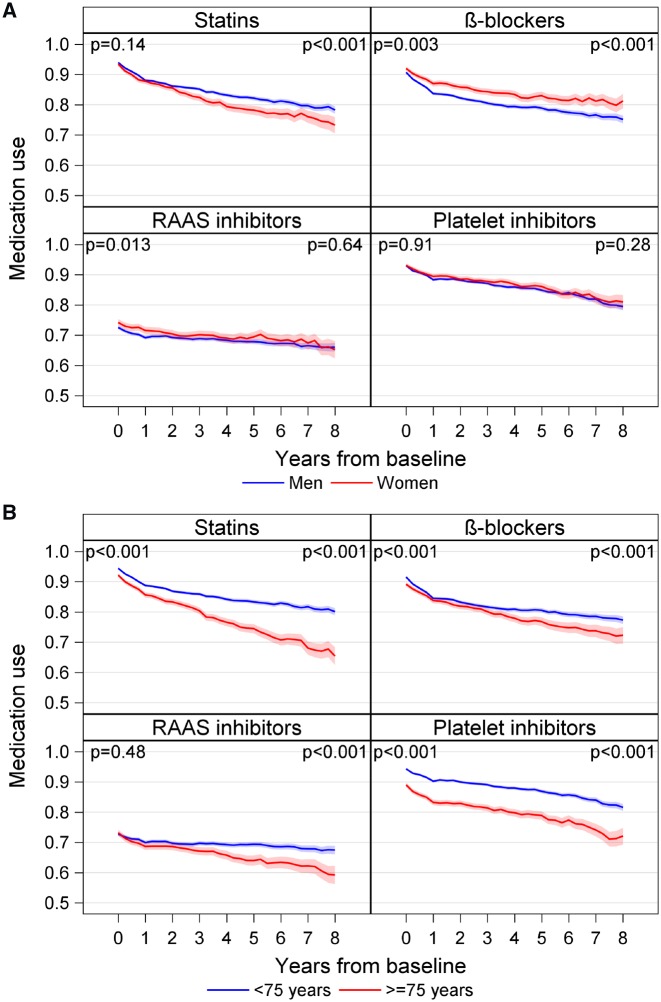
A higher proportion of women were dispensed β-blockers and RAAS inhibitors at baseline (Figure 2A and Supplementary material online, Table S5). After 8 years, a higher proportion of women was dispensed β-blockers and a lower proportion was dispensed statins. There was no significant difference between men and women in dispensed prescriptions of platelet inhibitors, either at baseline or after 8 years.
Figure 2.
Graphs showing percentage of patients with dispensed prescriptions over time grouped by: (A) sex and (B) age category. Shaded area represents 95% confidence intervals based on binomial distribution, P-values are obtained from the Fisher’s exact test for proportion of patients using secondary prevention medications between men and women, and patients <75 and ≥75 years old, respectively, at baseline and 8 years. RAAS, renin-angiotensin-aldosterone system.
Statins, β-blockers, and platelet inhibitors were dispensed less often to patients who were ≥75 years of age at baseline. After 8 years, all four classes of medications were less often dispensed to patients aged ≥75 years (Figure 2B and Supplementary material online, Table S6).
Mortality
Crude mortality rates and associations between secondary prevention medications and mortality are presented in Table 2. All classes of medication were individually associated with reduced mortality risk in model 1, adjusted for age and sex, and in model 2, with adjustment also for patient characteristics and comorbidities at baseline. In model 3, with additional adjustment for time-updated use of the other secondary prevention drugs, statins (HR 0.56, 95% CI 0.52–0.60; P < 0.001), RAAS inhibitors (HR 0.78, 95% CI 0.73–0.84; P < 0.001), and platelet inhibitors (HR 0.74, 95% CI 0.69–0.81; P < 0.001) were significantly associated with a lower mortality risk, whereas there was no significant association between the use of β-blockers and mortality risk (HR 0.97, 95% CI 0.90–1.06; P = 0.54).
Table 2.
Crude mortality rates with 95% confidence interval based on time-updated exposure and adjusted effects of time-updated use of secondary prevention therapy on mortality evaluated by Cox regression
| Crude mortality rate without treatment | Crude mortality rate with treatment | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|---|
| Per 100 person-years (95% CI) | Per 100 person-years (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| P-value | P-value | P-value | |||
| Statins | 5.84 (5.51–6.18) | 2.21 (2.12–2.29) | 0.48 (0.45–0.52) | 0.51 (0.48–0.55) | 0.56 (0.52–0.60) |
| <0.001 | <0.001 | <0.001 | |||
| β-Blockers | 3.33 (3.10–3.57) | 2.60 (2.51–2.69) | 0.90 (0.83–0.97) | 0.82 (0.75–0.88) | 0.97 (0.90–1.06) |
| 0.007 | <0.001 | 0.54 | |||
| RAAS inhibitors | 3.10 (2.94–3.28) | 2.56 (2.46–2.66) | 0.87 (0.81–0.93) | 0.70 (0.65–0.75) | 0.78 (0.73–0.84) |
| <0.001 | <0.001 | <0.001 | |||
| Platelet inhibitors | 5.57 (5.21–5.94) | 2.33 (2.25–2.42) | 0.55 (0.51–0.60) | 0.67 (0.62–0.72) | 0.74 (0.69–0.81) |
| <0.001 | <0.001 | <0.001 |
Model 1 is adjusted for age and sex.
Model 2 is Model 1 additionally adjusted for following variables at baseline: body mass index category, diabetes, hypertension, hyperlipidaemia, previous stroke, atrial fibrillation, heart failure, previous myocardial infarction, chronic obstructive pulmonary disease, history of cancer, peripheral arterial disease, pulmonary hypertension, acute coronary syndrome or stable coronary artery disease as indication for CABG, left ventricular function, estimated glomerular filtration rate category (CKD-EPI formula), year of CABG.
Model 3 is Model 2 additionally adjusted for all other time-updated secondary prevention medications than the main effect variable.
CABG, coronary artery bypass grafting; CI, confidence interval; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration, HR, hazard ratio; RAAS, renin–angiotensin–aldosterone system.
The accumulated medication exposure time was associated with lower mortality risk for statins (adjusted HR 0.90, 95% CI 0.88–0.92; P < 0.001), platelet inhibitors (adjusted HR 0.93, 95% CI 0.91–0.95; P < 0.001), and RAAS inhibitors (adjusted HR 0.98, 95% CI 0.96–1.00; P = 0.015) for each additional year using the medication. There was no association between total exposure time and mortality risk for β-blockers in model 3 (adjusted HR 0.99, 95% CI 0.96–1.01; P = 0.21).
A sensitivity analysis investigating the association between dispensed prescriptions and adjusted mortality risk separately for every year after the start of follow-up is presented in Supplementary material online, Figure S3. No significant interaction with the follow-up time could be shown for statins, β-blockers, and platelet inhibitors. However, for RAAS inhibitors the calculated effect increased over time, adjusted HR at 1 year was 0.86 (95% CI 0.77–0.97), at 2 years 0.81 (95% CI 0.75–0.88) and at 5 years 0.75 (95% CI 0.70–0.81), P-value for interaction 0.024.
Subgroup analyses
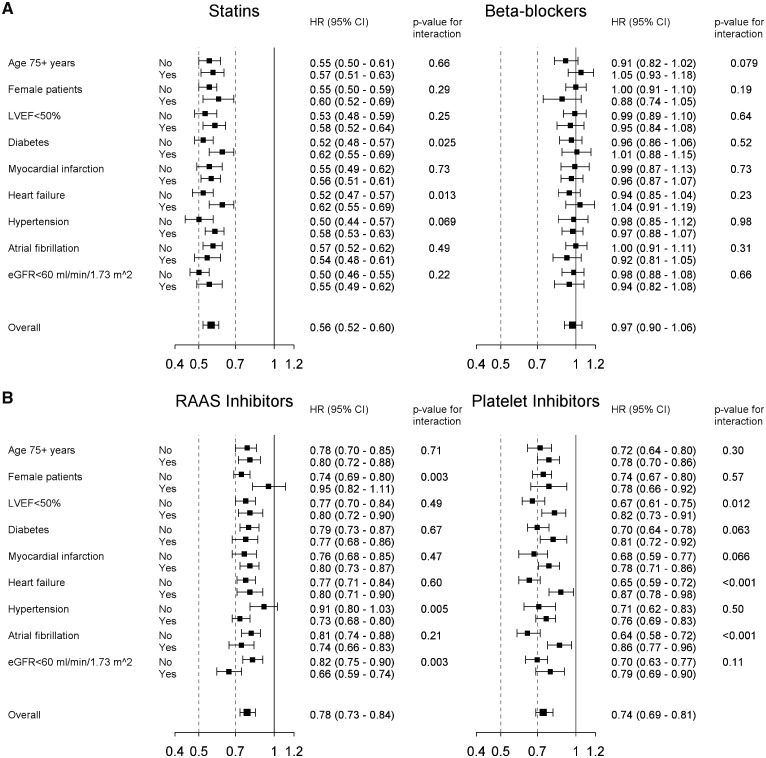
Forest plots showing associations between mortality and time-updated use of secondary prevention medications in specified subgroups are presented in Figure 3. The association between use of statins and lower mortality risk was consistent among all the subgroups investigated. There were more pronounced reductions in mortality in statin-treated patients without diabetes and in patients without heart failure. The lack of association between the use of β-blockers and mortality risk was consistent across all the subgroups. For RAAS inhibitors, there were significant interactions with sex and hypertension, with significantly reduced mortality risk only in patients with hypertension and in men. There was also a significant interaction for treatment with RAAS inhibitors and renal function, showing more pronounced reductions in adjusted mortality risk in patients with impaired renal function. Use of platelet inhibitors was associated with lower adjusted mortality risk across all subgroups, but with significantly greater reductions in adjusted mortality risk in patients with no heart failure, no atrial fibrillation and in those with LVEF ≥50%.
Figure 3.
Forest plots showing the results from the interaction analyses of the impact of secondary prevention medication on mortality overall and for selected subgroups. (A) Statins and β-blockers; (B) RAAS inhibitors and platelet inhibitors. RAAS, renin-angiotensin-aldosterone system.
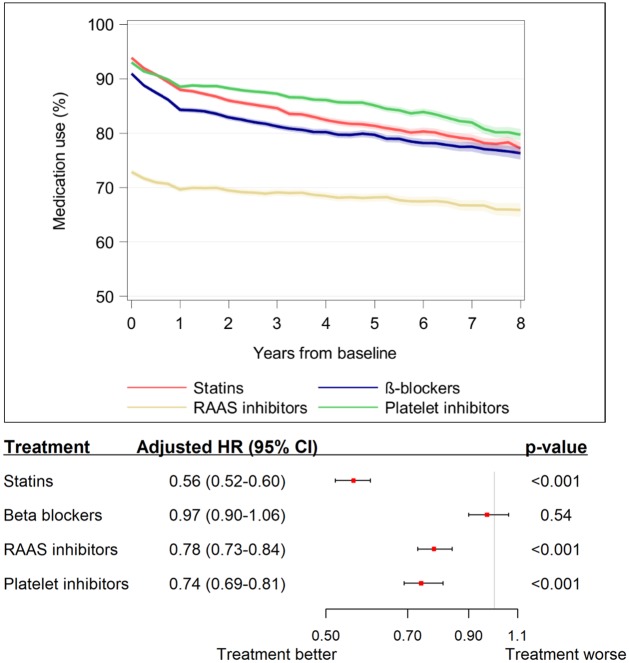
Take home figure.
The graph displays the declining use of secondary prevention medications over time after surgery and the forest plot displays associations between use of secondary prevention medications and mortality risk.
Discussion
The main findings of this study using real-world data were that: (i) the use of secondary prevention medications after CABG in Sweden was high early after surgery; (ii) the use of all secondary prevention drugs investigated decreased over time after surgery and was lowest in elderly patients, and (iii) the use of statins, platelet inhibitors, and RAAS inhibitors was individually associated with significantly better long-term survival whereas there was no association between the use of β-blockers and survival.
In the present study, statins, β-blockers, and platelet inhibitors were dispensed to over 90% of the patients at baseline, which is higher than in most previous real-world registry studies,5–9 and even higher than what has been achieved in many prospective clinical trials.10,17,18 The high utilization of secondary prevention medication at baseline most likely reflects an increased awareness of the importance of optimizing secondary prevention medications after CABG, as reported from several studies10,16,18–21 and as recommended in guidelines. There was, however, a gradual and significant decline in dispense over time for all the medications. This is alarming, since we observed a strong and consistent association between mortality and the use of statins, RAAS inhibitors, and platelet inhibitors, with greater reductions in mortality risk with longer exposure time. The lower utilization of secondary prevention medications in elderly patients at baseline and the faster decline (Figure 2B) is worrying for the same reasons. Our findings indicate that all secondary prevention medications were underutilized in patients aged ≥75 years, since a larger proportion of older patients have comorbidities warranting treatment with β-blockers and RAAS inhibitors (Table 1). There was no interaction with age for any of the medications, and therefore no support in the present study for treating elderly patients differently (Figure 3). The small gender differences in the use of medication may be related to differences in baseline characteristics, such as women being older on average and more often having comorbidities warranting treatment with β-blockers and RAAS inhibitors, but overall no major and consistent differences in the use of secondary prevention medication in relation to gender were observed.
The absence of any association between the use of RAAS inhibitors and mortality in normotensive patients, and women in general, is a finding at the subgroup level and should be interpreted with caution. However, in contrast to the strong and consistent associations between reduced mortality and the use of statins and platelet inhibitors, there was no significant association between β-blocker-use and mortality in the fully adjusted models—neither overall, nor in any of the subgroups reported in Figure 3. These findings are in accordance with the results of a randomized trial,22 and call into question the routine use of β-blockers after CABG.
This study had strengths, but it also had several limitations. The strengths include the real-world setting, the large study cohort with national coverage, the complete follow-up, and the time-updated data on dispensed prescriptions included in the statistical models. However, the adherence to secondary prevention medications in Sweden may not be representative for other countries and we had no information on the reason for patients not receiving a particular secondary prevention drug, and could thus not differentiate legitimate reasons for not taking the drug (i.e. contraindications) from doctors’ non-prescription or patients’ non-adherence to prescription. We had insufficient data on smoking and could therefore not include this well-known risk factor in our statistical models. The lack of data on tobacco use is important since it is known that smoking is a strong predictor of poor outcome after CABG23 and smokers have lower adherence to medications.24 Allowing two consecutive 3-month periods without a dispensed prescription before a patient was considered not to be on treatment minimized the risk of reverse causality (i.e. that terminally ill patients stopped using prescriptions) but may have led to underestimation of the effect of the medication. Finally, like all retrospective studies, there was a risk of selection bias and additional unmeasured confounders that were not adjusted for in the models.
Conclusions
The use of secondary prevention medications after CABG was high early after surgery but decreased significantly over time. Furthermore, this study highlights the importance of continuous secondary prevention medication with statins, platelet inhibitors, and RAAS inhibitors after CABG to improve long-term survival whereas the routine use of β-blockers is questionable.
Funding
The study was supported by the Swedish Heart-Lung Foundation (20150587 and 20180560 to A.J.); the Swedish state under the agreement between the Swedish government and the county councils concerning economic support of research and education of doctors (ALF agreement) (ALFGBG-725131 to A.J.); and Västra Götaland Region (VGFOUREG-847811 to A.J.). The supporting bodies had no influence on the analysis and interpretation of data, on the writing of the report, or on the decision to submit the paper for publication.
Conflict of interest: A.J. has received research grants, speaker’s honorarium, and consultancy fee from AstraZeneca and speaker’s and consultancy honorarium from Boehringer-Ingelheim. E.C.H. has received speaker’s honorarium from AstraZeneca and Boehringer-Ingelheim. C.J.M has received speaker’s honorarium from AstraZeneca. None of the other co-authors have any conflicts of interests.
Supplementary Material
References
- 1. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group.. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165.30165437 [Google Scholar]
- 2. Kulik A, Ruel M, Jneid H, Ferguson TB, Hiratzka LF, Ikonomidis JS, Lopez-Jimenez F, McNallan SM, Patel M, Roger VL, Sellke FW, Sica DA, Zimmerman L; American Heart Association Council on Cardiovascular Surgery and Anesthesia . Secondary prevention after coronary artery bypass graft surgery: a scientific statement from the American Heart Association. Circulation 2015;131:927–964. [DOI] [PubMed] [Google Scholar]
- 3. Sousa-Uva M, Head SJ, Milojevic M, Collet J-P, Landoni G, Castella M, Dunning J, Gudbjartsson T, Linker NJ, Sandoval E, Thielmann M, Jeppsson A, Landmesser U.. 2017 EACTS Guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg 2018;53:5–33. [DOI] [PubMed] [Google Scholar]
- 4. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ; ESC Scientific Document Group.. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2019;doi:10.1093/eurheartj/ehz245. [Google Scholar]
- 5. Bradshaw PJ, Jamrozik K, Gilfillan I, Thompson PL.. Preventing recurrent events long term after coronary artery bypass graft: suboptimal use of medications in a population study. Am Heart J 2004;147:1047–1053. [DOI] [PubMed] [Google Scholar]
- 6. Kulik A, Levin R, Ruel M, Mesana TG, Solomon DH, Choudhry NK.. Patterns and predictors of statin use after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg 2007;134:932–938. [DOI] [PubMed] [Google Scholar]
- 7. Hiratzka LF, Eagle KA, Liang L, Fonarow GC, LaBresh KA, Peterson ED.. Atherosclerosis secondary prevention performance measures after coronary bypass graft surgery compared with percutaneous catheter intervention and nonintervention patients in the Get With the Guidelines Database. Circulation 2007;116:I-207–212. [DOI] [PubMed] [Google Scholar]
- 8. Hlatky MA, Solomon MD, Shilane D, Leong TK, Brindis R, Go AS.. Use of medications for secondary prevention after coronary bypass surgery compared with percutaneous coronary intervention. J Am Coll Cardiol 2013;61:295–301. [DOI] [PubMed] [Google Scholar]
- 9. Riley RF, Don CW, Aldea GS, Mokadam NA, Probstfield J, Maynard C, Goss JR.. Recent trends in adherence to secondary prevention guidelines for patients undergoing coronary revascularization in Washington State: an analysis of the clinical outcomes assessment program (COAP) registry. J Am Heart Assoc 2012;1:e002733.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iqbal J, Zhang YJ, Holmes DR, Morice MC, Mack MJ, Kappetein AP, Feldman T, Stahle E, Escaned J, Banning AP, Gunn JP, Colombo A, Steyerberg EW, Mohr FW, Serruys PW.. Optimal medical therapy improves clinical outcomes in patients undergoing revascularization with percutaneous coronary intervention or coronary artery bypass grafting: insights from the Synergy Between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) trial at the 5-year follow-up. Circulation 2015;131:1269–1277. [DOI] [PubMed] [Google Scholar]
- 11. Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L.. The Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART). Heart 2010;96:1617–1621. [DOI] [PubMed] [Google Scholar]
- 12. Vikholm P, Ivert T, Nilsson J, Holmgren A, Freter W, Ternström L, Ghaidan H, Sartipy U, Olsson C, Granfeldt H, Ragnarsson S, Friberg Ö.. Validity of the Swedish Cardiac Surgery Registry. Interact Cardiovasc Thorac Surg 2018;27:67–74. [DOI] [PubMed] [Google Scholar]
- 13. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO.. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP.. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 16. Farooq V, Serruys PW, Bourantas C, Vranckx P, Diletti R, Garcia Garcia HM, Holmes DR, Kappetein AP, Mack M, Feldman T, Morice MC, Colombo A, Morel MA, de Vries T, van Es GA, Steyerberg EW, Dawkins KD, Mohr FW, James S, Ståhle E.. Incidence and multivariable correlates of long-term mortality in patients treated with surgical or percutaneous revascularization in the Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX) trial. Eur Heart J 2012;33:3105–3113. [DOI] [PubMed] [Google Scholar]
- 17. Park S-J, Ahn J-M, Kim Y-H, Park D-W, Yun S-C, Lee J-Y, Kang S-J, Lee S-W, Lee CW, Park S-W, Choo SJ, Chung CH, Lee JW, Cohen DJ, Yeung AC, Hur SH, Seung KB, Ahn TH, Kwon HM, Lim D-S, Rha S-W, Jeong M-H, Lee B-K, Tresukosol D, Fu GS, Ong TK; BEST Trial Investigators . Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med 2015;372:1204–1212. [DOI] [PubMed] [Google Scholar]
- 18. Goyal A, Alexander JH, Hafley GE, Graham SH, Mehta RH, Mack MJ, Wolf RK, Cohn LH, Kouchoukos NT, Harrington RA, Gennevois D, Gibson CM, Califf RM, Ferguson TB Jr, Peterson ED; PREVENT IV Investigators. Outcomes associated with the use of secondary prevention medications after coronary artery bypass graft surgery. Ann Thorac Surg 2007;83:993–1001. [DOI] [PubMed] [Google Scholar]
- 19. Christensen J. Preoperative lipid-control with simvastatin reduces the risk of postoperative thrombocytosis and thrombotic complications following CABG. Eur J Cardiothorac Surg 1999;15:394–399. [DOI] [PubMed] [Google Scholar]
- 20. Kulik A, Brookhart MA, Levin R, Ruel M, Solomon DH, Choudhry NK.. Impact of statin use on outcomes after coronary artery bypass graft surgery. Circulation 2008;118:1785–1792. [DOI] [PubMed] [Google Scholar]
- 21. Kurlansky P, Herbert M, Prince S, Mack M.. Coronary artery bypass graft versus percutaneous coronary intervention. Meds matter: impact of adherence to medical therapy on comparative outcomes. Circulation 2016;134:1238–1246. [DOI] [PubMed] [Google Scholar]
- 22. Effect of metoprolol on death and cardiac events during a 2-year period after coronary artery bypass grafting. The MACB Study Group. Eur Heart J 1995;16:1825–1832. [PubMed] [Google Scholar]
- 23. Zhang YJ, Iqbal J, van Klaveren D, Campos CM, Holmes DR, Kappetein AP, Morice MC, Banning AP, Grech ED, Bourantas CV, Onuma Y, Garcia-Garcia HM, Mack MJ, Colombo A, Mohr FW, Steyerberg EW, Serruys PW.. Smoking is associated with adverse clinical outcomes in patients undergoing revascularization with PCI or CABG: the SYNTAX trial at 5-year follow-up. J Am Coll Cardiol 2015;65:1107–1115. [DOI] [PubMed] [Google Scholar]
- 24. Warren JR, Falster MO, Fox D, Jorm L.. Factors influencing adherence in long-term use of statins. Pharmacoepidemiol Drug Saf 2013;22:1298–1307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.