Abstract
Polycystic ovary syndrome (PCOS) is characterized by hyperandrogenism, oligo-anovulation and polycystic ovarian morphology, with metabolic dysfunction from insulin resistance and abdominal fat accumulation worsened by obesity. As ancestral traits, these features could have favored abdominal fat deposition for energy use during starvation, but have evolved into different PCOS phenotypes with variable metabolic dysfunction. Adipose dysfunction in PCOS from hyperandrogenemia and hyperinsulinemia likely constrains subcutaneous (SC) fat storage, promoting lipotoxicity through ectopic lipid accumulation and oxidative stress, insulin resistance and inflammation in non-adipose tissue. Recent findings of inherently exaggerated SC abdominal stem cell development to adipocytes in women with PCOS, and PCOS-like traits in adult female monkeys with natural hyperandrogenemia, imply common ancestral origins of PCOS in both human and nonhuman primates.
Keywords: polycystic ovary syndrome, hyperandrogenism, insulin resistance, adipocyte, adipose stem cells, developmental programming
1.1. Introduction
As the most common reproductive-metabolic disorder of reproductive-aged women, polycystic ovary syndrome (PCOS) is characterized by ovarian hyperandrogenism from altered hypothalamic-pituitary-ovarian function in combination with hyperinsulinemia from insulin resistance. Its major endocrine-metabolic manifestations include hirsutism, oligo-anovulation and polycystic ovarian morphology (PCOM) in association with glucose intolerance, dyslipidemia and preferential abdominal fat accumulation worsened by obesity [1]. These complex endocrine-metabolic interactions determine the risks of women with PCOS developing subfertility, diabetes and/or cardiovascular disease.
While a heritable etiology for PCOS is suggested by its peripubertal onset and familial clustering, several gene candidates, including those regulating insulin action, androgen biosynthesis and gonadal function, contribute only a minor effect to the PCOS phenotype [2]. Instead, heritability of PCOS interacts with risk-increasing environmental factors to fully explain its prevalence. Such genetic-environmental interactions likely begin before birth when an altered maternal endocrine-metabolic environment causes epigenetic modifications of fetal genetic susceptibility to PCOS, which then continue after birth into adulthood. These interactions alter the capacity of women with PCOS to safely store fat and, in the presence of excess energy intake versus energy expenditure, predispose to lipotoxicity, defined as the ectopic deposition of lipid in non-adipose tissue where it induces oxidative stress linked with insulin resistance and inflammation [3].
This review emphasizes the basic science and clinical medical research conducted over the past two years to clarify endocrine-metabolic interactions underlying PCOS, how they predispose to lipotoxicity as a link between metabolism dysfunction and impaired reproduction, and if they have developmental origins that favored survival of humans, and their close nonhuman primate relatives, in ancient times.
1.2. PCOS phenotypic expression
The current Rotterdam criteria for PCOS include at least two of the following three features: clinical or biochemical hyperandrogenism, oligo-anovulation and PCOM, excluding other endocrinopathies [4]. These Rotterdam criteria create different PCOS phenotypes, including classic PCOS (i.e., hyperandrogenism with oligo-anovulation with or without PCOM [previous 1990 NIH criteria]), ovulatory PCOS (hyperandrogenism and PCOM) and non-androgenic PCOS (oligo-anovulation and PCOM) [5] (Table 1). As another modification, the Androgen Excess and PCOS (AE-PCOS) Society considers hyperandrogenism as the cardinal feature of PCOS, defining PCOS as hyperandrogenism with ovarian dysfunction (either oligo-anovulation and/or PCOM), excluding other androgen excess-related disorders [5]. Consequently, the 6–10 percent prevalence of PCOS by 1990 NIH criteria has doubled by using the broader Rotterdam criteria, with 1990 NIH-defined PCOS being the most common phenotype [5].
Table 1.
Different PCOS phenotypes
| Hyperandrogenism and oligo-anovulation with or without PCOM1 (1990 NIH criterion) | Hyperandrogenism and PCOM1 (Ovulatory PCOS) | Oligo-anovulation and PCOM1 (Non-androgenic) | |
|---|---|---|---|
| Hirsutism | ++ | ++ | + |
| Infertility | ++ | + | ++ |
| Obesity | ++ | ++ | + |
| Glucose Intolerance | ++ | ++ | + |
| Dyslipidemia | ++ | ++ | + |
PCOM: Polycystic ovarian morphology
Such variable PCOS phenotypic expression is crucial when considering endocrine-metabolic interactions in women with PCOS. Women with NIH-defined PCOS are at greatest risk of developing menstrual irregularity, anovulatory infertility, type 2 diabetes mellitus and metabolic syndrome, as defined by increased abdominal (android) obesity, hypertension, hypertriglyceridemia, hyperglycemia and decreased serum high-density lipoprotein (HDL) cholesterol levels. Ovulatory women with PCOS (by Rotterdam and AE-PCOS criteria) have a lower body mass index (BMI) and milder hyperinsulinemia and hyperandrogenism, which lowers the risks of developing similar reproductive and metabolic abnormalities, while women with non-androgenic PCOS have the least metabolic risk [5].
1.3. Insulin resistance
Given the worldwide obesity epidemic, 60–95% of all women with PCOS have insulin resistance from perturbed insulin receptor/post receptor signaling, altered adipokine secretion and abnormal steroid metabolism [1] in combination with increased total abdominal fat over a wide BMI range [5, 6, 7]. In women with PCOS, moreover, hyperinsulinemia in the presence of obesity interacts with hyperandrogenism to worsen the PCOS phenotype [1, 5, 8, 9]. Consequently, almost one-half of women with PCOS in the United States have metabolic syndrome, which is higher in prevalence than that of age-matched normal women in this country [5, 10] and of women with PCOS in other countries where obesity is less prevalent [11].
In addition, the amount of abdominal fat and its relative proportion to total body fat are greater in normal-weight women with PCOS by NIH criteria compared to age- and BMI-matched normal women [6]. In all these women combined, abdominal fat mass and its relative proportion to total body fat positively correlate with circulating androgen and fasting insulin levels, and remain related to androgen levels, adjusting for insulin levels [6]. These findings emphasize important androgen-insulin interactions governing abdominal fat and its distribution with implications for both subcutaneous (SC) abdominal adipose that normally stores lipid as protection against insulin resistance, and intra-abdominal adipose that has the opposite effect [12].
1.4. Subcutaneous abdominal adipose
Subcutaneous abdominal adipose normally maintains metabolic homeostasis by balancing lipogenesis (lipid formation) and lipolysis (lipid breakdown) in mature adipocytes with the formation of new adipocytes. Central to these events is adipogenesis, a process whereby multipotent adipose stem cells (ASCs) initially undergo commitment to preadipocytes and then differentiate into newly-formed adipocytes [13, 14, 15]. In this manner, SC adipose can increase its fat storage capacity through both enlargement of mature adipocytes and formation of new adipocytes to buffer fatty acid influx when energy intake exceeds energy expenditure [16, 17].
Subcutaneous abdominal adipose is altered by hyperandrogenism in women with PCOS. Within this adipose depot, androgen normally inhibits early-stage adipogenesis, diminishes insulin-stimulated glucose uptake and impairs catecholamine-stimulated lipolysis through reduced β2-adrenergic receptor and hormone-sensitive lipase (HSL) protein expression independent of BMI or age [13, 18, 19, 20]. In agreement, SC abdominal adipose of women with PCOS shows diminished insulin-mediated glucose uptake, reduced glucose transporter type 4 (GLUT-4) expression [21] and lipolytic catecholamine resistance from diminished protein levels of β2-adrenergic receptor, HSL and protein kinase A regulatory-IIβ component (PKA-RegIIβ) [22, 23].
Using glycerol as a biomarker of lipolysis, moreover, serum glycerol levels are reduced in normal-weight women with PCOS and normal insulin sensitivity (22), but are slightly elevated in overweight women with PCOS and insulin resistance [24]. This variable lipolytic activity of adipose can be explained in part by androgen-induced lipolytic catecholamine resistance in normal-weight women with PCOS and normal insulin sensitivity [22] that is antagonized by impaired suppression of lipolysis by insulin in overweight women with PCOS and insulin resistance [24].
Adipose responsiveness to insulin in vivo can also be quantified using adipose insulin resistance (adipose-IR) as the product of fasting circulating insulin and total fatty acid levels [25]. Adipose-IR is increased in healthy normal-weight women with PCOS and low-normal insulin sensitivity (Si), as measured by a frequently sampled intravenous glucose tolerance test (IVGTT), compared to age- and BMI-matched normal women [26] (Figure 1). Furthermore, adipose-IR positively correlates with serum androgen and fasting triglyceride (TG) levels, and negatively correlates with Si and serum adiponectin levels in these women combined. The inverse relationships of adipose-IR with Si and TG levels under these conditions remain significant, adjusting for serum androgen levels [26].
1.
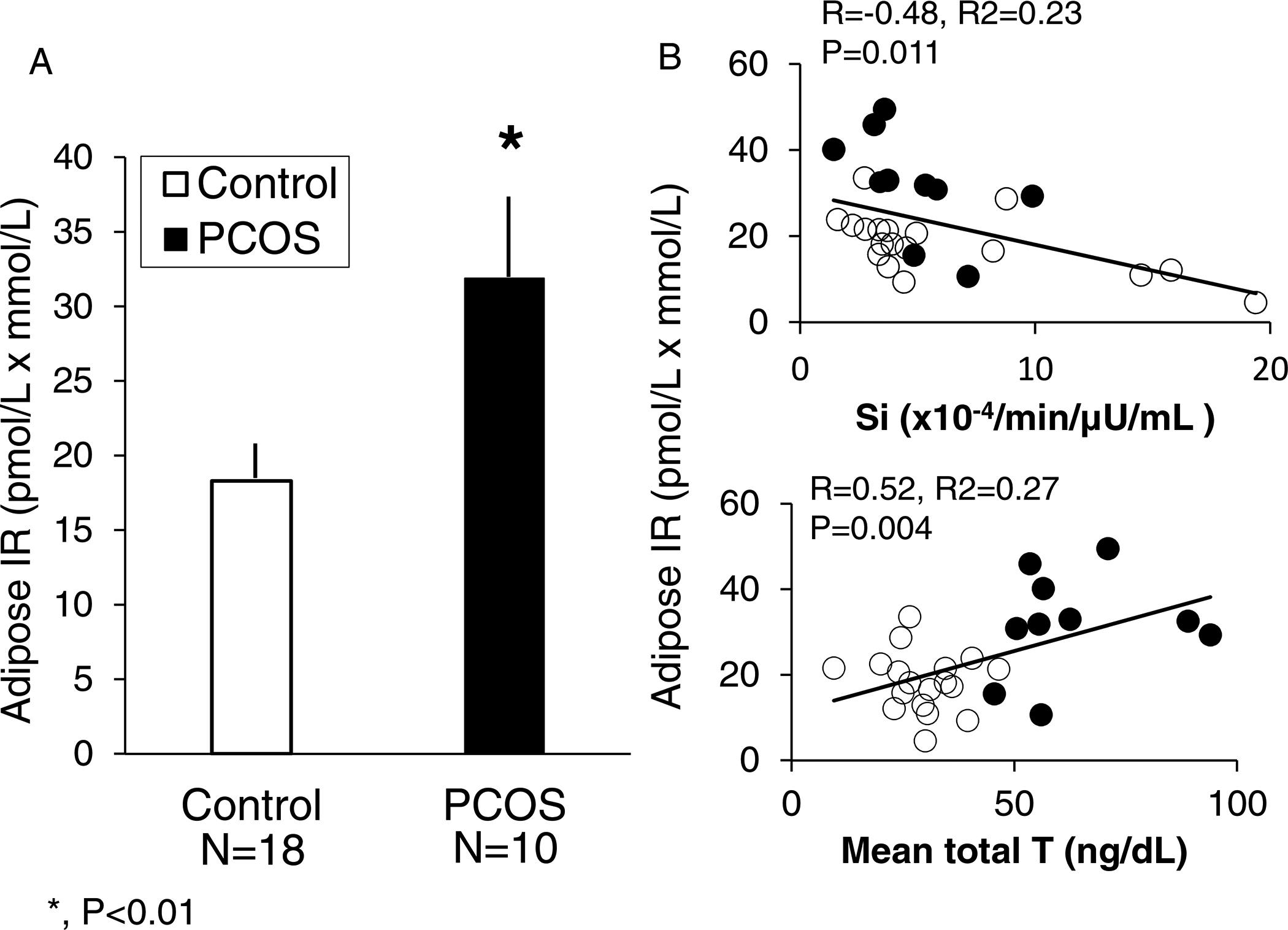
A) Differences in adipose insulin resistance (adipose-IR) between normal-weight women with PCOS (N=10) and controls (N=18) (P<0.01). B) Significant negative and positive correlations of adipose-IR with and insulin sensitivity (Si) and serum total testosterone (T), respectively, in the same normal-weight women with PCOS and controls combined. Adipose-IR is the product of fasting circulating insulin (pmol/L) and total fatty acid (mmol/L) levels. Filled circles and columns, women with PCOS; Open circles and columns, controls. *, P < 0.01 by Student’s t-test [Modified from reference 6]. SI units: T ng/dL × 0.0347.
This relationship of adipose-IR with low-normal Si in normal-weight women with PCOS may be linked with androgen inhibition of early-stage adipogenesis, through insulin-induced androgen production within adipose by intracellular aldo-ketoreductase type 3 1C3 (AKR1C3) activity [27]. Such amplification of androgen action within adipose itself appears to constrain SC fat storage capacity [13, 26, 28–30], causing a greater proportion of small SC abdominal adipocytes to form through enhanced adipocyte hyperplasia in an attempt to balance adipogenesis with glucose-insulin homeostasis [6, 26, 31]. As evidence, a similar population of small SC abdominal adipocytes occurs in other individuals with different forms of metabolic dysfunction [32, 33, 34], in whom it protects against insulin resistance by enhancing ASC commitment to preadipocyte differentiation through upregulation of ZFP423 due to epigenetic changes in its promotor region [35].
1.5. Intra-abdominal adipose
Although SC abdominal adipose protects against insulin resistance, intra-abdominal adipose has the opposite effect (12). Human intra-abdominal adipose normally has increased lipolytic activity and resists androgen inhibition of catecholamine-induced lipolysis compared to SC abdominal fat, despite both fat depots expressing androgen receptors [20].
Intra-abdominal fat mass, as determined by abdominal magnetic resonance imaging, is greater in normal-weight women with PCOS by NIH criteria compared to age- and BMI-matched normal women [6]. In all these women combined, intra-abdominal fat mass positively correlates with circulating levels of androgens as well as fasting insulin, TG, non-HDL cholesterol and total cholesterol [6]. Within intra-abdominal adipose of nonobese women with PCOS, moreover, exaggerated catecholamine-induced lipolysis from increased PKA-HSL complex activity accompanies normal insulin suppression of lipolysis [19, 36]. In women with PCOS, therefore, increased intra-abdominal adipose with high lipolytic activity enhances free fatty acid delivery to the liver and muscle for energy storage, but also can induce insulin resistance if increased fatty acid availability overwhelms the capacity of these tissues to oxidize fat or convert diacylglycerols to triacylglycerols [19, 36, 37].
1.6. Lipotoxicity
Lipotoxicity refers to the ectopic lipid accumulation in non-adipose tissue where it induces oxidative/endoplasmic reticulum stress tightly linked with insulin resistance and inflammation [3]. Women with PCOS who have a constrained SC adipose storage capacity are at increased risk of developing lipotoxicity due to excess free fatty acid uptake into non-adipose cells, including the muscle, liver, pancreas and ovary, that is exacerbated by preferential accumulation of intra-abdominal fat with high lipolytic activity [19, 28, 29, 36, 38, 39]. This is because excess fatty acid influx into skeletal muscle and liver can promote diacylglycerol-induced insulin resistance, which impairs insulin signaling via increased serine phosphorylation of insulin receptor substrate, and is worsened by disrupted mitochondrial oxidative phosphorylation [37, 40].
Lipotoxicity explains why insulin resistance occurs in some normal-weight women with PCOS when a constrained SC fat storage capacity is insufficient to balance excess energy intake with energy expenditure [5]. Interestingly, SC abdominal adipose of normal-weight women with PCOS resembles that of overweight/obese individuals by having an increased number of small adipocytes, while lacking enlarged adipocytes as seen in overweight/obese individuals with decreased serum adiponectin levels [6, 24, 41].
1.7. SC Abdominal stem cells
Sufficient evidence also suggests that SC fat storage capacity is developmentally programmed [34]. Subcutaneous abdominal ASCs from normal-weight PCOS by NIH criteria versus age- and BMI-matched normal women exhibit altered gene expression of adipogenic/angiogenic functions involving androgen-insulin interactions through TGF-b signaling [26]. Moreover, when SC abdominal ASCs from these normal-weight PCOS are cultured without androgen, exaggerated ZFP423-induced ASC commitment to preadipocytes negatively correlates with circulating fasting glucose levels. Enhanced lipid accumulation in these newly, in vitro-formed adipocytes also positively correlates with circulating androgen levels. Taken together, these findings suggest a programmed mechanism to maintain glucose-insulin homeostasis when fat accretion is accelerated [31] (Figure 2). Furthermore, the increased proportion of small SC abdominal adipocytes observed in normal-weight women with PCOS (see Section 1.4) [6] resembles a similar increase in the proportion of small SC abdominal adipocytes found in PCOS-like prenatally-T treated adult rhesus monkeys with increased visceral adiposity and insulin resistance [2] and in prenatally-T treated sheep with insulin resistance [11]. The recent observation in adult female monkeys with natural hyperandrogenemia of comparable PCOS-like traits strongly suggests an evolutionary origin of PCOS-like characteristics in both human and nonhuman primates [2].
2.
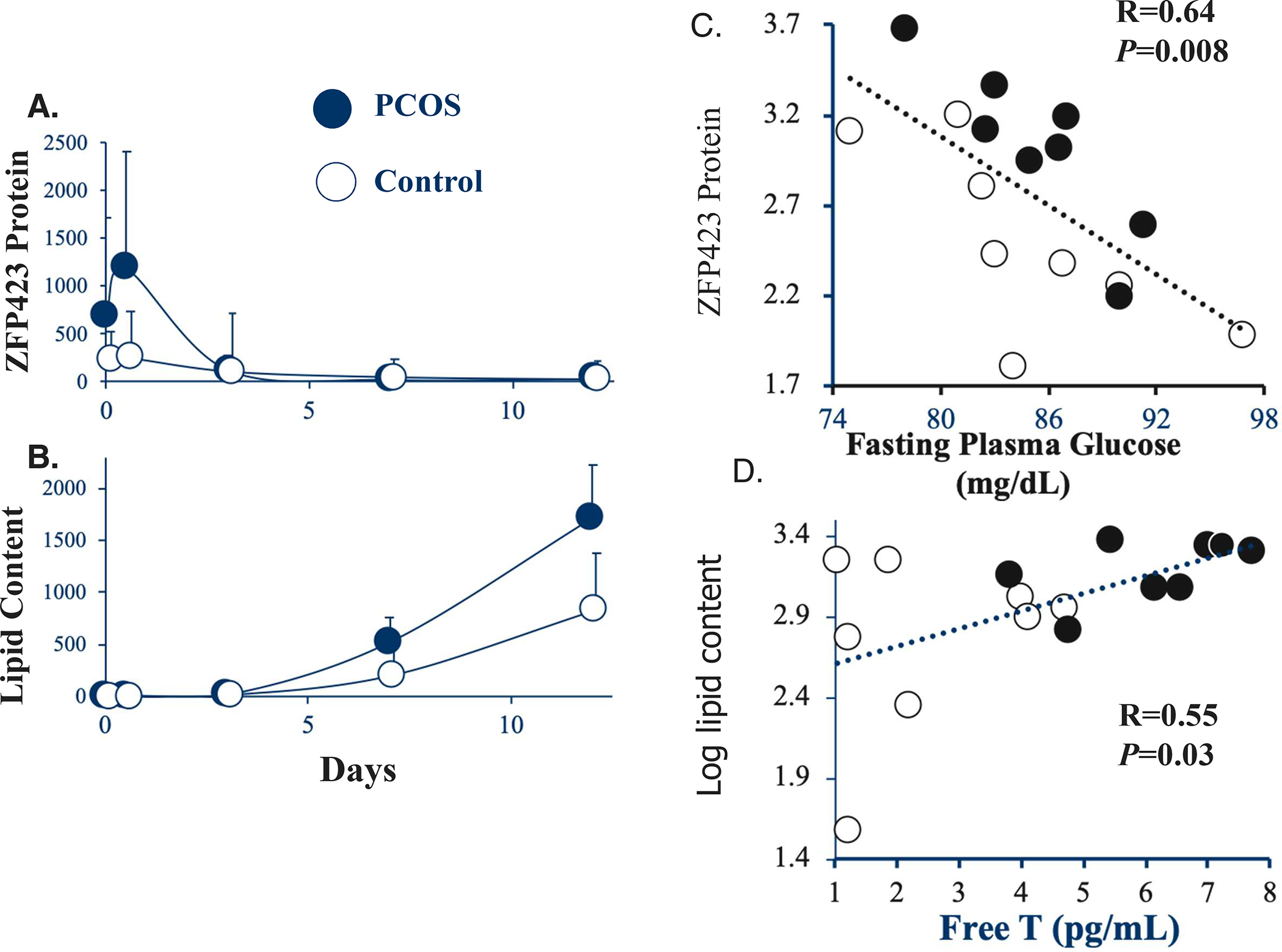
Temporal changes of A) ZFP423 protein expression and B) lipid content in subcutaneous (SC) abdominal ASCs cultured in adipogenic medium for up to 12 days. Values are expressed as median ± 95% confidence intervals of 8 age and BMI pair-matched normal-weight women with PCOS and controls. Significant correlations of C) log ZFP423 protein expression (day 0.5) with fasting plasma glucose levels, and of D) log lipid content (day 12) with serum free T levels in the same pair-matched normal-weight women with PCOS and controls. ZFP423 protein expression and lipid content values are expressed as Texas Red and Oil-Red-O fluorescence, respectively, divided by DAPI [Modified from reference 31]. Filled circles, women with PCOS; Open circles, controls. SI units: glucose mg/dL × 0.056; free T pg/mL × 3.47.
1.8. Developmental programming
Viewed through the perspective of evolutionary genetics, the high worldwide prevalence of PCOS in today’s environment and its negative impact on reproduction should have disappeared over millennia unless a beneficial effect favoring reproduction coexisted [42]. One such concept (i.e., metabolic thrift) is that ancestral traits originally favored PCOS in hunter-gatherers during the late Pleistocene, when food deprivation in pregnant women programmed enhanced adipogenesis for greater fat storage in the fetus to meet the increased metabolic demands of reproduction in later life. These same ancestral traits that originally favored PCOS are now at increased risk of elimination from the population due to the increased energy availability and reduced physical activity within many modern societies.
From this perspective, it is interesting that amniotic fluid T levels are elevated in female fetuses of PCOS mothers [43] at a time when regional fat depots in the human fetus develop between 14 and 28 weeks of gestation [44]. Moreover, umbilical cord testosterone levels at birth are elevated in some, but not all, female infants of PCOS mothers, while elongated anogenital distance, as a reliable postnatal biomarker of mid-gestational fetal hyperandrogenism, occurs in both female infants of PCOS mothers and in women with PCOS [11].
In normal-weight women with PCOS, therefore, inherently enhanced SC adipogenesis within a constrained fat depot that promotes free fatty acid uptake into muscle, liver and highly-lipolytic intra-abdominal fat may be an ancestral metabolic adaptation to food deprivation that promotes fatty acid oxidation and increased glucose availability through a balance between hyperandrogenism and insulin resistance for optimal energy use during reproduction (Figure 3). In modern societies, furthermore, such insulin resistance might also be an important vestigial mechanism promoting fatty acid oxidation to curtail excess fat accretion, as found in nondiabetic Pima Indians at risk for weight gain [45].
3.
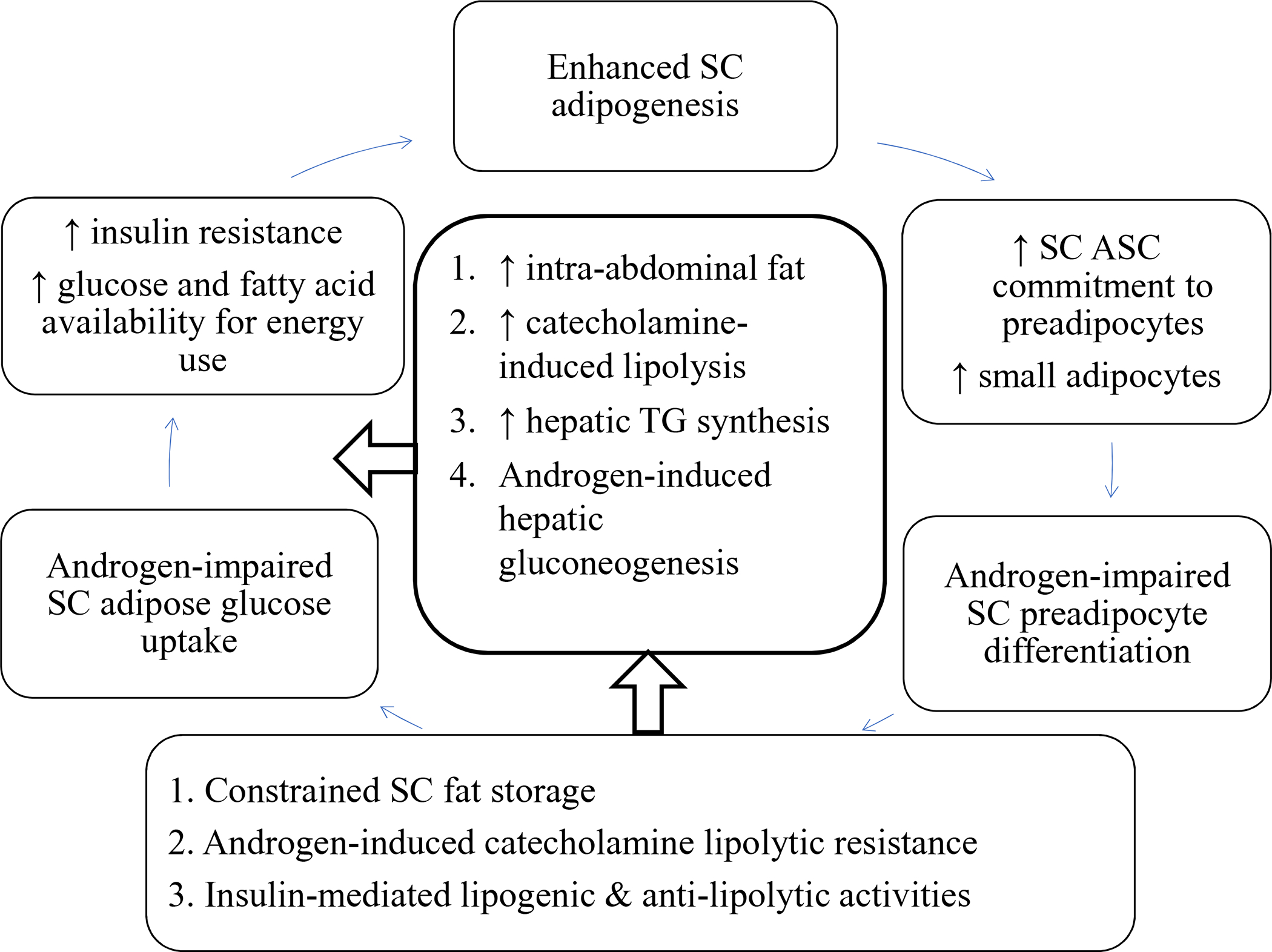
Metabolic thrift in PCOS. Inherently enhanced subcutaneous (SC) abdominal adipogenesis in the presence of hyperandrogenism constrains SC fat storage and promotes preferential intra-abdominal fat accumulation. In SC abdominal adipose, the combination of androgen-induced catecholamine lipolytic resistance with insulin-mediated lipogenic/anti-lipolytic activities favors fat storage and can be counterbalanced by insulin resistance that favors increased circulating glucose and free fatty acid levels for energy use. An increased amount of highly-lipolytic intra-abdominal adipose also enhances free fatty acid delivery to the liver and muscle for energy storage or use. When energy intake exceeds energy utilization, increased free fatty acid availability overwhelms the capacity of target tissues to oxidize fat or convert diacylglycerol to triacylglycerol, worsening insulin resistance and increasing the risks of developing metabolic syndrome and lipotoxicity [6, 13, 18–20, 36, 37, 40].
Alternative yet complementary evolutionary origins of PCOS may also exist. Nonthrifty ancestral traits related to androgen excess include increased bone/muscle strength, enhanced aggression to improve survival, and subfertility from infrequent ovulation, all of which allow women more time and strength for childrearing of fewer offspring, thereby enhancing the probability of offspring survival [42].
1.9. Conclusion and future directions
Polycystic ovary syndrome has persisted from antiquity to become the most common reproductive-metabolic disorder of reproductive-aged women. Its ancestral traits once favored abdominal fat deposition and increased energy availability through hyperandrogenism and insulin resistance, respectively, for reproduction during food deprivation. These same traits in today’s environment, however, give rise to the different PCOS phenotypes with variable risks for subfertility and metabolic dysfunction that are worsened by obesity. Future studies will examine how heritable PCOS characteristics are influenced by today’s environment through epigenetic chromosomal changes that alter metabolic function. They will approach these investigations from a new perspective that PCOS may have evolutionary origins in both human and nonhuman primates.
Highlights.
PCOS is defined by androgen excess, oligo-anovulation and polycystic ovaries.
PCOS phenotypes are associated with insulin resistance and are worsened by obesity.
Women with PCOS have preferential abdominal fat accumulation and increased visceral fat.
Constrained subcutaneous fat storage in women with PCOS predisposes to lipotoxicity.
Enhanced stem cell development to fat cells in PCOS suggests ancestral origins.
Acknowledgements
We thank Karla Largaespada for subject recruitment strategies and administrative responsibilities that were crucial for the successful studies of the normal-weight PCOS subjects,
Grants
This work was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health (NIH) under awards P50HD071836 and P51 ODO11092 for the Endocrine Technologies Support Core (ETSC) through the Oregon National Primate Research Center and P50HD028934 through the Wisconsin National Primate Research Center and the University of Virginia; statistical analyses by the NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881; and the Santa Monica Bay Woman’s Club. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Human rights and informed consent
Human studies at UCLA were performed according to the Declaration of Helsinki after approval by the UCLA Institutional Review Board and signed informed consent by each subject
Declaration of Interests: none
References
- 1.Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr Rev 2015;36(5):487–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott DH, Dumesic DA, Levine JE Hyperandrogenic Origins of Polycystic Ovary Syndrome – Implications for Pathophysiology and Therapy. Expert Rev Endocrinol Metab 2019;14(2):131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This paper investigates the pathogenic origins of PCOS through animal models derived from experimentally-induced hyperandrogenism during gestation, or from naturally-occurring PCOS-like traits that demonstrate similar reproductive, neuroendocrine and metabolic abnormalities.
- 3.Brennan KM, Kroener LL, Chazenbalk GD, Dumesic DA. Polycystic Ovary Syndrome: Impact of Lipotoxicity on Metabolic and Reproductive Health. Obstet Gynecol Surv 2019;74(4):223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ on behalf of the International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 2018;33:1602–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]; * These international evidence-based guidelines include several recommendations that address the assessment and management of polycystic ovary syndrome (PCOS) with the goal of improving health outcomes of women with PCOS.
- 5.Chang RJ, Dumesic DA. Polycystic Ovary Syndrome and Hyperandrogenic States In: Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management, Eighth Edition Strauss JF III, Barbieri RL (eds). Elsevier Saunders, Philadelphia, 2018; 520–555. [Google Scholar]
- 6.Dumesic DA, Akopians AL, Madrigal VK, Ramirez E, Margolis DJ, Sarma MK, Thomas AM, Grogan TR, Haykal R, Schooler TA, Okeya BL, Abbott DH, Chazenbalk GD. Hyperandrogenism Accompanies Increased Intra-Abdominal Fat Storage in Normal Weight Polycystic Ovary Syndrome Women. J Clin Endocrinol Metab 2016;101(11):4178–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This paper demonstrates that androgen excess in normal-weight healthy women with PCOS is associated with preferential intra-abdominal fat deposition and subcutaneous (SC) abdominal adipose structure-function changes that could constrain SC adipose storage and promote metabolic dysfunction.
- 7.Tosi F, Di Sarra D, Kaufman JM, Bonin C, Moretta R, Bonoro E, Zanolin E, Mogetti P. Total body fat and central fat mass independently predict insulin resistance but not hyperandrogenemia in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2015;100:661–9. [DOI] [PubMed] [Google Scholar]
- 8.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endo Rev 2012;33(6):981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim SS, Norman RJ, Davies MJ, Moran LJ. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev 2013;14:95–109 [DOI] [PubMed] [Google Scholar]
- 10.Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Repro Update. 2010;16(4):347–363 [DOI] [PubMed] [Google Scholar]
- 11.Dumesic DA, Hoyos LR, Chazenbalk GD, Naik R, Padmanabhan V, Abbott DH. Mechanisms of Intergenerational Transmission of Polycystic Ovary Syndrome. Reproduction, 2019. August 1 pii: REP-19–0197.R1. doi: 10.1530/REP-19-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** The review examines the ontogeny of PCOS through the developmental origins of adult disease (DoHAD) model. Clinical PCOS studies and findings from relevant animal models are integrated to explain how intergenerational transmission of PCOS symptomatology can be programmed through an altered maternal endocrine-metabolic environment to adversely affect the female fetus and long-term offspring health.
- 12.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab 2011; 96:E1756–E1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chazenbalk GD, Singh P, Irge D, Shah A, Abbott DH, Dumesic DA. Androgens inhibit adipogenesis during human adipose stem cell commitment to predipocyte formation. Steroids 2013;78:920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol 2011;12(11):722–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annual Rev Biochem 2012; 7(81):715–36. [DOI] [PubMed] [Google Scholar]
- 16.Saponaro C, Gaggini M, Carli F, Gastaldelli A. The subtle balance between lipolysis and lipogenesis: a critical point in metabolic homeostasis. Nutrients 2015;7(11):9453–9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romacho T, Elsen M, Rohrborn D, Eckel J. Adipose tissue and its role in organ crosstalk. Acta Physiol (Oxf), 2014;210(4):733–53. [DOI] [PubMed] [Google Scholar]
- 18.Corbould A Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J Endocrinol 2007;192(3):585–94. [DOI] [PubMed] [Google Scholar]
- 19.Arner P Effects of testosterone on fat cell lipolysis. Species differences and possible role in polycystic ovarian syndrome. Biochimie 2005;87:39–43. [DOI] [PubMed] [Google Scholar]
- 20.Dicker A, Ryden M, Naslund E, Muehlen IE, Wiren M, Lafontan M, Arner P. Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia 2004;47:420–428. [DOI] [PubMed] [Google Scholar]
- 21.Rosenbaum D, Harber RS, Dunaif A. Insulin resistance in polycystic ovary syndrome: decreased expression of GLUT-4 glucose transporters in adipocytes. Am J Physiol 1993;264(2 Pt 1):E197–202. [DOI] [PubMed] [Google Scholar]
- 22.Faulds G, Rydén M, Ek I, Wahrenberg H, Arner P. Mechanisms behind lipolytic catecholamine resistance of subcutaneous fat cells in the polycystic ovarian syndrome. J Clin Endocrinol Metab 2003; 88:2269–2273. [DOI] [PubMed] [Google Scholar]
- 23.Ek I, Arner P, Bergqvist A, Carlstrom K Wahrenberg H. Impaired adipocyte lipolysis in nonobese women with the polycystic ovary syndrome: a possible link to insulin resistance? J Clin Endocrinol Metab 1997;82:1147–53. [DOI] [PubMed] [Google Scholar]
- 24.Mannerås-Holm L, Leonhardt H, Kullberg J, Jennische E, Odén A, Holm G, Hellström M, Lönn L, Olivecrona G, Stener-Victorin E, Lönn M. Adipose tissue has aberrant morphology and function in PCOS: Enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab 2011;96:E304–E311. [DOI] [PubMed] [Google Scholar]
- 25.Søndergaard E, Espinosa De Ycaza AE, Morgan-Bathke M, Jensen MD. How to measure adipose tissue insulin sensitivity. J Clin Endocrinol Metab 2017;102(4):1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dumesic DA, Phan JD, Leung KL, Grogan TR, Ding X, Li X, Hoyos LR, Abbott DH, Chazenbalk GD. Adipose Insulin Resistance in Normal-Weight Polycystic Ovary Syndrome Women. J Clin Endocrinol Metab 2019;104(6):2171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This paper demonstrates that normal-weight, healthy women with PCOS have increased resistance of adipose to insulin in vivo that is accompanied by altered subcutaneous abdominal adipose stem cell gene expression related to both hyperandrogenism and insulin resistance.
- 27.O’Reilly MW, Kempegowda P, Walsh M, Taylor AE, Manolopoulos KN, Allwood JW, Semple RK, Hebenstreit D, Dunn WB, Tomlinson JW, Arlt W. AKR1C3-Mediated Adipose Androgen Generation Drives Lipotoxicity in Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2017;102(9):3327–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Virtue S, Vidal-Puig A. It’s not how fat you are, it’s what you do with it that counts. PLoS Biol 2008; 6:e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta 2010; 1801:209–214. [DOI] [PubMed] [Google Scholar]
- 30.Anik llhan G, Yildizhan B, Pekin T. The impact of lipid accumulation product (LAP) and visceral adiposity (VAI) on clinical, hormonal and metabolic parameters in lean women with polycystic ovary syndrome. Gynecol Endocrinol 2018;35(3):233–236. doi: 10.1080/09513590.2018.1519794 [DOI] [PubMed] [Google Scholar]
- 31.Fisch SC, Farzan Nikou A, Wright EA, Phan JD, Leung KL, Grogan TR, Abbott DH, Chazenbalk GD, Dumesic DA. Precocious Subcutaneous Abdominal Stem Cell Development to Adipocytes in Normal-Weight Polycystic Ovary Syndrome Women. Fertil Steril 2018;110:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This paper shows that subcutaneous abdominal adipose stem cells of normal-weight women with PCOS show exaggerated commitment to preadipocytes and enhanced adipocyte lipid content during maturation in vitro that negatively and positively correlate with circulating fasting glucose and androgen levels, respectively, as a possible mechanism to maintain glucose-insulin homeostasis during accelerated fat accretion.
- 32.Tandon P, Wafer R, Minchin JE. Adipose morphology and metabolic disease. J Expt Biol 2018; 221(Pt Suppl 1), jeb164970. doi: 10.1242/jeb.164970. [DOI] [PubMed] [Google Scholar]
- 33.Arner E, Westermark PO, Spalding, KL, Britton T, Ryden M, Frisen J, Bernard S, Arner P. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes 2010;59(1):105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffsted J, Naslund E, Britton T, Concha H, Hassan M, Rydén M, Frisén J, Arner P. Dynamics of fat cell turnover in humans. Nature 2008; 453(7196):783–787. [DOI] [PubMed] [Google Scholar]
- 35.Longo M, Raciti GA, Zatterale F, Parrillo L, Desiderio A, Spinelli R, Hammarstedt A, Hedjazifar S, Hoffmann JM, Nigro C, Mirra P, Fiory F, Formisano P, Miele C, Smith U, Beguinot F. Epigenetic modifications of the Zfp/ZNF423 gene control murine adipogenic commitment and are dysregulated in human hypertrophic obesity. Diabetologia 2018;61:369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The paper shows how epigenetic events controlling ZNP423 expression affect precursor cell commitment and differentiation into mature adipocytes and how dysregulation of these events adversely affects subcutaeous abdominal adipose structure-function in humans.
- 36.Ek I, Arner P, Rydén M, Holm C, Thörne A, Hoffstedt J, Wahrenberg H. A unique defect in the regulation of visceral fat cell lipolysis in the polycystic ovary syndrome as an early link to insulin resistance. Diabetes 2002;51:484–92. [DOI] [PubMed] [Google Scholar]
- 37.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unraveling the mechanism. Lancet 2010;375(9733):2267–77. Doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Zegher F, Lopez-Bermejo A, Ibáñez L. Adipose tissue expandability and the early origins of PCOS. Trends Endocrinol Metab 2009;20:418–423. [DOI] [PubMed] [Google Scholar]
- 39.Ramkissoon R, Gardner TB. Pancreatic Steatosis: An Emerging Clinical Entity. Am J Gastroenterol. 2019. May 23. doi: 10.14309/ajg.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 40.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Eng J Med 2014;371(23):1131–1141. [DOI] [PubMed] [Google Scholar]
- 41.McLaughlin T, Lamendola C, Coghlan N, Liu TC, Lerner K, Sherman A, Cushman SW. Subcutaneous adipose cell size and distribution: Relationship to insulin resistance and body fat. Obesity (Silver Spring) 2014; 22:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corbett S, Morin-Papunen L. Polycystic ovary syndrome and recent human evolution. Mol Cell Endocrinol 2013;373:39–50. [DOI] [PubMed] [Google Scholar]
- 43.Palomba S, Marotta R, Di Cello A, Russo T, Falbo A, Orio F, Tolino A, Zullo F, Esposito R, La Sala GB. Pervasive developmental disorders in children of hyperandrogenic women with polycystic ovary syndrome: a longitudinal case-control study. Clin Endocrinol (Oxf) 2012;77:898–904. [DOI] [PubMed] [Google Scholar]
- 44.Poissonnet CM, LaVelle M, Burdi AR. Growth and development of adipose tissue. J Pediatr 1988;113 (1 Pt 1):1–9. [DOI] [PubMed] [Google Scholar]
- 45.Swinburn BA, Nyomba BL, Saad MF, Zurlo F, Raz I, Knowler WC, Lillioja S, Bogardus C, Ravussin E. Insulin resistance associated with lower rates of weight gain in Pima Indians. J Clin Invest 1991;88:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]


