Summary
-
1
The accelerating extinction rate of plant species and its effect on ecosystem functioning is a hotly debated topic in ecological research. Most research projects concerning the relationship between species diversity and productivity have been conducted in artificial plant communities, with only a few in natural ecosystems. In this study we examined the relationship between species diversity and above‐ground net primary productivity (ANPP) over two consecutive growth seasons (2004 and 2005) in a semi‐arid steppe ecosystem of northern China, that were subjected to different land uses.
-
2
Land use affected the relationship between species diversity and ANPP in this semi‐arid steppe ecosystem. Exclusion of grazing without or with biomass removal by mowing increased ANPP, species richness and species diversity compared with free grazing; the effect was reflected mainly as enhanced importance of the perennial forbs functional group in terms of their relative contributions to ANPP, plant cover and plant abundance.
-
3
Many mechanisms regulate the relationship between species diversity and productivity. Differential effects of anthropogenic activities on biodiversity and ecosystem functioning greatly complicate the analysis of such relationships. On grazing‐exclusion sites the relationship between ANPP and species richness can be best described as an exponential growth function (R 2 = 0·99, P < 0·001, n = 24); whereas on the free‐grazing site the relationship takes the form of exponential decay (R 2 = 0·96, P < 0·001, n = 24). Our study concludes that the mode and severity of disturbance are important factors for interpreting the relationship between species diversity and productivity in semi‐arid steppe ecosystems.
Keywords: biodiversity, land use, local scale, productivity, steppe ecosystem
Introduction
Worldwide loss of biodiversity and its consequences for ecosystem functioning have emerged recently as a hotly debated topic in ecological research (Troumbis 2001; Wardle 2001; Loreau, Naeem & Inchausti 2002). In past decades losses of biodiversity have occurred at an unprecedented scale, with the current global extinction rate estimated at 100−1000 times greater than prehuman levels (Pimm et al. 1995). Widespread stressors, such as land‐use change, fragmentation, biotic invasions and climate change, are considered to be the major drivers of biodiversity losses (Chapin et al. 1997).
Interest in the effects of biodiversity on ecosystem stability and functioning has been heightened by the rapidly accelerating rate of species extinctions. A loss of biodiversity could significantly affect the functioning of many terrestrial ecosystems (Ehrlich & Ehrlich 1981; Schlapfer & Schmid 1999). Manipulation experiments on the relationship between plant diversity and ecosystem functioning (productivity, nutrient cycling, decomposition, nitrogen mineralization rate, nitrate leaching) have indicated that (i) more diverse plant communities are more productive; and (ii) more diverse ecosystems could make full use of the most limiting nutrient, soil mineral nitrogen, and lead to reduced leaching losses of this vital nutrient (Naeem et al. 1995; Naeem et al. 1996).
Although there have been extensive research efforts, controversy over the diversity–productivity relationship in natural communities still looms large. Positive relationships between plant species (or functional group diversity) and productivity (or other ecosystem processes) have been reported in a number of experimental studies (Tilman & Downing 1994; Symstad et al. 1998; Loreau et al. 2002). Detailed evidence from terrestrial habitats, particularly grasslands, suggests that productivity is often a non‐linear, concave function of the richness of species or functional groups (Waid et al. 1999). Mittelbach et al. (2001) found that, in 65% of the studies they examined, the relationship between biodiversity and productivity was described by a concave down function. In most recent laboratory and field experiments the effect of biodiversity on productivity was tested using artificial plant communities, where different diversity levels were established by drawing plant species from a random species pool (Kahmen et al. 2005). These experiments under artificial conditions are, by and large, constrained in their capacity for extrapolation to natural ecosystems. Cardinale, Nelson & Palmer (2000) noted that the cause of the diversity–productivity relationship could change with environmental context. In many manipulative experiments, the simplification of environmental conditions for testing specific hypotheses could unknowingly eliminate the very factors regulating the diversity–productivity relationship that exists in natural ecosystems. For example, the relatively homogenized soils in artificial grassland ecosystems could result in underestimation of the role of niche differentiation at a smaller spatial and/or temporal scale (He et al. 2003).
While several researchers have suggested that causal relationships exist between diversity and ecosystem functioning, particularly between species diversity and above‐ground productivity (1994, 1995), others believe that the key functional attributes or traits of dominant species in plant communities and the composition of functional types play a more prominent role than simply species richness in driving ecosystem properties (Hooper & Vitousek 1997; Berendse 1998; Grime 1998). Based on data from a 24‐year grazing‐exclusion study, Bai et al. (2004) reported a causal relationship between species diversity and productivity in a typical steppe of the Inner Mongolian Plateau, northern China. This finding, however, was questioned by Wang et al. (2005) and Guo (2005), who argued that the roles of spatial heterogeneity and functional groups were not sufficiently reflected in the analyses of Bai et al. (2004). In natural environments, spatio‐temporal heterogeneities of resources are ubiquitous features that regulate both richness and the distribution of biomass among taxa (Petraitis, Latham & Niesenbaum 1989). Stochastic environmental fluctuation and disturbance have been found to affect grassland biodiversity (McNaughton 1983; Collins 1987) and the spatial structure of communities (Seabloom & Richards 2003).
Research in the semi‐arid Mediterranean region has provided rich information regarding the effects of land use on plant diversity in semi‐arid grassland ecosystems (Osem, Perevolotsky & Kigel 2002; Bonet 2004; Armas & Pugnaire 2005). Bonet (2004) found that land‐use history played an important role in determining the ordination of communities, and that previous cropping influenced the pathway of succession in a semi‐arid Mediterranean region. In arid and semi‐arid environments, competition and facilitation can both affect the composition and structure of plant communities (Armas & Pugnaire 2005; Michalet 2006), and spatial variation in the balance of the two factors may, to a large extent, determine diversity–productivity relationships at a local scale. Osem et al. (2002) showed that diversity of annual plant community could be determined by interactions between grazing and small‐scale spatial and temporal variation in primary productivity. Despite the spatially wide occurrence and significant regional importance, the Eurasian steppe ecosystems have been largely under‐represented in the global analysis of diversity–productivity relationships.
Steppe ecosystems under natural conditions are constantly subjected to disturbance and environmental perturbation. The intensified anthropogenic activities in recent history have had a great impact on both the structure and functioning of the semi‐arid steppe ecosystems of northern China. Some land‐use practices, such as hay harvesting by mowing, have been demonstrated to cause shifts in the composition of plant functional groups and consequently to lead to changes in ecosystem functioning (Bao, Li & Zhong 2004). To determine further how land use affects the diversity–productivity relationship in semi‐arid steppe ecosystems, we conducted surveys on plant species diversity and community productivity over two growth seasons (2004−05) on sites of free grazing and grazing exclusion, with and without hay harvesting, in Inner Mongolia, northern China. We hypothesized that the mode and intensity of disturbance due to anthropogenic activities contributed to the relationship between species diversity and community productivity.
Materials and methods
study sites and experimental design
This study was conducted in Duolun County of Inner Mongolia (latitude 41°46′−42°39′ N, longitude 115°50′−116°55′ E, elevation 1150−1800 m a.s.l.), northern China. The long‐term mean annual, minimum and maximum air temperatures for the area are 1·6, −18·3 and 18·7 °C. Mean annual precipitation is 385 mm (67% falls between June and August). The soil in the top 40‐cm layer is classified as chestnut (FAO‐UNESCO 1974), below 40 cm a mixture of sandy soil and gravels. Vegetation of the region consists predominantly of common grassland plants of the steppe zone including Stipa krylovii Roshev., Agropyron cristatum (L.) Gaertner, Allium bidentatum Fisch. ex Prokh. & Ikonn.‐Gal., Artemisia frigida Willd., etc.
Traditional land uses in the study area have been a mixture of livestock grazing and farming. From the late 1950s to the late 1970s, the land uses of the region were managed under communal systems subject to strict government regulations. During the period 1970−77, Duolun County experienced rapid expansion of farmlands at the expense of grasslands (Baoyin & Liu 2001). The economic reforms and open policy since 1978 have resulted in private ownership of lands and free land‐use practices by the locals. Consequently, the area was subjected to intensive farming, grazing and land‐use changes that reduced the areas of grasslands (Liu & Tong 2003; Zhan et al. 2004). The extremely intensified land uses placed tremendous pressure on the regional grasslands, causing severe land degradation and extreme levels of desertification (Liu & Tong 2003; You et al. 2003; Zhan et al. 2004). In recognition of the environmental problems caused by overexploitation of the lands in this agro‐pastoral ecotone, in 2000 the local government imposed a policy banning livestock grazing, and sought alternative land uses that could help sustain the regional land productivity and economy. Hay production has been experimented with as one of the land‐use types potentially suitable for the region. However, some areas are still used for sheep and cattle grazing.
Three study sites were selected based on major land‐use types and pre‐existing experimental setups in the area, which included uncontrolled or free‐range grazing (FG); grazing exclusion without biomass removal (GE); or grazing exclusion with biomass removal by mowing ≈6 cm above the ground surface (MW). The FG site had been heavily grazed since 1979, with an estimated 75% of above‐ground biomass consumed by livestock (mainly cattle and sheep) each year (Wang, Wang & Chen 2003). The GE site was established in 2001 by constructing a fence around 21 ha of previously grazed grassland. The MW site, which also was previously grazed grassland, had been subjected to mechanical mowing in late August each year since 2001, with ≈80% of above‐ground biomass harvested as forage. Before 1978 the study area was not utilized/managed and the major disturbance resulted mainly from antelope and rabbit (occasionally sheep) grazing, or wildfire.
Our study area is in an agro‐pastoral zone, where interannual variation of climate is relatively small. For the past 10 years annual precipitation typically varied between 350 and 450 mm, and mean temperatures varied from around −15 °C in January (minimum) to ≈20 °C in July (maximum). The climatic conditions in both 2004 and 2005 at our study sites were close to the long‐term average (data not shown).
measurements
Four 30 × 30‐m plots were established on each site, representative of a land‐use type for measurements of above‐ground biomass, plant abundance, species composition and richness, and cover by plant functional groups. Those plots were located systematically along the north–south transect, taking into consideration the topography and spatial coverage of the given land‐use type at our study sites. Most studies concerning productivity in the steppe ecosystems of the region have been based on a plot size of 10 × 10 m. In this study we increased our plot size to 30 × 30 m, necessary to account for the potential spatial heterogeneity of vegetation pattern and resources based on our knowledge of the study area. The plant functional group was defined as by Bai et al. (2004) on the basis of life form: annuals and biennials (AB); shrubs and semishrubs (SS); perennial rhizome grass (PR); perennial bunchgrass (PR); perennial forbs (PF).
We determined the above‐ground net primary production (ANPP) by measuring the peak biomass, as our study sites contained mostly herbaceous plants with above‐ground tissues that die back annually. All living tissues were clip‐harvested by species in three replicated 1 × 1‐m quadrats on each plot between 15 and 25 August in 2004 and 2005, respectively, and oven‐dried at 65 °C to constant weight (≈48 h). The above‐ground biomass was measured by individual species, and grouped into categories of total community and plant functional group in our data analysis. The ANPP of shrubs and semishrubs (such as Artemisia frigida and Thymus serphyllum) was determined by clip‐harvesting subsamples at beginning (late April) and peak growth seasons (mid‐August) and taken as the difference between the two harvests.
Several terms were used for describing the biodiversity traits of plant communities: plant abundance, diversity (species richness), evenness (Pielou index), and Shannon–Wiener index of species. The Pielou Evenness index (E) was calculated as:
| (eqn 1) |
where P i is the relative importance value of species i, and S the total number of species. The Shannon–Wiener index was calculated as:
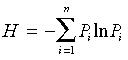 |
(eqn 2) |
On each site, species frequencies were measured from 25 randomly placed quadrats (0·5 × 0·5 m) by recording the presence of species within each quadrat. Species frequency was expressed as the proportion of quadrats containing a given species (Duncan et al. 1997).
statistical analysis
anova was used to analyse different treatment effects (land‐use types). Means of the main effects were compared using Duncan's multirange test at P < 0·05. The relationships of ANPP with all variables of biodiversity were examined using Pearson's correlation analysis. All statistical analyses were performed using spss (ver. 11·0) software.
Results
Land use affected both biodiversity and ANPP in the semi‐arid steppe ecosystem studied. The values of Pielou and Shannon–Wiener indices and species richness were significantly higher on both types of grazing‐exclusion plot (GE and MW) than on the FG plots, while the MW site had a markedly higher (P < 0·05) level of plant abundance than the other two land‐use types (GE and FG) (Table 1). The ANPP averaged (in g m−2 year−1) 199 ± 9 on the GE site, 144 ± 6 on the MW site, and 96 ± 5 on the FG site.
Table 1.
Values of biodiversity indices in three different land‐use types in a semi‐arid steppe ecosystem of northern China
| Land use* | Plant abundance (m−2) | Pielou index | Shannon–Wiener index | Species richness |
|---|---|---|---|---|
| GE | 182 ± 13 a | 0·67 ± 0·01 b | 1·78 ± 0·03 b | 14·5 ± 0·39 b |
| MW | 309 ± 27 b | 0·66 ± 0·01 b | 1·72 ± 0·04 b | 13·7 ± 0·59 b |
| FG | 164 ± 14 a | 0·57 ± 0·02 a | 1·36 ± 0·05 a | 11·3 ± 0·51 a |
GE, grazing exclusion without biomass removal; MW, grazing exclusion with biomass removal by mowing in August; FG, free grazing.
Values designated by the same letter are not significantly different at P = 0·05.
Pearson's correlation analysis revealed a significant (P < 0·05) and positive correlation between ANPP and Shannon–Wiener index on the MW site, and a significant (P < 0·05) but negative correlation on the FG site (Table 2). The correlation between ANPP and species richness was significant (P < 0·01) across all three land‐use types, with a positive correlation on the GE and MW plots, but a negative correlation on the FG plots. As illustrated in Fig. 1, the relationship between ANPP and species richness can be best described as an exponential growth function on the GE and MW plots, and as an exponential decay function on the FG plots.
Table 2.
Pearson's correlation coefficients for relationships of above‐ground net primary productivity with different biodiversity indices in three different land‐use types in a semi‐arid steppe ecosystem of northern China
| Land use† | Plant abundance (m−2) | Pielou index | Shannon–Wiener index | Species richness |
|---|---|---|---|---|
| GE | 0·30 | −0·34 | 0·11 | 0·87** |
| MW | 0·09 | −0·22 | 0·47* | 0·88** |
| FG | −0·51 | −0·21 | −0·48* | −0·55** |
GE, grazing exclusion without biomass removal; MW, grazing exclusion with biomass removal by mowing in August; FG, free grazing.
Correlation significant at *, P < 0·05; **, P < 0·01 (two‐tailed test).
Figure 1.
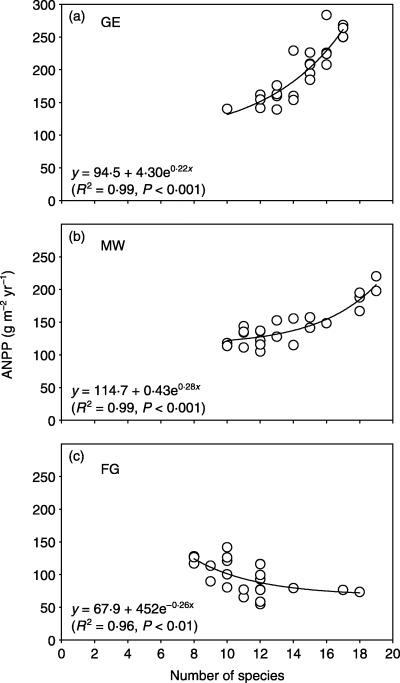
Relationship between above‐ground net primary productivity (ANPP) and species richness in three different land‐use types in a semi‐arid steppe ecosystem of northern China. GE, grazing exclusion without biomass removal; MW, grazing exclusion with biomass removal by mowing in August; FG, free grazing.
The species composition of plant communities varied with land‐use type. In total 31 and 34 species were found on the GE and MW plots, and 27 species on the FG plots (Table 3). Most of these species belonged to the PF functional group.
Table 3.
Species frequency (%) in three different land‐use types in a semi‐arid steppe ecosystem of northern China
| Plant functional group | Species | Land‐use type* | ||
|---|---|---|---|---|
| GE | MW | FG | ||
| Annual and biennial | Setaria viridis | 0 a | 0 a | 24 ± 3 b |
| Dontostemon micranthus | 3 ± 2 a | 8 ± 3 a | 3 ± 2 a | |
| Salsola collina | 60 ± 7 b | 4 ± 2 a | 8 ± 3 a | |
| Chenopodium aristatum | 9 ± 3 a | 25 ± 4 b | 12 ± 3 a | |
| Chenopodium glaucum | 0 a | 1 ± 1 a | 3 ± 2 a | |
| Fagopyrum tataricum | 0 a | 1 ± 1 a | 0 a | |
| Gentiana squarrosa | 4 ± 2 a | 11 ± 3 b | 0 a | |
| Orostachys fimbriatus | 0 a | 4 ± 2 b | 0 a | |
| Perennial rhizome grass | Agropyron michnoi | 66 ± 5 b | 39 ± 5 a | 57 ± 4 b |
| Leymus chinesis | 11 ± 3 b | 3 ± 2 a | 3 ± 3 a | |
| Aneurolepidium secalinum | 0 a | 0 a | 1 ± 1 a | |
| Perennial bunchgrass | Cleistogenes squarrosa | 91 ± 3 ab | 98 ± 2 b | 83 ± 4 a |
| Stipa krylovii | 100 a | 100 a | 100 a | |
| Poa angustifolia | 1 ± 1 a | 2 ± 2 a | 0 a | |
| Koeleria cristata | 0 a | 0 a | 30 ± 5 b | |
| Perennial forb | Oxytropis glabra | 3 ± 2 a | 5 ± 2 a | 30 ± 3 b |
| Carex duriuscula | 46 ± 4 a | 82 ± 4 b | 96 ± 2 c | |
| Melissitus ruthenica | 10 ± 3 a | 7 ± 2 a | 36 ± 4 b | |
| Astragalus scaberrimus | 36 ± 6 b | 8 ± 2 a | 65 ± 3 c | |
| Astragalus galactites | 0 a | 1 ± 1 a | 23 ± 2 b | |
| Heteropappus altaicus | 47 ± 5 b | 31 ± 5 a | 41 ± 5 ab | |
| Echinops latifolius | 0 a | 3 ± 3 a | 0 a | |
| Potentilla acauli | 6·7 ± 0·6 b | 7·9 ± 0·5 b | 4·8 ± 0·6 a | |
| Potentilla bifurca | 20 ± 4 b | 27 ± 3 b | 0 a | |
| Potentilla tanacetifolia | 1 ± 1 a | 1 ± 1 a | 12 ± 3 b | |
| Potentilla betonicaefolia | 1 ± 1 a | 0 a | 0 a | |
| Allium bidentatum | 98 ± 1 b | 99 ± 1 b | 2 ± 1 a | |
| Allium tenuissimum | 82 ± 3 b | 93 ± 3 c | 8 ± 2 a | |
| Allium neriniflorum | 34 ± 6 b | 30 ± 5 b | 9 ± 2 a | |
| Allium ramosum | 4 ± 2 a | 44 ± 2 b | 0 a | |
| Allium senescens | 10 ± 3 b | 54 ± 2 c | 0 a | |
| Phlomis mongolica | 0 a | 4 ± 2 b | 0 a | |
| Iris tenuifolia | 19 ± 4 b | 17 ± 2 b | 0 a | |
| Thalictrum petaloideum | 7 ± 3 a | 7 ± 2 a | 2 ± 1 a | |
| Silene jenisseensis | 1 ± 1 a | 2 ± 1 a | 1 ± 1 a | |
| Dianthus chinensis | 1 ± 1 a | 5 ± 3 a | 0 a | |
| Stellera chamaejasme | 1 ± 1 a | 2 ± 1 a | 2 ± 1 a | |
| Shrub and semishrub | Artemisia frigida | 100 a | 100 a | 100 a |
| Thymus serphyllum | 1 ± 1 a | 0 a | 6 ± 2 b | |
GE, grazing exclusion without biomass removal; MW, grazing exclusion with biomass removal by mowing in August; FG, free grazing.
Values designated by the same letter are not significantly different at P = 0·05.
Compared with FG plots, the GE and MW plots were characterized by significantly (P < 0·05) greater frequency of Liliaceae species (Allium bidentatum, Allium tenuissimum, Allium neriniflorum, Allium ramosum, Allium senescens), most of which grow close to the ground surface with low productivity. The FG plots had significantly (P < 0·05) greater numbers of legume species (Oxytropis glabra, Melissitus ruthenica, Astragalus scaberrimus, Astragalus galactites), and a higher frequency of the rhizomatous species, especially Carex duriuscula, than the other two sites. Two plant species, Setaria viridis and Koeleria cristata, were found exclusively on the FG plots with relatively high frequencies, whereas Orostachys fimbriatus, Echinops latifolius and Phlomis mongolica were exclusive to the MW plots with low freqencies (Table 3). Within the AB functional group, species numbers were, on average, 75 and 40% higher, respectively, on MW plots than on GE and FG plots.
The GE and MW plots differed from the FG plots by displaying greater importance of PF species and lesser importance of SS species in terms of relative contributions to ANPP, plant abundance and cover.
On both GE and MW plots, above‐ground production was comprised mainly of the PB, PF and SS functional groups, PF being the most dominant; whereas on the FG plots, above‐ground production was predominantly and equally comprised of the PB and SS functional groups (Fig. 2). On the FG plots, above‐ground production of the PB functional group increased with species richness, whereas above‐ground production of the SS functional group decreased with species richness (Fig. 3). The AB functional group contributed a meaningful fraction of total ANPP only on GE plots. For plant abundance, the PF functional group was most dominant on GE and MW plots, followed by the PR and PB functional groups (Fig. 4). On the FG plots, the PR functional group ranked highest and PB next to highest, followed by PF. The PF functional group on the MW plots had much higher abundance than on plots of the other two land‐use types (Fig. 4).
Figure 2.
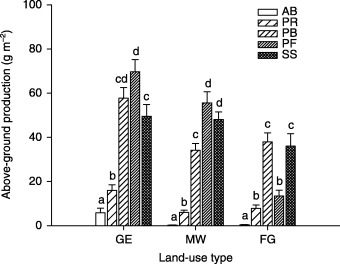
Above‐ground production by plant functional group in three different land‐use types in a semi‐arid steppe ecosystem of northern China. GE, grazing exclusion without biomass removal; MW, grazing exclusion with biomass removal by mowing in August; FG, free grazing; AB, annuals and biennials; PR, perennial rhizomes; PB, perennial bunchgrass; PF, perennial forbs; SS, shrubs and semishrubs. Letters above histograms indicate significant differences between plant functional groups within each land‐use type at P < 0·05.
Figure 3.
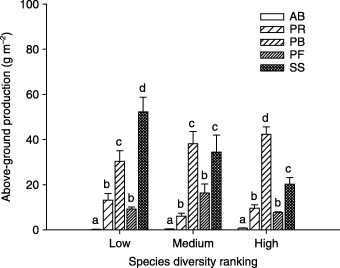
Above‐ground production by plant functional group under different species diversity ranking on free‐range grazing site in a semi‐arid steppe ecosystem of northern China. Species diversity was ranked as low (species richness 8–10); medium (10–12); high (13–18). Abbreviations as in Fig. 2. Letters above histograms indicate significant differences between plant functional groups within each land‐use type at P < 0·05.
Figure 4.
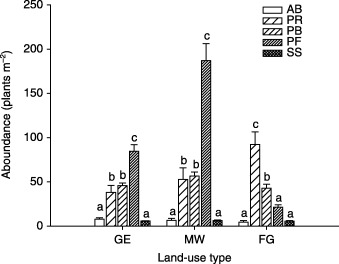
Abundance by plant functional group in three different land‐use types in a semi‐arid steppe ecosystem of northern China. Abbreviations as in Fig. 2. Letters above histograms indicate significant differences between plant functional groups within each land‐use type at P < 0·05.
Plant cover was almost the same for the PB, PF and SS functional groups on the GE plots, and about equal for the PF and SS functional groups on the MW plots, but was dominated mainly by the SS functional group on the FG plots (Fig. 5).
Figure 5.
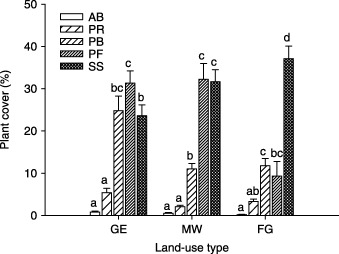
Cover by plant functional group in three different land‐use types in a semi‐arid steppe ecosystem of northern China. Abbreviations as in Fig. 2. Letters above histograms indicate significant differences between plant functional groups within each land‐use type at P < 0·05.
Discussion
The current study demonstrated that, at the local scale, the relationship between biodiversity and productivity could be strongly affected by the mode and severity of disturbance reflected in different land‐use types in the semi‐arid steppe ecosystems. The three land‐use types in this study represented three different modes and levels of disturbance: (1) minimal disturbance on the site of grazing exclusion without biomass removal (GE); (2) moderate disturbance on the site of grazing exclusion with biomass removal by mowing at ≈6 cm above the ground surface (MW); (3) severe disturbance on the site of free‐range grazing (FG). A positive relationship was found between species diversity and productivity at the community level on the minimally and moderately disturbed GE and MW plots, in line with generally recognized patterns (Tilman, Wedin & Knops 1996; Loreau et al. 2002). On the severely disturbed FG plots, however, this pervasive pattern between biodiversity and productivity was altered in such a way that a negative correlation was displayed between ANPP and species diversity.
A previous study by Osem et al. (2002) indicates that increase in species richness is related to increasing availability of soil resources in the low productivity range, and that primary productivity can reflect spatial and temporal variation in resource availability across plant communities in semi‐arid Mediterranean grassland ecosystems. In this study, the better restored soil resources may have contributed to the higher diversity and productivity on the GE and MW plots compared with the FG plots. Experimental studies on biodiversity have shown that productivity is often a decelerating monotonic function of biodiversity (Benedtti‐Cecchi 2005). At diversity levels typically found in natural plant communities, there has been no strong evidence for a direct relationship between plant diversity and productivity (Kahmen et al. 2005a; Kahmen, Perner & Buchmann 2005b). In a climate perturbation experiment, Kahmen et al. (2005b) found that increasing diversity enhanced below‐ground productivity during drought, but above‐ground productivity was reduced. In this study, we found that the relationship between ANPP and species richness was best described by an exponential growth function (R 2 = 0·99, P < 0·001, n = 24) on the GE and MW plots, and by an exponential decay function (R 2 = 0·96, P < 0·001, n = 24) on the FG plots. Our results provide further evidence that the precise mechanisms underlying the relationship between species diversity and productivity can be very complex (Huston 1997; Wardle et al. 1999; Aarssen, Laird & Pither 2003), and that the mode and severity of disturbance are critical in regulating such a relationship.
Changes in biodiversity can affect ecosystem processes through a variety of pathways, such as changes in community structure, loss of a keystone species or changes in resource‐use patterns among species (Huston 1997; Tilman 2000; Kinzig, Pacala & Tilman 2001; Hector et al. 2002). The positive diversity–productivity relationship has been largely based on the so‐called ‘insurance hypothesis’, that more diverse ecosystems are more likely to contain species that can thrive during a given environmental perturbation and compensate for declining competitors caused by that disturbance (Pimm 1984). In the semi‐arid Mediterranean rangeland, it was found that diversity of the annual plant community was determined mainly by the less abundant species (Osem et al. 2002), suggesting that higher species richness does not necessarily support higher productivity. On the less disturbed GE and MW plots, the positive relationship between species diversity and productivity may reflect the spatial variation in the relative balance between interspecific competition and facilitation among species in different functional groups.
In natural ecosystems, biodiversity can be insignificant in comparison with the overwhelming influences of environmental and anthropogenic factors on ecosystem functioning (Huston & McBride 2002). The mode and extent of disturbance could therefore dominate over the effects of biodiversity on productivity. For example, habitat conversion tends to exert a positive effect on NPP while having a negative effect on species richness (Williams et al. 2005). The pervasive effects of anthropogenic activities on both ecosystem functioning and biodiversity thus complicate the analysis of diversity–productivity relationship (Williams et al. 2005). One implication of this relationship is that an increase in the spatial variability of biodiversity can cause dramatic decreases in the mean productivity of the system (Benedtti‐Cecchi 2005). In this study, free‐range grazing represented a land‐use type with severe disturbance by grazers that intensifies the spatial variability of habitats at smaller spatial scales, creating a range of niches suitable only for specialist plant species, in addition to the regular habitats in the ecosystem. The localized patches of high species diversity on severely disturbed plots could simply reflect the greater habitat or resource heterogeneity, rather than a strategy for maintaining the integrity of ecosystem functioning when under repeated disturbance, such as free‐range grazing. On the FG plots, it was apparent that the SS functional group dominated above‐ground production, and this dominance decreased with species diversity (Fig. 3). This could further explain the negative relationship between species diversity and productivity on the severely grazed site.
Although a general decline in biodiversity occurs at a global scale, fragmentation, transformation of land and a variety of other anthropogenic disturbances may have contrasting effects on the number of species at regional or local scales (Sax, Gaines & Brown 2002; Sax & Gaines 2003). We found that, at the local scale, different land‐use practices, or severities of disturbance, caused shifts in species composition and plant functional group in the semi‐arid steppe ecosystem. The minimally disturbed GE site or moderately disturbed MW site facilitated greater species richness and plant species frequency, compared with the severely disturbed FG site, while the latter seemed to favour legume species.
Competition and facilitation for resources are two of the most decisive factors determining vegetation pattern (Armas & Pugnaire 2005; Michalet 2006). Under harsh environmental conditions, interspecific interactions, instead of facilitation, are expected to play a vital role in determining the community structure and species composition (Bellot et al. 2004; Maestre & Cortina 2004). On the FG plots, heavy grazing by sheep and cattle greatly suppressed the growth of dominant S. krylovii, and promoted the occurrence for less competitive plants such as S. viridis and K. cristata, which were completely absent on minimally or moderately disturbed GE and MW plots. Their specific biological characteristics may explain the occurrence of those species exclusively on the FG plots. Setaria viridis is an annual opportunistic species and propagates by seed (Li & Yan 2006). It can grow on bare ground created by sheep grazing and trampling. Koeleria cristata is a turfed bunchgrass that reproduces from seeds and tillers, and is primarily found in sandy or coarse‐textured soils (Wang et al. 1999). The leaf layer of K. cristata extends to only 10–15 cm above the ground surface, making the species poorly competitive on less disturbed sites where high grasses are abundant (Wang et al. 1999). Three species, O. fimbriatus, P. mongolica and E. latifolius, were found exclusively on the MW plots at relatively low frequencies. While a comprehensive explanation is not readily available for the exclusive occurrence of these species on MW plots, the activities associated with mowing may play a part in facilitating their spread under this land‐use type.
Some leguminous species, for example O. glabra, M. ruthenica, A. scaberrimus and A. galactites, had a higher frequency on the FG plots than on the GE and MW plots. These species could play important roles in maintaining site fertility by fixing atmospheric nitrogen and improving pastoral value (Bonet 2004). However, O. glabra is known to be poisonous to sheep. Several highly palatable species, including A. bidentatum, A. tenuissimum, A. neriniflorum, A. ramosum and A. senescens, were found to be better preserved in the absence of grazing on the GE and MW plots.
Anthropogenic pressure has produced quantifiable changes in the numbers, identities and abundances of species and functional groups in many habitats (Tilman & Lehman 2001). Species composition and diversity have proven to be significant determinants of ecosystem processes in grassland ecosystems (Tilman, Lehman & Thomson 1997). We found that, under the minimal or moderate disturbances such as on the GE and MW plots, there were greater contributions by perennial forbs to community ANPP, total abundance and plant cover than under severe disturbance, such as on the FG plots. Previous studies by others have shown increased diversity of native perennial plants after grazing exclusion in a grassland ecosystem in Australia (Conway 2000). On the MW plots, the improved light conditions by repeated harvesting of above‐ground biomass might be one of the major mechanisms through which species richness was maintained, as mowing decreased the cover of perennials and favoured the recruitment of short‐lived species. Bao et al. (2004) showed that, on a similar steppe ecosystem, repeated mowing resulted in replacement of the dominant rhizomatous species by short bunchgrasses after 17 years. This could account for the higher number of plant species found on the MW plots than on the GE plots.
There are many mechanisms underlying the changes in biodiversity and the relationship between diversity and productivity. Several studies have demonstrated that this relationship can arise from covariation of productivity with other abiotic or management factors, illustrating the complexity of environmental regulation of species diversity in natural communities (Gough, Grace & Taylor 1994; Schaffers 2002; Rajaniemi 2003). Moreover, the species‐redundancy hypothesis asserts that many species are so similar that ecosystem functioning is independent of diversity if major functional groups are present (Vitousek & Hooper 1993). Indeed, many factors will coexist in natural grassland ecosystems, and the relationship between biodiversity and productivity will depend largely on which driver plays a dominant role during the process. Based on the results of this study, we conclude that the mode and severity of disturbance are important factors for interpreting the relationship between species diversity and productivity in semi‐arid steppe ecosystems.
Acknowledgements
This study was partially supported by a ‘Talent Recruitment Fund’ of the Institute of Botany, Chinese Academy of Sciences to O.J.S., and by a grant from the National Natural Science Foundation of China (30521002). We wish to thank Wenming Bai, Wei Zhao, Ping Liu, Shihuan Song, Hongtao Zhao, Guangmei Wang, Zhiyou Yuan, Jin Liu, Jiaqian Tian and Wenyan Yang for assistance in carrying out field sampling, and Quansheng Chen for technical advice on data analysis. We extend our special thanks to Duolun Restoration Ecology Experimentation and Demonstration Station for permission to conduct this research on its experimental field sites. The associate editor and two anonymous reviewers are gratefully acknowledged for their critical and constructive comments on the previous manuscript.
References
- Aarssen, L.W. , Laird, R.A. & Pither, J. (2003) Is the productivity of vegetation plots higher or lower when there are more species? Variable predictions from interaction of the ‘sampling effect’ and ‘competitive dominance effect’ on the habitat templet. Oikos 102, 427–432. [Google Scholar]
- Armas, C. & Pugnaire, F.I. (2005) Plant interactions govern population dynamics in a semi‐arid plant community. Journal of Ecology 93, 978–989. [Google Scholar]
- Bai, Y.F. , Han, X.G. , Wu, J.G. , Chen, Z.Z. & Li, L.H. (2004) Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature 431, 181–184. [DOI] [PubMed] [Google Scholar]
- Bao, Y.J. , Li, Z.H. & Zhong, Y.K. (2004) Compositional dynamics of plant functional groups and their effects on stability of community ANPP during 17 yr of mowing succession on Leymus chinensis steppe of Inner Mongolia, China. Acta Botanica Sinica 46, 1155–1162. [Google Scholar]
- Baoyin, T. & Liu, D. (2001) The changing of ploughland and analysis of problem of Duo Lun County on agriculture–animal husbandry ecotone. Acta Scientiarum Naturalium Universitatis Neimongol 32, 657–660. [Google Scholar]
- Bellot, J. , Maestre, F.T. , Chirino, E. , Hernandez, E. & Ortiz de Urbina, J. (2004) Afforestation with Pinus halepensis reduces native shrub performance in a Mediterranean semi‐arid area. Acta Oecologica 25, 7–15. [Google Scholar]
- Benedtti‐Cecchi, L. (2005) Unanticipated impacts of spatial variance of biodiversity on plant productivity. Ecology Letters 8, 791–799. [Google Scholar]
- Berendse, F. (1998) Effects of dominant plant species on soils during succession in nutrient poor ecosystems. Biogeochemistry 42, 73–88. [Google Scholar]
- Bonet, A. (2004) Secondary succession of semi‐arid Mediterranean old‐fields in south‐eastern Spain: insights for conservation and restoration of degraded lands. Journal of Arid Environments 56, 213–233. [Google Scholar]
- Cardinale, B.J. , Nelson, K. & Palmer, M.A. (2000) Linking species diversity to the functioning of ecosystems: on the importance of environmental context. Oikos 91, 175–183. [Google Scholar]
- Chapin, F.S. , Walker, B.H. , Hobbs, R.J. , Hooper, D.U. , Lawton, J.H. , Sala, D.E. & Tilman, D. (1997) Biotic control over the functioning of ecosystems. Science 277, 500–504. [Google Scholar]
- Collins, S.L. (1987) Interaction of disturbances in tallgrass prairie: a field experiment. Ecology 68, 1243–1250. [Google Scholar]
- Conway, M.G. (2000) The effects of grazing exclusion on a long‐grazed species‐rich Riverina grassland. Honours Thesis, Charles Sturt University, Wagga Wagga, NSW, Australia.
- Duncan, R.P. , Colhoun, K.M. & Foran, D.B. (1997) The distribution and abundance of Hieracium species (hawkweeds) in the dry grasslands of Canterbury and Otago. New Zealand Journal of Ecology 21, 51–62. [Google Scholar]
- Ehrlich, P.R. & Ehrlich, A.H. (1981) Extinction. The Causes and Consequences of the Disappearance of Species. Random House, New York. [Google Scholar]
- FAO‐UNESCO (1974) Soil Map of the World at 1 : 5 000 000, I. Legend. UNESCO, Paris. [Google Scholar]
- Gough, L. , Grace, J.B. & Taylor, K.L. (1994) The relationship between species richness and community biomass – the importance of environmental variables. Oikos 70, 271–279. [Google Scholar]
- Grime, J.P. (1998) Benefits of plant diversity to ecosystems: immediate, filter and founder effects. Journal of Ecology 86, 902–910. [Google Scholar]
- Guo, Q.F. (2005) Ecosystem maturity and performance. Nature 435, E6. [DOI] [PubMed] [Google Scholar]
- He, J.S. , Fang, J.Y. , Ma, K.P. & Huang, J.H. (2003) Biodiversity and ecosystem productivity: why is there a discrepancy in the relationship between experimental and natural ecosystems? Acta Phytoecologica Sinica 27, 835–843. [Google Scholar]
- Hector, A. , Bazeley‐White, E. , Loreau, M. , Otway, S. & Schmid, B. (2002) Overyielding in grassland communities: testing the sampling effect hypothesis with replicated biodiversity experiments. Ecology Letters 5, 502–511. [Google Scholar]
- Hooper, D.U. & Vitousek, P.M. (1997) The effects of plant composition and diversity on ecosystem processes. Science 277, 1302–1305. [Google Scholar]
- Huston, M.A. (1997) Hidden treatments in ecological experiments: re‐evaluating the ecosystem function of biodiversity. Oecologia 110, 449–460. [DOI] [PubMed] [Google Scholar]
- Huston, M.A. & McBride, A.C. (2002) Evaluating the relative strengths of biotic versus abiotic controls on ecosystem processes Biodiversity and Ecosystem Functioning (eds Loreau M., Naeem S. & Inchausti P.), pp. 47–60. Oxford University Press, New York. [Google Scholar]
- Kahmen, A. , Perner, J. , Audorff, V. , Weisser, W. & Buchmann, N. (2005a) Effects of plant diversity, community composition and environmental parameters on productivity in montane European grasslands. Oecologia 142, 606–615. [DOI] [PubMed] [Google Scholar]
- Kahmen, A. , Perner, J. & Buchmann, N. (2005b) Diversity‐dependent productivity in semi‐natural grasslands following climate perturbations. Functional Ecology 19, 594–601. [Google Scholar]
- Kinzig, A. , Pacala, S.P. & Tilman, D. (2001) The Functional Consequences of Biodiversity. Princeton University Press, Princeton, NJ, USA. [Google Scholar]
- Li, R.P. & Yan, Q.L. (2006) Effects of grazing and mowing on the meadow vegetation succession in Kerqin steppe. Journal of Arid Land Resources and Environment 20, 180–184. [Google Scholar]
- Liu, Q.Y. & Tong, Y.P. (2003) The effects of land use on the eco‐environmental evolution of farming‐pastoral region in North China: with an emphasis on Duolun County in Inner Mongolia. Acta Ecologica Sinica 23, 1025–1030. [Google Scholar]
- Loreau, M. , Naeem, S. & Inchausti, P. (2002) Biodiversity and Ecosystem Functioning: Synthesis and Perspectives. Oxford University Press, Oxford, UK. [Google Scholar]
- Maestre, F.T. & Cortina, J. (2004) Do positive interactions increase with abiotic stress? A test from a semi‐arid steppe. Proceedings of the Royal Society of London B 271, S331–S333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton, S.J. (1983) Serengeti grassland ecology: the role of composite environmental factors and contingency in community organization. Ecological Monographs 53, 291–320. [Google Scholar]
- Michalet, R. (2006) Is facilitation in arid environments the result of direct or complex interactions? New Phytologist 169, 3–6. [DOI] [PubMed] [Google Scholar]
- Mittelbach, G.G. , Steiner, C.F. , Scheiner, S.M. et al. (2001) What is the observed relationship between species richness and productivity? Ecology 82, 2381–2396. [Google Scholar]
- Naeem, S. , Thompson, L.J. , Lawler, S.P. , Lawton, J.H. & Woodfin, R.M. (1994) Declining diversity can alter the performance of ecosystems. Nature 368, 734–737. [Google Scholar]
- Naeem, S. , Thompson, L.J. , Lawler, S.P. , Lawton, J.H. & Woodfin, R.M. (1995) Empirical evidence that declining species diversity may alter the performance of terrestrial ecosystems. Philosophical Transactions of the Royal Society B: Biology Sciences 347, 249–262. [Google Scholar]
- Naeem, S. , Hakansson, K. , Lawton, J.H. et al. (1996) Biodiversity and plant productivity in a model assemblage of plant species. Oikos 76, 259–264. [Google Scholar]
- Osem, Y. , Perevolotyky, A. & Kigel, J. (2002) Grazing effect on diversity of annual plant communities in a semi‐arid rangeland: interactions with small‐scale spatial and temporal variation in primary productivity. Journal of Ecology 90, 936–946. [Google Scholar]
- Petraitis, P. , Latham, R. & Niesenbaum, R. (1989) The maintenance of species diversity by disturbance. Quarterly Review of Biology 64, 393–418. [Google Scholar]
- Pimm, S.L. (1984) The complexity and stability of ecosystems. Nature 307, 321–325. [Google Scholar]
- Pimm, S.L. , Russell, G.J. , Gittleman, J.L. & Brooks, T.M. (1995) The future of biodiversity. Science 269, 347–350. [DOI] [PubMed] [Google Scholar]
- Rajaniemi, T.K. (2003) Explaining productivity–diversity relationships in plants. Oikos 101, 449–457. [Google Scholar]
- Sax, D.F. & Gaines, S.D. (2003) Species diversity: from global decreases to local increases. Trends in Ecology and Evolution 18, 561–566. [Google Scholar]
- Sax, D.F. , Gaines, S.D. & Brown, J.H. (2002) Species invasions exceed extinctions on islands worldwide: a comparative study on plants and birds. American Naturalist 160, 766–783. [DOI] [PubMed] [Google Scholar]
- Schaffers, A.P. (2002) Soil, biomass, and management of semi‐natural vegetation – Part II. Factors controlling species diversity. Plant Ecology 158, 247–268. [Google Scholar]
- Schlapfer, F. & Schmid, B. (1999) Ecosystem effects of biodiversity – a classification of hypotheses and cross‐system exploration of emperical results. Ecological Applications 9, 893–912. [Google Scholar]
- Seabloom, E.W. & Richards, S.A. (2003) Multiple stable equilibria in grasslands mediated by herbivore population dynamics and foraging behavior. Ecology 84, 2891–2904. [Google Scholar]
- Symstad, A.J. , Tilman, D. , Willson, J. & Knops, J.M.H. (1998) Species loss and ecosystem functioning: effects of species identity and community composition. Oikos 81, 389–397. [Google Scholar]
- Tilman, D. (2000) Causes, consequences and ethics of biodiversity. Nature 405, 208–211. [DOI] [PubMed] [Google Scholar]
- Tilman, D. & Downing, J.A. (1994) Biodiversity and stability in grasslands. Nature 367, 363–365. [Google Scholar]
- Tilman, D. & Lehman, C. (2001) Human‐caused environmental change: impacts on plant diversity and evolution. Proceedings of the National Academy of Sciences, USA 98, 5433–5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman, D. , Wedin, D. & Knops, J. (1996) Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 379, 718–720. [Google Scholar]
- Tilman, D. , Lehman, C.L. & Thomson, K.T. (1997) Plant diversity and ecosystem productivity: theoretical considerations. Ecology 94, 1857–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troumbis, A.Y. (2001) No observational evidence for diversity enhancing productivity in Mediterranean shrublands? A reply to Wardle. Oecologia 129, 622–623. [DOI] [PubMed] [Google Scholar]
- Vitousek, P.M. & Hooper, D.U. (1993) Biological diversity and terrestrial ecosystem biogeochemistry Biodiversity and Ecosystem Function (eds Schulze E.D. & Mooney H.A.), pp. 3–14. Springer‐Verlag, Berlin. [Google Scholar]
- Waid, R.B. , Willig, M.R. , Steiner, C.F. , Mittelbach, G. , Gough, L. , Dodson, S.I. , Judag, G.P. & Parmenter, R. (1999) The relationship between productivity and species richness Annual Review of Ecology & Systematics 30, 257–300. [Google Scholar]
- Wang, S.P. , Wang, Y.F. & Chen, Z.Z. (2003) Chapter 2. The relationship between plant community productivity and forage quality The Management of Grazing Ecosystems (eds Wang S.P., Wang Y.F. & Chen Z.Z.), pp. 45–59. China Science Press, Beijing. [Google Scholar]
- Wang, S.P. , Niu, H.S. , Cui, X.Y. et al. (2005) Ecosystem stability in Inner Mongolia. Nature 435, E5–E6. [DOI] [PubMed] [Google Scholar]
- Wang, W. , Liang, C.Z. , Liu, Z.L. & Hao, D.Y. (1999) Research on restoring succession of degenerated grassland in Inner Mongolia. IV. Analysis of plant population dynamics during restoring succession. Journal of Arid Land Resources and Environment 13 (4), 44–55. [Google Scholar]
- Wardle, D.A. (2001) No observational evidence for diversity enhancing productivity in Mediterranean shrublands. Oecologia 129, 620–621. [DOI] [PubMed] [Google Scholar]
- Wardle, D.A. , Bonner, K.I. , Barker, G.M. et al. (1999) Plant removals in perennial grassland: vegetation dynamics, decomposers, soil biodiversity, and ecosystem properties. Ecological Monographs 69, 535–568. [Google Scholar]
- Williams, J.W. , Seabloom, E.W. , Slayback, D. , Stoms, D.M. & Viers, J.H. (2005) Anthropogenic impacts upon plant species richness and net primary productivity in California. Ecology Letters 8, 127–137. [Google Scholar]
- You, L.Y. , Lu, J.F. , Chen, H. & Zhou, J.X. (2003) Adjustment of land use types for desertification control and prevention: taking Duolun County of Inner Mongolia as a typical case. Geographical Research 22, 680–686. [Google Scholar]
- Zhan, J.Y. , Deng, X.Z. , Yue, T.X. , Bao, Y.H. , Zhao, T. & Ma, S.N. (2004) Land use change and its environmental effects in the farming–pasturing interlocked areas of Inner Mongolia. Resources Science 26, 80–88. [Google Scholar]


