Abstract
Pennsylvania has the third highest rate of death due to drug overdose (44.3 per 100,000) in the country, which is significantly higher than the national rate. This continues to have drastic societal impact. Medication assisted treatment (MAT), which includes opioid agonist medications, is the gold standard in treatment for OUD; however, a significant gap remains between the number of individuals in need of treatment and the number of MAT providers. Penn State Health established a system to address the opioid epidemic through the Pennsylvania Coordinated Medication Assisted Treatment program utilizing lessons learned from existing validated models. Connecting primary care sites and hospital systems through a combination of Hub and Spoke, bridge clinic services provided at the Hub, peer recovery services, Project Extension for Community Health Outcomes (ECHO), and layered emergency department (ED) initiation of buprenorphine, this model is an innovative approach that addresses many known barriers to MAT treatment initiation. Early results within the first six months indicate significantly shortened wait time for patients seeking treatment, provision of waiver training to 70 local physicians to prescribe buprenorphine, and improved knowledge and ability to provide patient care for providers participating in our first Project ECHO cohort.
Keywords: Medication assisted treatment, Opioid, Addiction, Hub-and-spoke system
1. Introduction
Opioid use disorder (OUD) is one of the largest health crises of this generation. With drug overdose deaths increasing to over 70,000 in 2017 (Drug Overdose Death Data) and those resulting from opioid misuse at over 47,000 (Drug Overdose Death Data), the United States (US) has surpassed the number of deaths experienced at the peak of the HIV/AIDS epidemic in the 1990s (Case & Deaton, 2015). In 2017, Pennsylvania had the third highest rate of death due to drug—primarily but not exclusively opioid—overdose (44.3 per 100,000) in the country, which was significantly higher than the national rate (21.7 per 100,000, (Centers for Disease Control, 2019). Through the Pennsylvania Coordinated Medication Assisted Treatment (PacMAT) program, Centers of Excellence, and the expansion of Medicaid in Pennsylvania (72,675 OUD patients covered in 2017), treatment for OUD patients became more accessible. Medication assisted treatment (MAT), which includes opioid agonist and partial agonist medications such as methadone and buprenorphine respectively, is the gold standard in evidence-based treatment for OUD with survival and treatment retention rates up to seven times that of drug-free modalities (Connery, 2015). Despite having effective treatment, a significant gap exists between the number of individuals in need of treatment, approximately 2.5 million (Substance Abuse and Mental Health Administration, 2014), and the number actually receiving treatment. This disparity exists, at least in part, because of the relatively small number of MAT providers, approximately 68,000 (Substance Abuse and Mental Health Association, 2019). Certainly, increasing the number of providers would be a key element of any effective intervention, but it is not enough to have more providers; the providers must be where they are needed. The overdose death rates in rural counties is approaching those in urban settings, increasing the urgency to have MAT providers in rural communities. As of June 2018, the number of buprenorphine treatment providers (n = 24) within a 25-mile radius of the city of Harrisburg was the same (n = 24) within a 50-mile radius, demonstrating the geographic disparity in access to treatment (Substance Abuse and Mental Health Association, 2019).
While geography is a serious and common barrier to increasing access to care, it is not the only one. To be authorized to prescribe buprenorphine, the most commonly used medication for outpatient treatment of OUD, physicians must obtain a waiver under the Drug Addiction Treatment Act (DATA). Currently, about 5% of practicing US physicians are DATA-waivered, and most waivered providers do not prescribe to their maximum waiver capacity (Huhn & Dunn, 2017). Lack of confidence in providing MAT among physicians further compounds the problem (Bart, 2012), and is reinforced by limited access to addiction experts for consultation and referral if necessary. Other reasons include the lack of multiple providers within one clinic to prescribe buprenorphine, the lack of mental health and other psychosocial support within the clinic, and lack of institutional support (Hutchinson, Catlin, Andrilla, Baldwin, & Rosenblatt, 2014). Moreover, stigma against those with OUD remains prevalent among prescribers and among those struggling with OUD (Can & Tanrıverdi, 2015). Therefore, critical next steps to combatting the overdose epidemic include both increasing the number of providers who are DATA-waived and increasing provider confidence to provide MAT at full capacity.
Any system to intervene in the overdose epidemic must first understand and build on effective system-based models of MAT delivery. Vermont’s Hub and Spoke system is an example of capacity expansion in which a Hub (i.e., a specialty clinic with addiction medicine specialists) is connected with spoke clinics that provide frontline care and referral for patients with OUD. Vermont’s system, initiated in 2012, led to a 64% increase in DATA-waived physicians as well as a 50% increase in patients served per physician waivered (Brooklyn & Sigmon, 2017). With its origin at the University of New Mexico, Project ECHO (Extension for Community Healthcare Outcomes) is a collaborative model of medical education and care management, increasing access to treatment in rural and underserved areas by engaging specialist teams with primary care providers in virtual case-based discussion and brief lectures that has been readily deployed to expand MAT access. Project ECHO began addressing OUD in 2005 and increased the number of DATA-waived providers, elevating New Mexico’s status to fourth ranked state for DATA-waived physicians per capita (Komaromy et al., 2016; Komaromy, Bartlett, Manis, & Arora, 2017). Replications of this model have demonstrated improved provider self-efficacy and confidence in managing patients who are prescribed MAT (Dubin et al., 2015).
Increasing MAT capacity by expanding the number of waivered physicians and improving their confidence to treat addresses only one of the obstacles to overcoming the overdose epidemic. Logistical challenges connecting a patient to care rapidly must be solved: once a patient is identified, that patient must be referred to and started on treatment quickly to reduce the risk of overdose while awaiting care. In Massachusetts, the Massachusetts General Hospital’s bridge clinic has helped patients obtain buprenorphine quickly, leading to significant increases in treatment retention at 30 days (Hostetter & Klein, 2017). At the Yale emergency department, DATA-waived emergency medicine physicians facilitated a warm hand-off twice as successfully as matched controls who did not receive buprenorphine in the emergency room (D’Onofrio et al., 2015).
A public health challenge as serious at the overdose epidemic demands all demonstrated effective interventions be used simultaneously. This paper describes a systems approach to addressing MAT expansion and treatment initiation in Central Pennsylvania, a unique hybrid of Hub and Spoke, bridge clinic and emergency department (ED)-initiated buprenorphine. An OUD focused Project ECHO further supports the spoke providers to overcome inadequate MAT knowledge and experience, poor access to resources and support, and a negative perception of treating OUD patients.
2. Methods
Penn State established a system in 2017 (Fig. 1) to address the overdose epidemic, supported by the Pennsylvania Department of Health-funded PacMAT Program. Our mission was to provide and expand comprehensive, patient-centered and innovative evidence-based treatment to individuals with substance use disorder in south central Pennsylvania. Using existing validated models, this initiative connected primary care sites and hospital systems through a combination of Hub and spoke, bridge clinic services provided at the Hub, peer recovery services, and layered emergency department (ED) initiation of buprenorphine. Treatment retention was an important variable of interest, and the model was designed to maximize retention so that an impact could be made on the overdose death rates in the region. A year after the Penn State system started treating substance use disorder, it expanded its ability to reach rural communities of Pennsylvania through addition of Project ECHO to provide clinical support to our spokes and other providers across the region. The Hub and Spoke element of the system ameliorated the isolation of specialty addiction treatment from other medical and psychiatric services. The system’s bridge clinic services decreased patient wait time, allowed immediate engagement in treatment and decreased risk of treatment drop out while awaiting an appointment at the preferred treatment setting. As Project ECHO sessions wove together providers of care, peer recovery services removed barriers separating one level of care from another, and allowed patients to move efficiently among settings as appropriate and also access peer-led support groups. Further, the program partnered with Emergency Departments as first points of contact for many people suffering from OUD and established Emergency Department initiation of buprenorphine as a route to rapid MAT access. We describe components of this program below.
Fig. 1.
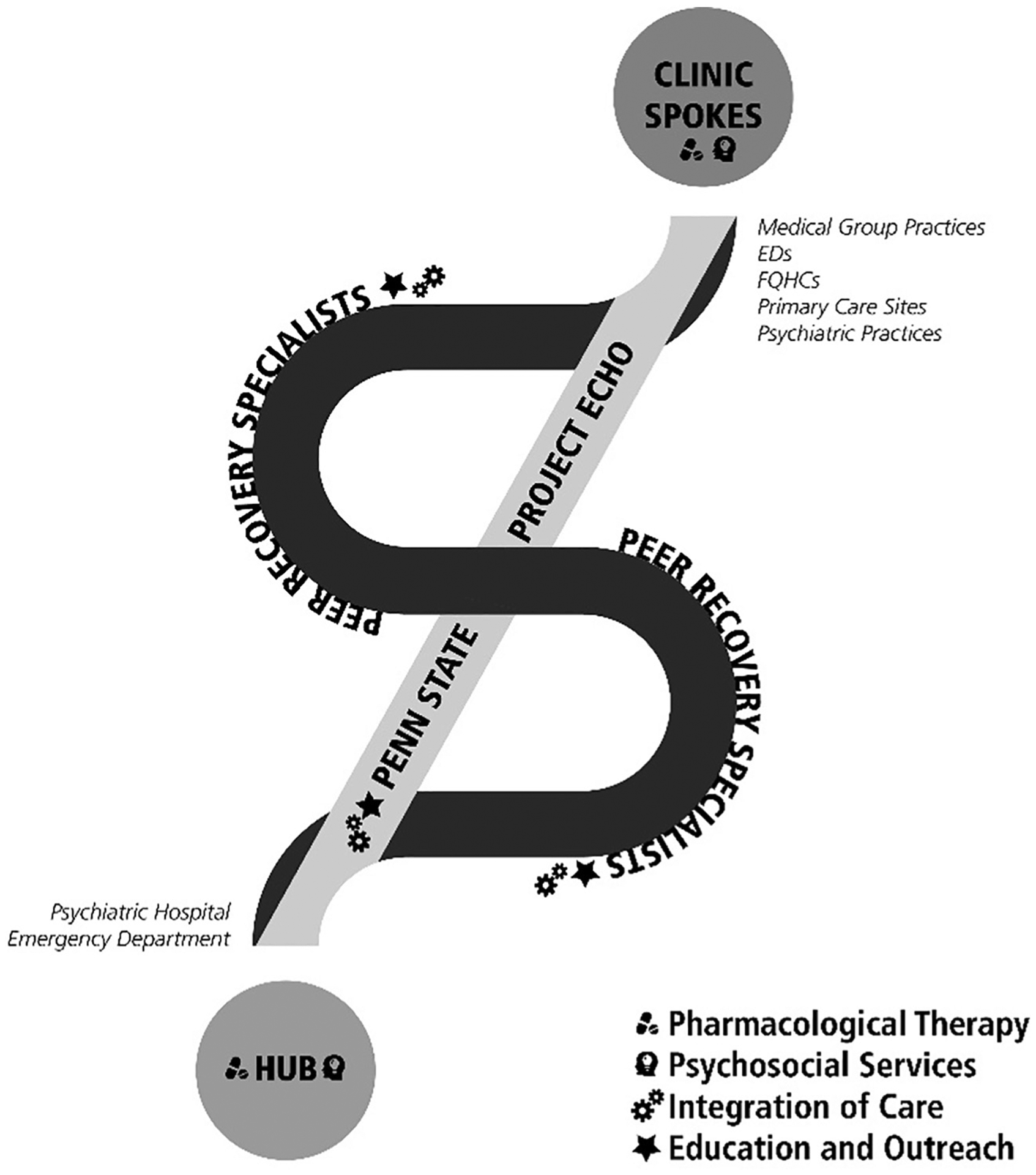
Central PA MAT system of care.
2.1. Catchment demographics
Paralleling the national trend, Pennsylvania’s high rates of drug overdose deaths were not restricted to urban areas, as rates of drug-related overdose deaths in rural counties approached those of urban settings. At the time treatment for substance use disorder launched at Penn State Health, the health system drew from a population in six south central Pennsylvanian counties, five of which are rural: Cumberland, Dauphin, Franklin, Lancaster, Lebanon, and York (Fig. 2). In the catchment area, rates of overdose deaths ranged from 21 to 39 per 100,000 residents. Although the catchment area focused in the six counties described above, the patients served came from as many as ten surrounding counties.
Fig. 2.
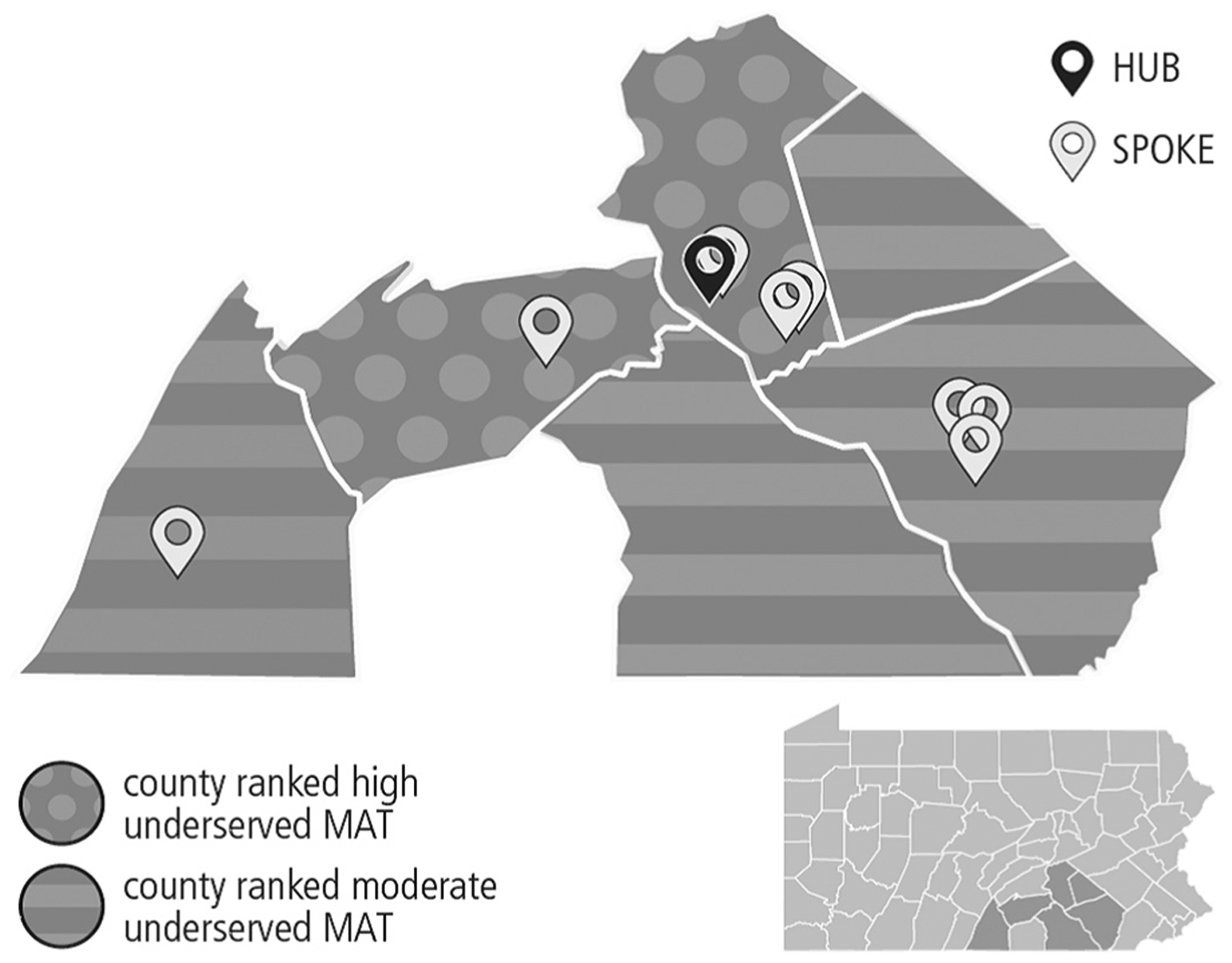
Hub and Spoke geographic catchment area
2.2. Protection of human subjects
The Human Subjects Protection Office at the Penn State College of Medicine determined the activities described did not require formal IRB review because the research met the criteria for exempt research according to the policies of the institution and provisions of applicable federal regulations.
2.3. Hub
The system Hub—located in the city of Harrisburg, Dauphin County—was licensed as an opioid treatment program (OTP) located within a psychiatric hospital. The OTP opened providing all forms of MAT (methadone, daily dosed buprenorphine-naloxone, prescribed buprenorphine-naloxone and extended-release naltrexone) in combination with comprehensive OUD psychosocial services. Two part-time physicians and a full-time certified nurse practitioner practiced on site, as well as several residents and fellow physicians from Penn State Health rotating through the site for teaching and patient care. Nine counselors practiced on site at a patient-counselor ratio of 35:1 to provide individual counseling and outpatient level services. In addition, the Hub linked with psychiatric services within the hospital, including inpatient treatment and partial hospitalization. A full-time certified recovery specialist worked with patients on vocational rehabilitation to assist patients who are ready to reenter the workforce. Additionally, the clinic used contingency management—an evidence-based behavioral intervention based on applied behavior analysis—as an incentive to keep patients participating at their recommended level of care (Petry, Alessi, Olmstead, Rash, & Zajac, 2017). Patient rewards total—at most—$50 per patient per year were budgeted in-kind.
The Hub provided daily, directly-observed therapy for patients, serving as an intensive outpatient clinic in support of 12 surrounding primary care, emergency department and primary psychiatric spoke sites. The Hub’s engagement with spoke sites primarily existed as a higher level of support with daily medication treatment and on-site counseling with the goal of returning patients to spoke sites once stabilized. Examples of Hub patients included pregnant women and patients requiring “urgent care” or “bridge appointments” in need of medication initiation within 24–48 h after identification prior to their first appointment at a spoke site. To improve the ability of the Hub to reach new patients, its affiliated emergency department was rendered capable of initiating buprenorphine on site and transferring patients to the Hub for treatment intake within 24–48 h. Finally, the Hub fostered strong relationships with a county jail, drug court and the local county drug and alcohol authority. Patients engaged in work-release or going through diversion courts, as well as those who need county funds for MAT, received services at this clinic.
2.4. Clinic spokes
The spoke clinics included 18 buprenorphine waivered physicians across 12 spoke sites from Penn State Medical Group practices, the Milton S. Hershey Medical Center Emergency Department, several federally qualified health centers, and primary care sites accepting Medicaid patients and psychiatric practices. Spoke sites accessed enhanced care management to help patients navigate from spoke to Hub and Hub to spoke, providing higher levels of care when needed. A phone call by the patient or provider to the care manager led to a hand-off that facilitated patients’ transition to Hub for medication stabilization, either with daily methadone or buprenorphine. This allowed for greater treatment retention for unstable patients. On those occasions when a patient at a spoke site wished to initiate care, but no appointment was available at the spoke—see Table 1— the spoke site could access the system’s urgent care or “bridge” services at the Hub, allowing patients to begin treatment rapidly and return to the spoke at the next available appointment for MAT. Bridge services reduced barriers and prevented patients from being lost to care. Potential patient visit wait times varied depending on the number of full-day clinics available per month, per spoke prior to our clinic opening (see Table 1). This system of care supported physicians with utilizing administrative time, completing paperwork needed for insurance companies or disability applications, and following up with any other question or patient need.
Table 1.
Example of 8 spoke clinic potential visit wait times prior to Hub-and-Spoke initiation.
| Clinic site | Number of full-day clinics per month | Range of potential wait until next available appointment |
|---|---|---|
| Site 1 | 4 | 1–21 days |
| Site 2 | 20 | 1–3 days |
| Site 3 | 20 | 1–3 days |
| Site 4 | 8 | 1–5 days |
| Site 5 | 4 | 1–14 days |
| Site 6 | 4 | 1–7 days |
| Site 7 | 2 | 7–21 days |
| Site 8 | 8 | 1–5 days |
2.5. Peer recovery specialist services
Peer-based recovery support services using peer recovery specialists, also referred to as care managers or care management support, facilitated hand-offs between spoke and Hub in this system. These specialists, who were in long-term recovery themselves, ensured that patients adhered to medical and behavioral health appointments while navigating them through care transitions. Each spoke site had a corresponding peer recovery specialist supported by Medicaid behavioral health reimbursement and grants, through a non-profit organization called the Recovery, Advocacy, Service and Empowerment (RASE) project. Peer recovery specialists assisted patient transitions to different care settings by providing transportation, help with scheduling appointments, and support at appointments. Their consistent involvement in a patient’s care reduced stigma and a patient’s feeling of being dismissed by spoke sites. Peer support specialists also coordinated location-appropriate and convenient MAT access based on individual patient needs, insurance enrollment navigation, and established connections with peer-led community support networks, including sober support networks.
2.6. Emergency department
All providers at Milton S. Hershey Medical Center Emergency Department (ED) received training to become buprenorphine waivered and prescribed buprenorphine to patients seen after overdose or in withdrawal since April 2018. As such, the affiliated ED became one of very few in Pennsylvania with a completely buprenorphine waivered provider staff. ED integration was only possible by combining a physician champion, support from senior leadership, and a protocol designed to ensure our ED providers did not feel they were practicing outside of their scope of practice. Importantly, our OTP clinic served as a reliable aftercare resource following treatment initiation. We also established procedures to minimize multiple ED visits within a 30 day period for patients, so that our ED providers would prescribe a maximum of 2 days of medication per patient. Once a patient with substance use disorder was identified, ED social workers called the 24-hour admissions line to make an appointment at the Hub within 1–2 days.
2.7. Project ECHO
Project ECHO is a model with the power to rapidly transfer knowledge and exponentially increase capacity to deliver best-practice care to underserved populations (Arora et al., 2014). The ECHO model has four core principles: (1) use technology to leverage scarce resources; (2) share best practices to reduce disparities; (3) employ case-based learning to master complexity; (4) monitor outcomes to ensure benefit. ECHO sessions use videoconferencing technology as a platform for telementoring and collaborative care, with a Hub and Spoke structure (Fig. 3). The ECHO model is not “telemedicine” where specialists assume the care of the patient; it is a guided practice model aimed at practice improvement, in which the PCP retains responsibility for managing the patient, operating with increasing independence as skills, confidence, and self-efficacy grow (Komaromy et al., 2016). The ECHO model evaluates outcomes within Moore’s Levels for CME framework (Davis, Barnes, & Fox, 2003). At the provider level, these outcomes include participation, satisfaction, learning, competence, and performance.
Fig. 3.
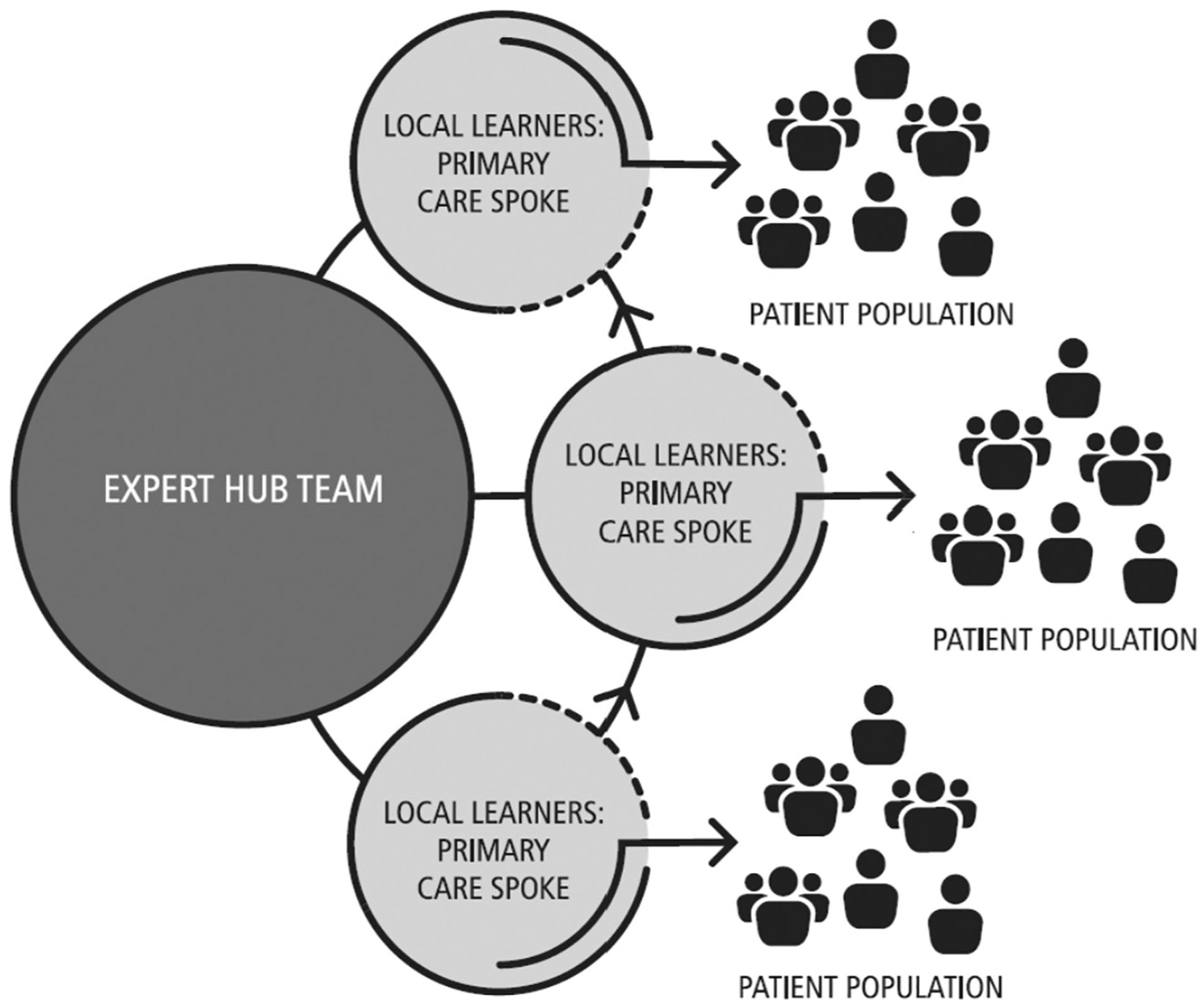
Hub-and-Spoke Project ECHO model.
Project ECHO launched at Penn State in November 2018 to address OUD within the PacMAT model. In the first cohort, twenty newly DATA-waivered providers enrolled to participate in twelve, one-hour sessions given every other week. Community-based providers (the spokes) participated in weekly teleECHO clinics with a multi-disciplinary specialty team at Penn State that included Addiction Medicine specialists, a pharmacist, a peer recovery specialist, social workers, and guest experts including an obstetrician, hepatologist, psychologist and psychiatrist. Weekly teleECHO sessions featured presentations of deidentified patient cases by primary care providers and team members. The ECHO specialist and primary care teams collaborated to discuss the case and develop recommendations for patient care. Following the case discussion, a brief lecture was given by one of the specialists. After each session, participants received a short survey asking questions about whether or not that particular session resulted in increased knowledge, decreased professional isolation, an improved ability provide appropriate care to patients, satisfaction with the information provided, and relevance to their practice.
3. Results
The Hub introduced different system components at different times, beginning in November 2017 with the introduction of Hub and Spoke clinics and bridge visits. ED initiation of buprenorphine commenced in April 2018 with 30 newly waivered physicians. Project ECHO sessions began in November 2018. Results reported reflect the first six months of clinic operation, including Hub and Spoke, bridge clinic, and one month of ED buprenorphine initiation.
3.1. Preliminary treatment patterns
Within the first 6 months of the Hub opening, 222 intakes were scheduled and 180 patients were seen, with a retention-to-intake rate of 81%. There were no recorded reasons for the 42 patients who did not appear for intake. Of the 180 patients, 62%, 37%, and 1% identified as male, female, and transgender, respectively (Table 2). The average age of this patient population was 36.3 years, with the majority identifying as Caucasian or white (82%). Of the 180 patients, 127 began buprenorphine treatment, 9 began extended-release naltrexone treatment, and 30 began methadone treatment. 14 patients declined any form of MAT. Of the 14, 3 were referred to an outside treatment center, 9 declined any MAT and 2 desired counseling only. When stratifying the population by MAT type, the distribution of methadone, buprenorphine and extended-release naltrexone were proportionate between male and female clients.
Table 2.
Demographics of patients seen within the first six months (November 2017–May 2018).
| Demographics | ||
|---|---|---|
| Age (M; SD) | 36.3 (11.33) | |
| Gender | ||
| Male | 112 | 62% |
| Female | 67 | 37% |
| Transgender | 1 | 1% |
| aRace | ||
| Caucasian | 147 | 82% |
| African American | 29 | 16% |
| Asian | 3 | 2% |
| Ethnicity | ||
| Non-Hispanic/Latino | 165 | 92% |
| Hispanic/Latino | 15 | 8% |
One reported race as “unknown”.
3.2. Current treatment pattern
As of April 2019, the Hub treated over 600 individuals with 352 in active treatment. In addition, 12 spoke sites treated 306 active patients. The spoke sites consisted of six primary care clinics, one psychiatric clinic, one pain clinic, two EDs and two probation/parole and county drug courts (Table 3). Ninety-two patients visited the Hub for a bridge appointment (seen within 24 h) only, subsequently followed up at a spoke site, and 36 patients continued treatment with the Hub after being initiated from the ED. Treatment retention rate was defined as appointments kept within thirty days of data capture. During the first six months of the hub program 43% of buprenorphine patients, 11% of extended-release naltrexone patients, and 63% of methadone patients were retained in treatment. Future analyses will determine treatment retention rates of each type of MAT for our total patient count at the end of the grant period in September 2019.
Table 3.
Total number of patients seen per clinic type (November 2017–May 2018).
| Spoke site type | Location | Total patients |
|---|---|---|
| Primary care FQHC-Clinic 1 | Cumberland County | 31 |
| Primary care FQHC-Clinic 2 | Cumberland County | 39 |
| Primary care FQHC-Clinic 1 | Lancaster county | 20 |
| Primary care FQHC-Clinic 2 | Lancaster county | 20 |
| Primary psychiatry | Lebanon County | 42 |
| Primary care-Clinic 1 | Dauphin County | 5 |
| Primary care-Clinic 2 | Dauphin County | 37 |
| Pain clinic | Franklin County | 21 |
| Emergency Department-1 | Dauphin County | 9 |
| Emergency Department-2 | Dauphin County | 27 |
| Probation/parole | Dauphin county | 5 |
| Opioid Intervention Court | Cumberland County | 10 |
3.3. Project ECHO
The first Penn State ECHO OUD cohort included twenty primary care providers from several specialties, including general internal medicine, family medicine and emergency medicine. Together, these participants treated a combined 373 OUD patients in their practices. The majority of ECHO participants (70%) provided care at our spoke clinics, but we did include several participants from outside our hub-and-spoke system. Through Project ECHO, we reviewed nine patient cases and provided 105 h of CME with positive provider feedback. Results included increased knowledge of evidence-based OUD management (n = 109/121, 90%), decreased sense of professional isolation (n = 101/121, 83%), and self-reported improvement in ability to provide MAT to OUD patients (n = 107/121, 88%). In addition, the Project ECHO platform, Zoom, provided virtual DATA-waiver training to 41 physicians in the state.
4. Discussion
Penn State implemented a system of care in which patients received timely treatment close to home and physicians felt supported and confident to treat opioid use disorder. Building from validated models for MAT treatment and capacity expansion, Penn State has launched an innovative system of care for OUD patients in central Pennsylvania that has the potential to address many known barriers to MAT treatment initiation. Offering all forms of approved MAT for OUD combined with advanced case management and treatment support services has allowed our program to begin to fill the gap between those who need treatment for OUD and those who actually receive it. We have begun to address the need for expanded MAT offering in the primary care and emergency medicine settings by supporting 70 local physicians to receive buprenorphine waiver training during our first year, including all of the ED physicians at Penn State to facilitate acute treatment protocols. For those newly waivered physicians, our OUD Project ECHO provides ongoing peer and expert support and education.
Our initial retention rates from the first six months of the Hub opening are consistent with other retention averages (Hser et al., 2014; Larochelle et al., 2018). We attribute these unremarkable results to several factors. First, we implemented a unique model of care as a new program. The launch of any clinical intervention demands careful navigation of state and federal regulations and de novo development of processes, procedures, and practice improvement. Establishing both our hub treatment programs and our coordination with the peer recovery specialist network out of the hub required six months’ effort, equivalent to the time of the data collection. Moreover, the launch of our Hub, hub-and-spoke and ED services were staggered, and not all were available at the time of data collection. We are hopeful that future data reviews will find a significant increase in our retention rates.
There are many reasons for the rise of the overdose epidemic, which has developed over time. Accordingly, addressing it will take a complex, multi-modal approach involving prevention as well as treatment. A recent review of 12 successful primary care based MAT models (Korthuis et al., 2017) showed all contained some degree of four key components: (1) pharmacological therapy; (2) psychosocial services; (3) integration of care; and (4) education and outreach. The Penn State model discussed here incorporates all components, with the core Hub and Spoke system aligned closely with the Vermont approach (Brooklyn & Sigmon, 2017) that brings care to a large area of small cities interspersed amidst a widely scattered rural population, similar to that in central Pennsylvania. The care continuum aspect of Hub and Spoke is an important model for both patients and providers; patients can receive access to medication in a primary care spoke site location convenient and familiar to them and providers are able to transfer patients requiring a higher level of care to the hub. There is a key difference between the Vermont and Pennsylvania model worth noting. Vermont’s model supports nurse care managers at every level of patient engagement provided by state funding (Brooklyn & Sigmon, 2017). Our model relies on peer care managers, a form of peer-based support that is widely used across a variety of healthcare settings and work to support patients by providing transitioning services to the community, employment, housing, education, and a connection to support networks (Gagne, Finch, Myrick, & Davis, 2018). We intend to show the sustainability of our approach by collecting data to demonstrate saved health care costs for patients through reduced ED visits and inpatient admissions through involvement of peer care managers. Demonstration of cost-savings may facilitate Medicaid and commercial payor assistance in supporting these valuable team members.
As discussed above, peer recovery services augment our Hub and Spoke system. We have partnered with two such specialists to work with patients within our system. Peer-based recovery support services are not new to the treatment of substance use disorder, but adapting these services to a hub and spoke system of care provides a specific structure and innovative approach to help individuals stay in treatment. Emerging evidence shows potential benefits in the use of peer-based support services across a variety of settings including substance abuse treatment (Gagne et al., 2018; Portillo, Goldberg, & Taxman, 2017; Rowe et al., 2007). The addition of these services aims to reduce known barriers to treatment such as transportation from rural areas, stigma, and confidentiality concerns. Before augmenting our Hub and Spoke system with peer-based support services, intake appointment wait times at spoke sites often exceeded two weeks, increasing the risk of loss-to-follow up. With the addition of peer-based care coordination we have been able to connect patient with intake appointments in 24–48 h. Future studies will be beneficial to see if actual wait times have been significantly impacted.
Provider and workforce barriers add another layer of complexity to effectively addressing the overdose epidemic. In addition to the low number of waivered providers, limited provider confidence and lack of specialty back up for complex cases and support services are the most common barriers to providing outpatient addiction treatment cited by physicians in rural locations (Andrilla, Coulthard, & Larson, 2017). The ECHO model has been used to address substance use disorders among rural and underserved populations in New Mexico since 2005 (Arora et al., 2014). In recent years its use has expanded across several states and institutions combating the overdose epidemic; however Penn State is the first to launch an OUD ECHO in Pennsylvania. ECHO has been shown to help providers overcome many of the barriers they face to implementing MAT. Providers in a substance use disorder-focused ECHO program reported that participation altered their care plan 77% of the time, a result duplicated in our model, and they rated the value of the clinical input they received as 4.8 out of 5 (Komaromy et al., 2017). A recent Agency for Healthcare Research and Quality technical brief on MAT models of care for OUD in primary care commended the ECHO model for enabling providers in rural communities to expand access to MAT as well as its emphasis on provision of psychosocial services (Chou et al., 2016). While the addition of Project ECHO to our model was recent, early results demonstrate increased knowledge, decreased sense of professional isolation, and self-reported improvement in ability to provide patient care that we hope will translate to increased capacity to treat patients with OUD.
Significant strengths of our model for MAT for OUD are its scalability and adaptability. With our OUD ECHO, we have trained 20 providers, but intend to reach up to 40 providers per year in rural locations where access to buprenorphine remains limited. Through our Hub, we hope to provide further expertise to other community clinics through models such as the Collaborative Opioid Prescribing Model (Stoller, 2015), with a goal to add an additional three new spoke clinics per year for the next three years. Drawing on our relationships with area hospitals, we will expand our offerings to include inpatient initiation of MAT (Liebschutz et al., 2014). Finally, we recognize the need to expand access to evidence-based MAT across the state and not merely in our region. Accordingly, we will engage policy makers in Pennsylvania to encourage state Medicaid and commercial payors to reimburse inpatient rehabilitation facilities that initiate MAT for OUD using buprenorphine or methadone, rather than detoxification which does not address ongoing symptoms of addiction.
5. Conclusions
Primary care providers are on the front lines of the overdose epidemic, but, sadly, the demand for treatment far exceeds the capacity to treat. Although MAT is the most effective approach for the treatment of OUD, there are many barriers to incorporating MAT into routine practice. Penn State’s Hub and Spoke MAT system of care is a unique answer to the crisis as it is the only program in Pennsylvania that offers peer recovery specialists, access to an entirely buprenorphine-waivered Emergency Department, and Project ECHO support for providers. Our system is built on collaborative models of treatment that have proven themselves effective both at reducing provider barriers to prescribing MAT and patient barriers to engaging in treatment. We are hopeful that in time our model will establish a standard of care for effective provision of MAT for OUD.
Funding
This work is supported by the Substance Abuse and Mental Health Services Administration through grant number 1H79TI081432-01, in part by the Department of Health’s targeted response and state opioid response funding streams, and in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant TR002014. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, DOH, or SAMHSA.
References
- Andrilla CHA, Coulthard C, & Larson EH (2017). Barriers rural physicians face prescribing buprenorphine for opioid use disorder. The Annals of Family Medicine, 15(4), 359–362. 10.1370/afm.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora S, Thornton K, Komaromy M, Kalishman S, Katzman J, & Duhigg D (2014). Demonopolizing medical knowledge. Academic Medicine, 89(1), 30–32. 10.1097/acm.0000000000000051. [DOI] [PubMed] [Google Scholar]
- Bart G (2012). Maintenance medication for opiate addiction: The foundation of recovery. Journal of Addictive Diseases, 31(3), 207–225. 10.1080/10550887.2012.694598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooklyn JR, & Sigmon SC (2017). Vermont hub-and-spoke model of care for opioid use disorder: Development, implementation, and impact. Journal of Addiction Medicine, 11(4), 286–292. 10.1097/ADM.0000000000000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can G, & Tanrıverdi D (2015). Social functioning and internalized stigma in individuals diagnosed with substance use disorder. Archives of Psychiatric Nursing, 29(6), 441–446. 10.1016/j.apnu.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Case A, & Deaton A (2015). Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proceedings of the National Academy of Sicnes of the United States of America, 112(49), 15078–15083. 10.1073/pnas.1518393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R, Korthuis T, Weimer M, Bougatsos C, Blazina I, Zakher B, … McCarty D (2016). Medication-assisted treatment models of care for opioid use disorder in primary care settings. (Retrieved from Rockville, MD: ). [PubMed] [Google Scholar]
- Connery HS (2015). Medication-assisted treatment of opioid use disorder: Review of the evidence and future directions. Harvard Review of Psychiatry, 23(2), 63–75. 10.1097/HRP.0000000000000075. [DOI] [PubMed] [Google Scholar]
- Davis D, Barnes B, & Fox R (2003). The continuing professional development of physicians: From research to practice. Chicago, Il: American Medical Association. [Google Scholar]
- D’Onofrio G, O’Connor PG, Pantalon MV, Chawarski MC, Busch SH, Owens PH, … Fiellin DA (2015). Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: A randomized clinical trial. The Journal of the American Medical Association, 313(16), 1636–1644. 10.1001/jama.2015.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin RE, Flannery J, Taenzer P, Smith A, Smith K, Fabico R, … Furlan AD(2015). ECHO Ontario chronic pain & opioid stewardship: Providing access and building capacity for primary care providers in underserviced, rural, and remote communities. Studies in Health Technology and Informatics, 209, 15–22. [PubMed] [Google Scholar]
- Gagne CA, Finch WL, Myrick KJ, & Davis LM (2018). Peer workers in the behavioral and integrated health workforce: Opportunities and future directions. American Journal of Preventive Medicine, 54(6S3), S258–S266. 10.1016/j.amepre.2018.03.010. [DOI] [PubMed] [Google Scholar]
- Hostetter M, & Klein S (2017). In focus: Expanding access to addiction treatment through primary care. The Commonwealth Fund. [Google Scholar]
- Hser YI, Saxon AJ, Huang D, Hasson A, Thomas C, Hillhouse M, … Ling W (2014). Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction, 109(1), 79–87. 10.1111/add.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn AS, & Dunn KE (2017). Why aren’t physicians prescribing more buprenorphine? Journal of Substance Abuse Treatment, 78, 1–7. 10.1016/j.jsat.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson E, Catlin M, Andrilla CH, Baldwin LM, & Rosenblatt RA (2014). Barriers to primary care physicians prescribing buprenorphine. The Annals of Family Medicine, 12(2), 128–133. 10.1370/afm.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaromy M, Bartlett J, Manis K, & Arora S (2017). Enhanced primary care treatment of behavioral disorders with ECHO case-based learning. Psychiatric Services, 68(9), 873–875. 10.1176/appi.ps.201600471. [DOI] [PubMed] [Google Scholar]
- Komaromy M, Duhigg D, Metcalf A, Carlson C, Kalishman S, Hayes L, … Arora S (2016). Project ECHO (Extension for Community Healthcare Outcomes): A new model for educating primary care providers about treatment of substance use disorders. Substance Abuse, 37(1), 20–24. 10.1080/08897077.2015.1129388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korthuis PT, McCarty D, Weimer M, Bougatsos C, Blazina I, Zakher B, … Chou R (2017). Primary care-based models for the treatment of opioid use disorder: A scoping review. Annals of Internal Medicine, 166(4), 268–278. 10.7326/M16-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle MR, Bernson D, Land T, Stopka TJ, Wang N, Xuan Z, … Walley AY (2018). Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: A cohort study. Annals of Internal Medicine, 169(3), 137–145. 10.7326/m17-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebschutz JM, Crooks D, Herman D, Anderson B, Tsui J, Meshesha LZ, … Stein M(2014). Buprenorphine treatment for hospitalized, opioid-dependent patients: A randomized clinical trial. The Journal of the American Medical Association Internal Medicine, 174(8), 1369–1376. 10.1001/jamainternmed.2014.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Olmstead TA, Rash CJ, & Zajac K (2017). Contingency management treatment for substance use disorders: How far has it come, and where does it need to go? Psychology of Addictive Behaviors, 31(8), 897–906. 10.1037/adb0000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo S, Goldberg V, & Taxman F (2017). Mental health peer navigators: Working with criminal justice-involved populations. The Prison Journal, 97(3), 318–341. [Google Scholar]
- Rowe M, Bellamy C, Baranoski M, Wieland M, O’Connell MJ, Benedict P, … Sells D (2007). A peer-support, group intervention to reduce substance use and criminality among persons with severe mental illness. Psychiatric Services, 58(7), 955–961. 10.1176/ps.2007.58.7.955. [DOI] [PubMed] [Google Scholar]
- Stoller K (2015). A collaborative opioid prescribing (CoOP) model linking opioid treatment programs with office-based buprenorphine providers. Addiction Science Clinical Practice, (A63), 10.25896576 [Google Scholar]
- Substance Abuse and Mental Health Association (2014). Results from the 2014 national survey on drug use and health: Detailed tabls. (Retrieved from Rockville, MD: ). [Google Scholar]
- Substance Abuse and Mental Health Association (2019). Practitioner and Program Data. Rockville, MD: Retrieved from https://www.samhsa.gov/medication-assisted-treatment/training-materials-resources/practitioner-program-data, Accessed date: 8 March 2019. [Google Scholar]
- https://www.cdc.gov/drugoverdose/data/statedeaths.html, Accessed date: 8 February 2019.
- Multiple Cause of Death, Accessed date: 10 March 2019.


