Abstract
Objective:
To evaluate the antidepressant effects of exercise in older adults, using randomized controlled trial (RCT) data.
Methods:
We conducted a meta-analysis of exercise in older adults, addressing limitations of previous works. RCTs of exercise interventions in older people with depression (≥ 60 years) comparing exercise vs. control were eligible. A random-effects meta-analysis calculating the standardized mean difference (SMD) (95% confidence interval [95%CI]), meta-regressions, and trim, fill, and fail-safe number analyses were conducted.
Results:
Eight RCTs were included, representing 138 participants in exercise arms and 129 controls. Exercise had a large and significant effect on depression (SMD = -0.90 [95%CI -0.29 to -1.51]), with a fail-safe number of 71 studies. Significant effects were found for 1) mixed aerobic and anaerobic interventions, 2) at moderate intensity, 3) that were group-based, 4) that utilized mixed supervised and unsupervised formats, and 5) in people without other clinical comorbidities.
Conclusion:
Adjusting for publication bias increased the beneficial effects of exercise in three subgroup analysis, suggesting that previous meta-analyses have underestimated the benefits of exercise due to publication bias. We advocate that exercise be considered as a routine component of the management of depression in older adults.
Keywords: Exercise, depression, older adults, publication bias, meta-analysis
Introduction
Depression in older adults is common, with prevalence estimates ranging from 4.6 to 9.3%.1 Late-life depression is a serious societal burden, resulting in increased health care costs,2 increased risk of morbidity and suicide, and impairments in physical, social, and cognitive functioning, all of which are associated with increased disability and mortality.3
Antidepressants remain the most common treatment choice, with selective serotonin re-uptake inhibitors con-sidered the first-line option.4 However, antidepressants are associated with many side effects, including falls,5 cardiovascular events, fractures, epilepsy, hyponatremia, and increased risk of all-cause mortality.6 Hence, there is a need for alternative strategies to improve depression in older adults.
Exercise has been studied as a potential non-pharmacological treatment for late-life depression.7 A meta-analysis including seven studies7 found a small to moderate effect (standardized mean difference [SMD] = -0.34, 95% confidence interval [95%CI]) -0.52 to -0.17) of exercise on depression. However, this review was conducted on data available 5 years ago and the authors included one large trial in which half of participants were not depressed or did not meet standardized criteria for depression or elevated depressive symptoms.8 Other pertinent questions remain unanswered in the literature on exercise and depression in older adults. For instance, no meta-regression of potential moderators of the antidepressant effect of exercise in randomized controlled trials (RCTs) in older adults with depression has been reported, despite high heterogeneity across studies. Although publication bias is known to be a potential threat to the validity of meta-analysis,9 no previous meta-analysis has considered its impact on the results obtained. A meta-analysis of psychotherapies for depression found that publication bias resulted in overstatement of effect sizes (ESs).10 In the literature on exercise for depression, one recent meta-analysis including adults demonstrated that publication bias resulted in underrepresentation of the true effect of exercise; adjusting for publication bias increased the ES from 0.98 to 1.15 (95%CI 0.68-1.27).11 A systematic review and meta-analysis focused on older adults also found evidence of publication bias; however, adjusted ES estimates were not provided.7
The present review aimed to address these limitations. Specific aims were: 1) to establish the effects of exercise on depression in older people with depression, using all available data, comparing exercise vs. non-active control groups; 2) to identify moderators, including sample characteristics (gender, medication use, and severity of baseline symptoms) and exercise intervention variables (length of intervention, frequency of exercise sessions, and supervision), that could influence the effects of exercise on depression; 3) to assess the possible influence of publication bias on the relationship between exercise and depression in older people; and 4) to evaluate the strength of the current evidence by calculating the number of negative studies required to nullify our conclusions.
Methods
This systematic review followed the PRISMA statement12 and the MOOSE guidelines.13
Inclusion criteria
Studies were eligible for inclusion in this meta-analysis if they met the following criteria:
1) Investigated older adults (minimum age of participants = 60 years) with a primary diagnosis of major depressive disorder (MDD), according to established criteria (e.g., DSM14 or ICD15), or those with increased depressive symptoms determined by a validated screening measure (e.g., the Hamilton Depression Scale16 [HAM-D], Beck Depression Inventory17 [BDI], Geriatric Depression Scale18 [GDS], or others). Studies included in this criteria were those that included participants with at least mild (or equivalent) scores on validated scales or, in case the scale did not have a validated cutoff, the cutoff used by the author was accepted. We also included studies that included some participants with other related diagnoses, such as dysthymia, that is classified into the category of chronic persistent depressive disorders.14
2) Measured depressive symptoms before and after intervention using a validated measure (e.g., HAM-D, BDI, and GDS).
3) Were RCTs investigating exercise, as defined by Caspersen et al.19 as planned, structured, repetitive, and purposive physical activity, in the sense that improvement or maintenance of one or more components of physical fitness is an objective in the active arm of the trial. Trials that used yoga, tai chi, or qigong were not included since previous studies found significant heterogeneity in these trials when compared with conventional aerobic or strength exercises.7
4) Included a non-active control group, such as usual-care/usual-treatment, wait-list control conditions, placebo pills, or other social activities (trials that included any other exercise interventions - such as stretching or low-dose exercise - as comparators were excluded).
5) Were published in peer-reviewed journals or as dissertations.
Information sources and searches
Potentially eligible studies were identified in a two-step process. First, three authors (BS, FBS, SR) reviewed all articles identified (both included and excluded with reasons) by the recent Cochrane review on exercise for depression.20 Second, three independent reviewers (BS, FBS, SR) searched the Academic Search Premier, MEDLINE, Psychology and Behavioral Sciences Collection, PsycINFO, SPORTDiscus, CINAHL Plus, and PubMed databases, without language restrictions, from January 2013 until August 1, 2015, using the keywords ([exercis* OR aerobic* OR running OR jogging OR walk* OR hiking OR swim* OR aquatic* OR cycling OR bicycl* OR strength* and activit* OR fitness OR train* OR "physical medicine" OR resistance OR lift*] AND [depression OR dysthymia]). In addition, the reference lists of all eligible articles of recent reviews investigating the effectiveness of exercise vs. control in adults or older people were screened to identify potentially eligible articles.7,20 -22
Study selection
Three authors (BS, FBS, SR) determined potentially eligible articles that met the inclusion criteria. After removal of duplicates, two independent reviewers screened all potentially eligible articles on the basis of titles and abstracts. After obtaining the full texts, the three authors then applied the eligibility criteria and, through consensus, generated a final list of articles for inclusion.
Outcomes
Our primary outcome of interest was the mean change in depressive symptoms in the exercise group, assessed by any validated scale, from baseline to post-intervention, in comparison with the mean change observed in the control group, calculated as the SMD and respective 95%CI. If an author reported the results of two outcome measures meeting our criteria (i.e., mean change/pre- and post-test change in depressive symptoms according to two different measures), we used the primary outcome chosen by the author. If this was not clear, we attempted to use the HAM-D or BDI to increase homogeneity in our results. For studies reporting the effects of two or more different exercise groups (home-based and supervised, aerobic and anaerobic, high- and low-dose), the arm reporting the greater ES was included in the analysis.
Data extraction
Two authors (FBS, SR) independently extracted data using a data extraction form designed to collect sample-related (number of participants, percentage of women, percentage of participants taking antidepressants, presence of clinical comorbidities, severity of baseline symptoms), exercise-related (trial duration, intensity of intervention according to the American College of Sports Medicine [ACSM] classification,23 weekly frequency), and methodological factors (study quality, instruments used for diagnosis and symptom assessment, supervision). Lastly, we extracted data for the primary outcome (means and standard deviation [SDs]) from both groups, pre- and post-intervention. If this was not available, we used the pre- and post-test mean change and SD, if reported within the study. In the event that a study reported two or more exercise groups, the group exposed to the highest intensity, volume, or dose was considered for analysis.
Risk of bias and quality assessment
Three authors (FBS, JR, BS) assessed study quality in terms of the presence of high, low, or unclear risk of bias, according to the Cochrane Handbook definition.24 The risk of bias was assessed by considering random sequence generation, allocation concealment, blinding of participants, blinding of those delivering the intervention, blinding of outcome assessors, incomplete data by outcome, selective reporting, and other factors. To be considered of high quality, studies had to report adequate allocation concealment AND presentation of outcomes data according to intention-to-treat principles AND blinding of outcome assessors. The criteria used for risk-of-bias assessment were based on previous studies.20
Meta-analysis
We performed meta-analysis using a random effects models due to expected heterogeneity, with SMD and 95%CIs used as the ES. First, we calculated the SMD statistic and corresponding 95%CIs to establish the effects of exercise on depression in older adults across all studies, using Comprehensive Meta-Analysis version 3 software (CMA Biostat, Englewood, NJ, USA). We then conducted a sensitivity analysis, computing the effects of exercise on depression in high-quality studies only. Subsequently, we conducted meta-regression analyses to investigate potential moderators of the antidepressant effects of exercise. Potential moderators were chosen a priori and included sex, age, use of medication, trial duration, and weekly intervention frequency. Next, we conducted subgroup analyses to compare exercise response according to depression diagnosis, study setting (inpatient, outpatient, mixed), high quality (low risk of bias) vs. low quality, presence of other major clinical comorbidities (yes or no), supervision (yes, no, unclear), exercise type (aerobic, resistance, mixed), and exercise intensity. Heterogeneity was assessed with Cochran’s Q and the I2 statistic for each analysis.24 Publication bias was assessed with the Begg-Mazumdar rank correlation test (yielding Kendall’s tau)25 and Egger’s bias test.26 In addition, we conducted a trim and fill adjusted analysis27 to remove the most extreme small studies from the positive side of the funnel plot and recalculated the ES at each iteration, until the funnel plot was symmetric about the (new) ES for all analysis. Finally, the classic fail-safe number of negative studies that would be required to nullify (i.e., make p > 0.05) our ES was calculated.28 SMDs ≤ 0.4 were con-sidered indicative of small effects, those between 0.41 and 0.7 were considered moderate, and those > 0.7 were considered large effects.29
Results
Search results
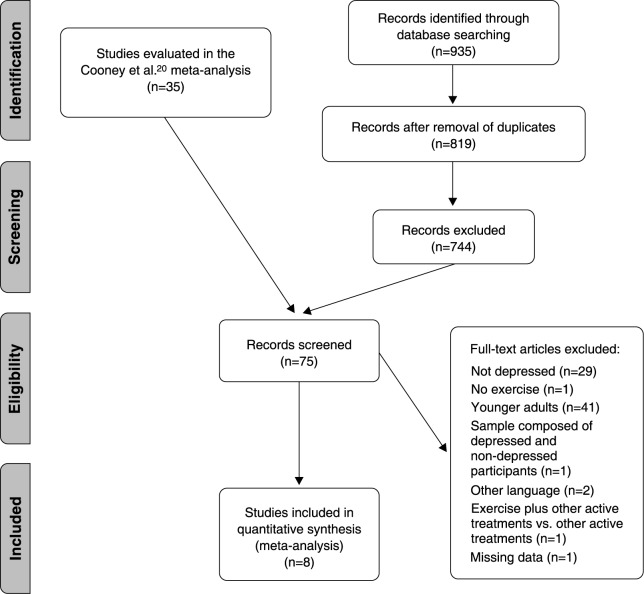
In the first stage of the search strategy, eight RCTs were identified from a previous review.30 -37 In the second stage, following the removal of duplicates, our search identified 819 potentially relevant articles. At the full-text review stage, we reviewed 49 articles (all eight from the first stage and 41 from our second-stage searches); of these, 40 were excluded with reasons. This yielded nine full-text, peer-reviewed articles that met the eligibility criteria.30 -38 Eight of these studies were from the first stage and one was from the second stage (details summarized in Figure 1). Of these, eight30,32 -38 provided complete data and were included in our meta-analysis.
Figure 1. Flowchart of study selection.
Characteristics of included trials and participants
The eight included studies assessed a population of 267 adults with depression, of whom 138 and 129 were randomized to exercise and control conditions, respectively. The mean (SD) age was 69.5 (0.71) for exercise and 70.5 (2.12) for control groups, respectively. No study was conducted in a sample exclusively limited to participants with MDD. Two studies33,34 included participants with other psychiatric diagnosis (dysthymia) and two others included participants with depression as well as participants with additional comorbid diagnoses, such as cardiovascular or neurological diseases.34,37 The most commonly used measures of depressive symptoms were the HAM-D (n=2), BDI (n=2), and GDS (n=2). The mean trial duration was 12 weeks. Participant details and symptom measures are presented in Table 1. Full details of other characteristics can be obtained from the authors upon request.
Table 1. Summary of included studies.
| Study | Sample size (n) | Age (mean or range) | Sex (% female) | Antidepressant use (% taking) | Outcome | Trial duration (weeks) | Diagnosis | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise | Control | Exercise | Control | Exercise | Control | Exercise | Control | ||||
| Brenes30 | 14 | 12 | 73.5 | 73.9 | 64 | 50 | 0 | 0 | HAM-D | 16 | Depressive symptoms |
| Huang38 | 19 | 20 | 76.42 | 75.85 | 57.9 | 55 | 0 | 0 | GDS-15 | 12 | Depressive symptoms |
| McNeil32 | 10 | 10 | ? | ? | ? | ? | 0 | 0 | BDI | 6 | Depressive symptoms |
| Shahidi33 | 20 | 20 | 65.7 | 68.4 | 100 | 100 | ? | ? | GDS | ? | Depressive symptoms |
| Sims34 | 23 | 21 | 67.95 | 66.27 | 39 | 41 | ? | ? | PHQ-9 | 10 | Depressive symptoms |
| Singh35 | 17 | 15 | 70 | 72 | 70.5 | 53.3 | 0 | 0 | BDI | 10 | MDD + dysthymia |
| Singh36 | 18 | 19 | 69 | 69 | 55 | 50 | 0 | 42 | HAM-D | 8 | MDD + dysthymia |
| Williams & Tappen37 | 17 | 12 | 71-101 | 71-101 | ? | ? | ? | ? | CSDD | 16 | Depressive symptoms |
BDI = Beck Depression Inventory; CSDD = Cornell Scale for Depression in Dementia; GDS = Geriatric Depression Scale; HAM-D = Hamilton Depression Scale; MDD = major depressive disorder; PHQ-9 = Patient Health Questionnaire.
Main analysis
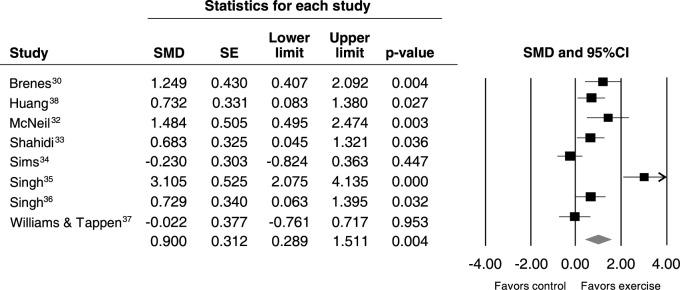
Data pooled from the eight included studies showed a large improvement in symptoms of depression favoring the exercise groups (SMD = -0.90, 95%CI 0.28-1.51, p = 0.004, Q = 38, I2 = 81.63, p < 0.001) (Figure 2). The Begg (tau = 0.71, p = 0.01) and Egger tests indicated publication bias (intercept = 9.66, p = 0.01), likely due to a greater presence of studies reporting a significant association between exercise and improvement in depression. A funnel plot is available from the authors upon request. The ES remained unchanged after adjustment for the trim and fill analysis.
Figure 2. Meta-analysis of all included studies. 95%CI = 95% confidence interval; SE = standard error; SMD = standardized mean difference.
Subgroup analyses
All subgroup analyses are presented in Table 2. Briefly, exercise had significant effects in samples without clinical comorbidities (SMD = -1.25, 95%CI -0.62 to -1.87, p < 0.001), in which mixed interventions combined aerobic exercise and strength training (SMD = -0.92, 95%CI -0.41 to -1.43, p = 0.02) or moderate intensity (SMD = -0.73, 95%CI 0.08 to -1.38, p < 0.001), in a mixed supervised and unsupervised format (SMD = -1.48, 95%CI -0.49 to -2.47, p = 0.003).
Table 2. Subgroup meta-analysis of all studies.
| Analysis | RCTs (n) | SMD | 95%CI | p-value | Heterogeneity (I2) | Trim and fill effect size (95%CI, adjusted studies) | Fail-safe number (n) |
|---|---|---|---|---|---|---|---|
| Main analysis | |||||||
| Exercise vs. control | 8 | -0.900 | -0.29 to -1.51 | 0.004 | 81.63 | Unchanged | 71 |
| Depression classification | |||||||
| MDD/dysthymia/MiDD | 2 | -1.883 | 0.44 to -4.21 | 0.11 | 93.06 | N/A | N/A |
| Depressive symptoms | 6 | -0.560 | -0.14 to -0.97 | 0.008 | 61.00 | Unchanged | 26 |
| Study setting | |||||||
| Outpatient/community | 7 | -1.037 | -0.37 to -1.74 | 0.002 | 82.33 | Unchanged | 73 |
| Nursing homes | 1 | -0.022 | 0.71 to -0.76 | 0.953 | 0 | N/A | N/A |
| Intensity of exercise | |||||||
| Moderate | 1 | -0.73 | 0.08 to -1.38 | 0.02 | 0 | N/A | N/A |
| Vigorous | 3 | -1.15 | 0.50 to -2.80 | 0.17 | 93.47 | Unchanged | 11 |
| Exercise type | |||||||
| Aerobic only | 3 | -0.66 | -0.10 to -1.42 | 0.09 | 65.82 | Unchanged | 4 |
| Resistance only | 3 | -1.15 | 0.50 to -2.80 | 0.174 | 93.40 | Unchanged | 11 |
| Mixed | 2 | -0.92 | -0.41 to -1.43 | < 0.0001 | 0 | N/A | N/A |
| Group exercise | |||||||
| Yes | 6 | -0.97 | -0.24 to -1.71 | 0.009 | 84.35 | -1.24 (0.38-2.10) (1) | 49 |
| No | 2 | -0.69 | 0.78 to -2.36 | 0.356 | 82.49 | N/A | N/A |
| Supervision | |||||||
| Supervised | 6 | -0.86 | -0.07 to -1.66 | 0.032 | 85.19 | -1.11 (0.24-1.98) (1) | 34 |
| Supervised and unsupervised | 1 | -1.48 | -0.49 to -2.47 | 0.003 | 0 | N/A | N/A |
| Comorbidities | |||||||
| No major comorbidities | 6 | -1.25 | -0.62 to -1.87 | < 0.001 | 74.10 | -1.38 (0.78-1.99) (1) | 81 |
| Participants with comorbidities | 2 | -0.14 | 0.61 to -0.31 | 0.52 | 0 | N/A | N/A |
95%CI = 95% confidence interval; MDD = major depressive disorder; MiDD = minor depressive disorder; N/A = not available; RCTs = randomized clinical trials; SMD = standardized mean difference.
Values in bold are significant.
Adjustment of publication bias and fail safe number of studies
Upon adjustment for potential publication bias, three analyses were adjusted by the Duval and Tweedie trim and fill method.27 ESs for all three analyses (group exercise, supervised, and no major comorbidities) all increased after adjustment. The fail-safe number of studies required to nullify the ESs were also relatively higher. In particular, the fail-safe number for the main analysis was n=71, while higher numbers were required to nullify the effect in studies conducted in people without clinical comorbidities (n=81) and in outpatients (n=73) (Table 2).
Meta-regression of antidepressant effects in the main analysis
Baseline depressive symptoms had borderline significance as moderators of the antidepressant effects of exercise (B = 0.156, 95%CI 0.008-0.3223, p = 0.06, R2 = 0.09). A summary of all meta-regression analyses is presented in Table 3. No other significant moderators were identified.
Table 3. Meta-regression of moderators/correlates of effects of exercise on depression.
| Moderator | RCTs (n) | β | 95%CI | p-value | R2 |
|---|---|---|---|---|---|
| Mean age, control | 6 | 0.122 | -0.083 to 0.327 | 0.24 | 0.02 |
| Mean age, exercise | 6 | 0.049 | -0.209 to 0.308 | 0.70 | 0.00 |
| Females, exercise (%) | 6 | 0.017 | -0.020 to 0.058 | 0.40 | 0.00 |
| Females, control (%) | 6 | 0.005 | -0.042 to 0.042 | 0.99 | 0.00 |
| Baseline depressive symptoms, exercise | 8 | 0.156 | 0.008 to 0.3223 | 0.06 | 0.09 |
| Baseline depressive symptoms, control | 8 | -0.021 | -0.150 to 0.107 | 0.74 | 0.00 |
| Dropout, exercise (%) | 7 | -0.050 | -0.195 to 0.095 | 0.49 | 0.28 |
| Dropout, control (%) | 7 | -0.058 | -0.168 to 0.052 | 0.30 | 0.00 |
| Trial duration | 7 | -0.017 | -0.209 to 0.173 | 0.85 | 0.00 |
| Weekly intervention frequency | 7 | -0.524 | -1.268 to 0.219 | 0.16 | 0.00 |
95%CI = 95% confidence interval; RCTs = randomized clinical trials.
Mean change in depressive symptoms
The mean improvement on the HAM-D (three studies) was -5.21 points (95%CI 3.15-7.26, p < 0.001), and in the BDI (two studies), -6.19 points (95%CI 4.39-7.99, p < 0.001).
Risk of bias
All studies were considered to be of low quality (high risk of bias). Full details on risk-of-bias assessment can be obtained from the authors upon request.
Discussion
The present study found a large and significant antidepressant effect of exercise in older adults. Specifically, large and significant effects were found for moderate-intensity exercise, in studies that used mixed aerobic and strength training, in both supervised and unsupervised formats, and in samples without major comorbidities. Moreover, our analyses suggested that some results were underestimated due to publication bias, suggesting that previous meta-analyses might have inadvertently underestimated the antidepressant effect of exercise. However, the small number of trials included in this review suggests that caution is warranted in interpretation of our findings.
Our results corroborate the findings of a previous study of depressed older adults experiencing significant depressive symptoms, which revealed significant antidepressant effects of exercise.7 The magnitude of the findings, how-ever, differs somewhat. Bridle et al.7 identified a small to moderate reduction in depressive symptoms (SMD = -0.34, 95%CI -0.52 to -0.17), while our analysis found a large effect (SMD = -0.90, 95%CI -0.28 to -1.51). Several factors that may account for the larger ES found in the present analysis. First, the studies used different strategies to calculate ESs. Bridle et al.7 based estimation of ESs on the difference of the post-intervention value between the intervention and control groups using the SMD ES. In the present review, we estimated the standardized difference in means from baseline to post-intervention and the change in SD. This strategy increased the ES of some studies (such as the Brenes et al.30 study, from an SMD of -0.55 to an SMD of -1.49). Second, there were important differences in inclusion criteria. In the present study, we did not include studies that enrolled participants who did not meet criteria for elevated depressed symptoms, as assessed by a validated instrument such as the HAM-D, BDI, or GDS. This criterion resulted in the exclusion of the Kerse et al.8 study from our review, because approximately 47% of the sample did not meet criteria for depressive disorder or increased depressive symptoms. Lastly, we also considered studies that used short-term interventions (less than 3 months’ duration) and excluded trials evaluating acute responses to a single exercise session, which resulted in the exclusion of the Singh et al. study39 (SMD = -0.67, 95%CI -1.43 to 0.08) and the inclusion of two trials by the same author, with SMDs of -3.10 and -0.72, respectively.35,36
This was the first study to calculate mean-difference changes in depressive symptoms across two commonly used measures in older adults. Specifically, we found a 5.2-point reduction in HAM-D and a 6.2-point reduction in BDI scores. While this reduction is smaller than that reported in a recent meta-analysis focusing on adults,11 it exceeds the threshold for clinically meaningful change proposed by the National Institute for Health and Care Excellence40 guidelines for depression treatment.
Our preliminary findings regarding exercise program variables in older adults may be used as inputs to design interventions for clinical practice, as currently available recommendations are based on specific studies rather than on meta-analytical data.41 First, moderate-intensity exercise promoted large, significant reductions in depressive symptoms, while no significant effect was found for vigorous exercise. This result differs from the findings of a previous meta-analysis of adults (age > 18 years),11 which showed that both moderate and vigorous exercise promote significant effects on depressive symptoms. We hypothesize that this effect may be because, in older adults, vigorous exercise is actually of higher relative intensity due to the deconditioning that occurs during aging. This issue may be even more pronounced in individuals with depression, who experience decreased aerobic capacity when compared to non-depressed participants.42
Second, mixed aerobic exercise and strength training, but not strength training or aerobic exercise alone, were effective treatments for depression in older adults. These findings are potentially attributable to the small number of studies included in each subgroup. However, even with two studies, the mixed interventions had significant effects on depression, and should thus be considered as an option to improve muscle strength and cardiorespiratory fitness. Indeed, improvement in fitness should be considered a target in exercise treatment for depression.43
Third, group exercise was effective for older patients, while exercise sessions not conducted in a group setting were not significantly efficacious. This may be particularly important in a population known to be at high risk of social isolation.44 Indeed, future research should consider the mental health benefits of exercise not only as a means of increasing physical activity, but also as a vehicle for promoting social interaction.
Lastly, our data show that special attention should be given to older people with significant comorbidities, such as cardiovascular or neurological diseases. We hypothesize that these comorbidities may limit functional capacity to engage in exercise, and that this limitation may be particularly relevant in older adults, who have an inherently lower exercise capacity due to the impacts of aging on neuromuscular structure and function.45
Several theories have been advanced to explain the antidepressant effect of exercise, including hormonal changes (e.g., increased beta-endorphins, serotonergic system adaptations, impact on hormone levels) and effects on neurogenesis (e.g., as demonstrated by increased brain-derived neurotrophic factor [BDNF] levels), inflammation (decreased levels of pro-inflammatory markers and increased levels anti-inflammatory markers), and oxidative stress (decreased levels of pro-oxidative markers and increased levels of antioxidant markers), as well as changes in cortical activity and structure.46 Indeed, exercise appears to promote adaptations in copeptin and thiobarbituric acid reactive species (TBARS) and total mean frequency in people with depression.46,47 In addition, preliminary evidence suggests associations of increases in hippocampal volume and serum levels of interleukin-1 beta with symptom improvement.46 However, exactly which specific mechanisms account for the antidepressant effects of exercise in MDD remains unclear; further research should attempt to elucidate this.
The present review has some limitations. As only eight trials were analyzed, some subgroups were very small, including only one or two trials. In addition, all included studies had small sample sizes (n < 50). Therefore, the likelihood that the Egger and Begg tests would detect publication bias was decreased.48,49 Considering this limitation, the preliminary subgroup analysis provides only initial directions for future research, and should be interpreted with caution.
In conclusion, exercise can be considered an effective non-pharmacological treatment for depression in older adults. This result is especially relevant because late-life depression is a major societal burden, resulting in increased health care costs, increased risk of morbidity, suicide, cognitive and functional decline, as well as increased mortality. Some results might be sensitive to publication bias, and previous meta-analyses may have inadvertently presented conservative ESs for the impact of exercise on depression in older adults. To ensure optimal effectiveness, moderate-intensity, mixed aerobic and anaerobic exercise sessions in a group format appear to have the greatest impact on reduction of depressive symptoms.
Acknowledgements
This study received financial support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Disclosure
The authors report no conflicts of interest.
References
- 1.Luppa M, Sikorski C, Luck T, Ehreke L, Konnopka A, Wiese B, et al. Age- and gender-specific prevalence of depression in latest-life-systematic review and meta-analysis. J Affect Disord. 2012;136:212–21. doi: 10.1016/j.jad.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 2.Luppa M, Sikorski C, Motzek T, Konnopka A, Konig HH, Riedel-Heller SG. Health service utilization and costs of depressive symptoms in late life - a systematic review. Curr Pharm Des. 2012;18:5936–57. doi: 10.2174/138161212803523572. [DOI] [PubMed] [Google Scholar]
- 3.Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci. 2003;58:249–65. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- 4.Alexopoulos GS. Pharmacotherapy for late-life depression. J Clin Psychiatry. 2011;72:e04. doi: 10.4088/JCP.7085tx2cj. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stubbs B. A meta-analysis investigating falls in older adults taking selective serotonin reuptake inhibitors confirms an association but by no means implies causation. Am J Geriatr Psychiatry. 2015;23:1098. doi: 10.1016/j.jagp.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ. 2011;343:d4551. doi: 10.1136/bmj.d4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bridle C, Spanjers K, Patel S, Atherton NM, Lamb SE. Effect of exercise on depression severity in older people: systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry. 2012;201:180–5. doi: 10.1192/bjp.bp.111.095174. [DOI] [PubMed] [Google Scholar]
- 8.Kerse N, Hayman KJ, Moyes SA, Peri K, Robinson E, Dowell A, et al. Home-based activity program for older people with depressive symptoms: DeLLITE--a randomized controlled trial. Ann Fam Med. 2010;8:214–23. doi: 10.1370/afm.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ioannidis JP, Munafo MR, Fusar-Poli P, Nosek BA, David SP. Publication and other reporting biases in cognitive sciences: detection, prevalence, and prevention. Trends Cogn Sci. 2014;18:235–41. doi: 10.1016/j.tics.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuijpers P, Smit F, Bohlmeijer E, Hollon SD, Andersson G. Efficacy of cognitive-behavioural therapy and other psychological treatments for adult depression: meta-analytic study of publication bias. Br J Psychiatry. 2010;196:173–8. doi: 10.1192/bjp.bp.109.066001. [DOI] [PubMed] [Google Scholar]
- 11.Schuch FB, Vancampfort D, Richards J, Rosenbaum S, Ward PB, Stubbs B. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res. 2016;77:42–51. doi: 10.1016/j.jpsychires.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- 15.World Health Organization (WHO). The ICD-10 Classification of Mental and Behavioural Disorders - Diagnostic criteria for research [Internet]. 1993 [cited 2016 Apr 08]. who.int/classifications/icd/en/GRNBOOK.pdf. [Google Scholar]
- 16.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 17.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 18.Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24:709–11. [PubMed] [Google Scholar]
- 19.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–31. [PMC free article] [PubMed] [Google Scholar]
- 20.Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;9:CD004366. doi: 10.1002/14651858.CD004366.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Josefsson T, Lindwall M, Archer T. Physical exercise intervention in depressive disorders: meta-analysis and systematic review. Scand J Med Sci Sports. 2014;24:259–72. doi: 10.1111/sms.12050. [DOI] [PubMed] [Google Scholar]
- 22.Silveira H, Moraes H, Oliveira N, Coutinho ES, Laks J, Deslandes A. Physical exercise and clinically depressed patients: a systematic review and meta-analysis. Neuropsychobiology. 2013;67:61–8. doi: 10.1159/000345160. [DOI] [PubMed] [Google Scholar]
- 23.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–59. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 24.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions, Version 5.1.0 [Internet]. 2011 [cited 2016 Apr 08]. handbook.cochrane.org/ [Google Scholar]
- 25.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 28.Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86:638–41. [Google Scholar]
- 29.Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Chichester: WileyBlackwell; 2008. pp. 359–87. [Google Scholar]
- 30.Brenes GA, Williamson JD, Messier SP, Rejeski WJ, Pahor M, Ip E, et al. Treatment of minor depression in older adults: a pilot study comparing sertraline and exercise. Aging Ment Health. 2007;11:61–8. doi: 10.1080/13607860600736372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mather AS, Rodriguez C, Guthrie MF, McHarg AM, Reid IC, McMurdo ME. Effects of exercise on depressive symptoms in older adults with poorly responsive depressive disorder: randomised controlled trial. Br J Psychiatry. 2002;180:411–5. doi: 10.1192/bjp.180.5.411. [DOI] [PubMed] [Google Scholar]
- 32.McNeil JK, LeBlanc EM, Joyner M. The effect of exercise on depressive symptoms in the moderately depressed elderly. Psychol Aging. 1991;6:487–8. doi: 10.1037//0882-7974.6.3.487. [DOI] [PubMed] [Google Scholar]
- 33.Shahidi M, Mojtahed A, Modabbernia A, Mojtahed M, Shafiabady A, Delavar A, et al. Laughter yoga versus group exercise program in elderly depressed women: a randomized controlled trial. Int J Geriatr Psychiatry. 2011;26:322–7. doi: 10.1002/gps.2545. [DOI] [PubMed] [Google Scholar]
- 34.Sims J, Galea M, Taylor N, Dodd K, Jespersen S, Joubert L, et al. Regenerate: assessing the feasibility of a strength‐training program to enhance the physical and mental health of chronic post stroke patients with depression. Int J Geriatr Psychiatry. 2009;24:76–83. doi: 10.1002/gps.2082. [DOI] [PubMed] [Google Scholar]
- 35.Singh NA, Clements KM, Fiatarone MA. A randomized controlled trial of progressive resistance training in depressed elders. J Gerontol A Biol Sci Med Sci. 1997;52:M27–35. doi: 10.1093/gerona/52a.1.m27. [DOI] [PubMed] [Google Scholar]
- 36.Singh NA, Stavrinos TM, Scarbek Y, Galambos G, Liber C, Fiatarone Singh MA. A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. J Gerontol A Biol Sci Med Sci. 2005;60:768–76. doi: 10.1093/gerona/60.6.768. [DOI] [PubMed] [Google Scholar]
- 37.Williams CL, Tappen RM. Exercise training for depressed older adults with Alzheimer's disease. Aging Ment Health. 2008;12:72–80. doi: 10.1080/13607860701529932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang TT, Liu CB, Tsai YH, Chin YF, Wong CH. Physical fitness exercise versus cognitive behavior therapy on reducing the depressive symptoms among community-dwelling elderly adults: a randomized controlled trial. Int J Nurs Stud. 2015;52:1542–52. doi: 10.1016/j.ijnurstu.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 39.Singh NA, Clements KM, Singh MA. The efficacy of exercise as a long-term antidepressant in elderly subjects: a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2001;56:M497–504. doi: 10.1093/gerona/56.8.m497. [DOI] [PubMed] [Google Scholar]
- 40.National Institute for Health and Care Excellence (NICE). Depression: the treatment and management of depression in adults, NICE Clinical Guidelines, No. 90. Leicester: British Psychological Society; 2009. [Google Scholar]
- 41.American College of Sports Medicine. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–30. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 42.Papasavvas T, Bonow RO, Alhashemi M, Micklewright D. Depression symptom severity and cardiorespiratory fitness in healthy and depressed adults: a systematic review and meta-analysis. Sports Med. 2016;46:219–30. doi: 10.1007/s40279-015-0409-5. [DOI] [PubMed] [Google Scholar]
- 43.Stubbs B, Rosenbaum S, Vancampfort D, Ward PB, Schuch FB. Exercise improves cardiorespiratory fitness in people with depression: a meta-analysis of randomized control trials. J Affect Disord. 2016;190:249–53. doi: 10.1016/j.jad.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Cattan M, White M, Bond J, Learmouth A. Preventing social isolation and loneliness among older people: a systematic review of health promotion interventions. Ageing Soc. 2005;25:41–67. doi: 10.7748/nop.17.1.40.s11. [DOI] [PubMed] [Google Scholar]
- 45.Conley KE, Cress ME, Jubrias SA, Esselman PC, Odderson IR. From muscle properties to human performance, using magnetic resonance. J Gerontol A Biol Sci Med Sci. 1995;50:35–40. doi: 10.1093/gerona/50a.special_issue.35. [DOI] [PubMed] [Google Scholar]
- 46.Schuch FB, Deslandes AC, Stubbs B, Gosmann NP, Silva CT, Fleck MP. Neurobiological effects of exercise on major depressive disorder: a systematic review. Neurosci Biobehav Rev. 2016;61:1–11. doi: 10.1016/j.neubiorev.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 47.Schuch FB, Vasconcelos-Moreno MP, Borowsky C, Zimmermann AB, Wollenhaupt-Aguiar B, Ferrari P, et al. The effects of exercise on oxidative stress (TBARS) and BDNF in severely depressed inpatients. Eur Arch Psychiatry Clin Neurosci. 2014;264:605–13. doi: 10.1007/s00406-014-0489-5. [DOI] [PubMed] [Google Scholar]
- 48.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 49.Sterne JA, Egger M, Moher D, Cochrane Bias Methods Group Addressing reporting biases [Internet]. 2011 [cited 1016 Apr 08]. community-archive.cochrane.org/sites/default/files/Handbook510pdf_Ch10_ReportingBias.pdf. [Google Scholar]