Abstract
Ethanol has been found to affect pulmonary cells by interfering with vitamin D metabolism and pulmonary defense mechanisms. The objective of this study was to understand the mechanisms of ethanol’s disruptive influence on the vitamin D pathway and inhibition of anti-microbial peptide cathelicidin (LL-37). Bronchial epithelial cells (BEAS-2Bs), primary human bronchial epithelial cells (HBECs), primary human alveolar epithelial cells (HPAEpiCs), and human monocyte cells (THP-1s) were used in this study. These cells were cultured and exposed to different treatment groups: medium-only control, ethanol (70 mM) only, diallyl disulfide (DADS) (10 μM) -only, and a co-exposure of ethanol (70 mM) and DADS (10 μM) for 10 or 24 hours. Calcidiol (50 ng/mL) and calcitriol (0.05 ng/mL) dose-response studies were conducted for 48 hours. After incubation, cells were trypsinized, lysed, and centrifuged, and the cellular lysate was prepared for assay. Protein was quantified, and levels of inactive vitamin D [25(OH)D3], active vitamin D [1, 25(OH)2 D3], and anti-microbial peptides (cathelicidin/ LL-37) in the samples were assayed using commercially available ELISA kits. In the ethanol-exposed group, cellular lysate concentrations of 25(OH)D3 and LL-37 were significantly reduced by 30%, and 40% in BEAS-2B cells, and 35% and 80% in HPAEpi cells respectively. Overall 1, 25(OH)2D3 cellular lysate levels were lower but followed a similar trend as the 25(OH)D3 response. LL-37 levels in primary bronchial, alveolar cells, and ThP-1 cells were statistically reduced in ethanol-exposed groups (60%, 80%, and 65%, respectively) when compared with control. Following the addition of DADS, levels of LL-37 were recovered to within control levels for all three cell types.
This study establishes two clinically relevant observations: that the exposure of pulmonary epithelial and monocyte cells to physiologically relevant levels of excessive ethanol selectively disrupts the activation of pulmonary vitamin D and inhibits the presence of antimicrobial peptide (LL-37) in vitro, and the co-exposure of DADS significantly attenuates ethanol-induced intracellular LL-37 depletion.
Keywords: alcohol, active vitamin D, inactive vitamin D, cathelicidin/LL-37, anti-microbial peptides, pneumonia, alcohol use disorder (AUD), pulmonary system
Introduction
Chronic ethanol over-consumption behavior characterized by alcohol use disorder (AUD) has been associated with several airway diseases such as asthma, bronchitis, and chronic obstructive pulmonary disease (COPD) (Jones, 2008). The severity of these immune response-modulated inflammatory diseases is associated with the concentration, duration, and route of exposure to ethanol and its numerous deleterious biochemical interactions in the pulmonary system. According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA), excessive exposure to inhaled or ingested ethanol increases susceptibility to respiratory infections, specifically, bacteria pneumonia, among vulnerable populations, such as people suffering from AUDs (Huber, First, & Grubner, 1991; Hughes & Norton, 2009; Jones, 2008).
Excessive dietary exposure to ethanol has been linked to nutritional deficiencies, including malabsorption of essential nutrients, vitamins (B, C, D, A, E), and pantothenic acid (Hlastala, 1998; Hughes & Norton, 2009). Although ethanol use-dependent malnutrition has a direct influence on essential nutrient availability, when malnutrition rates and severity were adjusted for socioeconomic confounders, vitamin D deficiencies remained (Goldsmith, Iber, & Miller, 1983). These published observations lend credence to the concept of nutritionally independent ethanol-induced metabolic interference of vitamin D conversion in chronic ethanol abusers.
Cholecalciferol, vitamin D3, more commonly referred to as vitamin D, is classified as a fat-soluble “vitamin” naturally present in relatively low quantities in a few foods (20–600 IU/serving size), e.g., cold water fishes, mushrooms, and dairy products, with much higher levels of exposure available in dietary or prescribed supplements (400 IU–2,000 IU/day) (Holick, 2007). In cases of severe vitamin D deficiency, large single bolus oral doses of cholecalciferol (250,000 IU) (Kearns et al., 2014) have been safely administered, with a risk of hypercalcemia occurring at levels greater than 300,000 IU (Cesur, Caksen, Gündem, Kirimi, & Odabaş, 2003). However, when skin melanin content is in concert with behavior and solar ultraviolet B levels, humans in most locations on the planet can generate physiologically healthy levels of vitamin D from exposure to sunlight (Hlastala, 1998). Newly generated vitamin D3 selectively binds to a hepatic protein similar to serum albumin and alpha-fetoprotein originally named Gc-globulin, contemporarily referred to vitamin D binding protein (VDBP) (Haddad, Matsuoka, Hollis, Hu, & Wortsman, 1993). Vitamin D binding protein stabilizes and escorts the newly formed D3 to the liver, where hydroxylation at the 25th carbon occurs. Inactive vitamin D [25(OH)D3] is generated in this reaction and is released into the blood where VDBP not only plays the role of carrier protein, but due to its high affinity and binding capacity for 25(OH)D3, VDBP serves as a short-term storage depot and provides rapid availability of 25(OH) D3 to blood-perfused tissues of the body (Cooke & Haddad, 1989; Haddad et al., 1993). Vitamin D binding protein-25(OH)D3 complexes are selectively endocytosed from glomerulus filtrate into the proximal nephron tubules of the kidneys, where a second hydroxylation reaction at the 1st carbon occurs, which converts 25(OH)D3 to 1,25(OH)2 D3, the active form of vitamin D (Cooke & Haddad, 1989). In the nephrotic system and as well in vitamin D-responsive tissue, 1-alpha-hydroxylation of 25(OH)D3 is mediated by cytochrome p450 27B1 (CYP27B1) activity. Over-production of 1,25(OH)2D3 is regulated by the simultaneous expression and subsequent activity of 25(OH) D3 and 1,25(OH)2 D3-degrading enzyme CYP24A1 (Holick, 2007) (Fig. 1). In addition to the nephrotic-mediated vitamin D activation pathway, several studies have shown that vitamin D activation mediated by the hydroxylation at the 1st carbon can also be mediated by extra-nephrotic/hepatic issues (Bouillon, De Groot, & Jameson, 2001).
Fig. 1.

Illustration of vitamin D circulation in the blood, and its metabolism in the skin, liver, kidney, and lungs.
For example, several types of pulmonary cells – bronchial epithelial cells, alveolar epithelial cells, macrophages, and monocytes – are 1,25(OH)2 D3-responsive via vitamin D receptor activity, but also can convert 25(OH) D3 to biologically active 1,25(OH)2 D3 via 1α-hydroxylase (CYP27B1) activity. Pulmonary cells also utilize 24-hydroxylase reactions to regulate vitamin D-VDR signaling and downstream vitamin D-mediated cellular activities (Holick, 2007; Jacobs et al., 2008; Jones, 2008; McDonald, Bald, & Cassatella, 1997). Of the many endogenous activities of active vitamin D3, one of the most important roles include upregulation and activation of broad-spectrum endogenous antimicrobial peptides such as cathelicidin/LL-37 in pulmonary macrophages and epithelial cells. In individuals with healthy circulating levels of vitamin D, endogenous antimicrobial peptides play significant roles protecting the human pulmonary system from infection and harmful pathogens by assisting the innate immune system to directly eliminate or indirectly recruit other peptides to fight invading pulmonary pathogens (Jones, 2008). In contrast, low levels of circulating inactive vitamin D3 have been associated with increased susceptibility to infections, for example, Mycobacterium tuberculosis infection, with documented severities in cases with more severe vitamin D3 deficiencies (Jacobs et al., 2008; Litonjua & Weiss, 2007).
Cathelicidin/LL-37 is an anti-microbial peptide that has been identified in monocytes, neutrophils, airway epithelium, and bronchial alveolar lavage fluid (Holick, 2007; Jones, 2008; Lo, Paris, & Holick, 1986). Endogenous antimicrobial peptides play important roles in the innate immune system by activating reactive oxygen species in macrophages as well as by inducing the release of proteins from other antimicrobial peptides including alpha (α) -defensin (Janssens et al., 2010; Johns Hopkins Medical Institutions, 2011). They also bind lipopolysaccharide residues, disrupt foreign cellular membranes, and recruit other immune cells to sites of injury or infection (Jerrells et al., 2007; Jin & Baillie, 1997; Kent, Devlin, Gutteridge, & Retallack, 1979). Cathelicidin/LL-37 proteins are positively regulated by active vitamin D [1,25(OH)2D3] stimulation of vitamin D-response elements (VDRE) encoded in the promoter region of cathelicidin/LL-37 genes (Jacobs et al., 2008; Janssen-Heininger, Poynter, & Baeuerle, 2000; Kasama, Strieter, Standiford, Burdick, & Kunkel, 1993; Lo et al., 1986; Lowenthal & Levy, 1999; White, 2010). Published studies report that excessive exposure to ethanol disrupts vitamin D metabolism and its activation. Subsequently, vitamin D dysmetabolism leads to many downstream inflammatory and immune-related effects, such as generation of endogenous antimicrobial peptides, e.g., defensins, tumor necrosis factor-alpha (TNF-α), cathelicidin, and others (Lee et al., 2004; Mehls, Wolf, & Wille, 1989; Schwalfenberg, 2011; Wang, Walter, Herting, Agerberth, & Johansson, 2004).
Diallyl disulfide (DADS) is an organosulfur compound found in garlic (Allium sativum). DADS has been found to be an efficient agent for detoxification and has chemopreventive properties in vivo and in vitro (McCaskill, Hottor, Sapkota, & Wyatt, 2015). With the ability to modulate CYP2E1-mediated bioactivation of chemicals, CYP2E1 preferentially catalyzes the oxidation of the sulfur atom to form the diallyl sulfoxide and sulfone (DASO and DASO2). This metabolic conversion suggests that DADS inhibits the metabolism of the CYP2E1 substrate by competitive inhibition mechanisms and by inactivating CYP2E1 via a suicide-inhibitory action of sulfone (Gao, Jiang, Wang, Zhao, & Wang, 2013).
The primary objective of this study is to characterize effects of physiologically relevant levels of ethanol on vitamin D metabolism and activation of anti-microbial peptides based on the observations from our published in vivo study (McCaskill, Hottor, Sapkota, & Wyatt, 2015). The secondary objective of this study is to further elucidate roles of organosulfur DADS (diallyl disulfide) in attenuating ethanol-mediated vitamin D and cathelicidin/LL-37 dysregulation in the human pulmonary system, specifically in bronchial epithelial, alveolar epithelial, and monocyte cells.
Materials and methods
Cell culture and treatment
Bronchial epithelial virus-transformed cells (BEAS-2B, ATCC CRP-9606) were cultured under serum-free conditions using the ATCC’s recommended protocol. BEAS-2B cells were grown in 25-cm2 culture flasks in BEBM medium (Lonza CC-3171) supplemented with BEGM (Lonza CC-3170) under controlled conditions (37 °C and 5% CO2).
Acute monocytic cells (THP-1, ATCC TIB-202) were cultured according to ATCC’s protocol. THP-1 cells were grown in 25-cm2 culture flasks in RPMI medium supplemented with fetal bovine serum (FBS, ATCC 30–2020) under controlled conditions (37 °C and 5% CO2). Human pulmonary alveolar epithelial cells (HPAEpiC; ScienCell Research Laboratories catalog no. 3200) were cultured according to the protocol from ScienCell Research Laboratory. HPAEpiC cells were grown in 25-cm2 culture flask in alveolar epithelial cell medium (AEpiCM, Cat. #3201) under controlled conditions (37 °C and 5% CO2).
Primary bronchial/tracheal epithelial cells (HBEC, ATCC PCS-300–010) were cultured under serum-free conditions using ATCC’s recommended protocol. HBEC cells were grown in 25-cm2 culture flask in airway epithelial basal medium (ATCC PCS-300–030) supplemented with bronchial epithelial cell growth kit (ATCC PCS-300–040) under controlled conditions (37 °C and 5% CO2).
After attaining confluency, the cell lines were divided into different treatment groups: medium only, medium supplemented with physiologically relevant levels of ethanol (70 mM) (Gao et al., 2013; Lamminpaa & Vilska, 1991; Olson, Smith, Kloss, Ho, & Apple, 2013; Pelissier, Lauque, Charpentier, & Franchitto, 2014; Urso, Gavaler, & Van Thiel, 1981), medium supplemented with 10-μM DADS, and medium supplemented with a mixture of 70-mM ethanol and 10-μM DADS. As a vehicle, 0.1% dimethyl sulfoxide (DMSO) was added to the medium for all treatment groups, including the control group. Loss of ethanol to vaporization was minimized by the maintenance of constant temperature and pressure in a closed environment. Steady-state equilibrium can be maximized in a cell culture flask, which results in ethanol evaporation of no more than 30% during the 24-hour incubation (Eysseric et al., 1997). However, the fluctuation of solubilized ethanol in vitro may mimic ethanol elimination via vaporization observed in in vivo systems (Dolganiuc & Szabo, 2009). Moreover, BEAS-2B cells were treated with increasing concentrations of calcidiol/25(OH)D3 dissolved in 0.1% DMSO (0, 5, 20, 50, and 100 ng/mL) for a dose-response relationship assessment. Cells were exposed in a designated treatment medium for either 10 hours or 24 hours, after which 25(OH)D3 and 1,25(OH)2D3 levels were quantified. In separate experiments, LL-37 expression levels were quantified after a 48-hour experimental time course. Dose concentration of ethanol was chosen to model high pulmonary ethanol exposure levels documented by blood alcohol content (BAC) that is commonly observed in admitted emergency department alcohol use disorder patients (Olson et al., 2013; Sisson, 2007).
Enzyme-linked immunosorbent assay (ELISA) for 25(OH)D3, 1, 25(OH)2D3, cathelicidin/LL-37, CYP2E1, and CYP27B1 quantification
Following completion of treatment, cells were scraped, re-suspended in phosphate-buffered saline (PBS), and then lysed/homogenized to prepare for the assay. 25-hydroxy vitamin D EIA (Immuno-diagnostic-systems, AC-57F1) was used according to manufacturer’s instructions to quantify 25(OH)D3 levels in the cellular lysate samples. In addition, 1, 25(OH)2D3 levels were quantified using 1, 25-hydroxy vitamin D EIA (Immuno-diagnostic-systems, AC-62F1). To quantify levels of cathelicidin/LL-37, LL-37, a human ELISA kit (Hycults Biotech, catalog no. HK321–02) was used according to manufacturer’s instructions. For CYP2E1 quantification, a human cytochrome P450 2E1 ELISA kit (MyBioSource, MBS2021746) was used according to the manufacturer’s protocol. CYP27B1 levels in cellular lysate were analyzed using a human cytochrome P450 27B1 ELISA kit (MyBioSource, MBS053337). The eluted lysates were quantified and normalized using spectrophotometry (Nanodrop, Thermo-Scientific, Wilmington, Delaware, United States) absorbance ratios (Greenfield, 2018).
Western blot
Following completion of treatment, BEAS-2B cells were scraped, re-suspended in phosphate-buffered saline (PBS), and then lysed/homogenized to prepare for the assay. An equal amount of protein of BEAS-2B cell lysate was separated on 4–12% Bis-Tris gel, transferred to a PVDF membrane, and blocked with Starting Block (TBS) blocking buffer (ThermoFisher-Scientific, catalog number 37543). The membrane was incubated with mouse monoclonal anti-cathelicidin antibody (ThermoFisher-Scientific, MA5–18048, 1:1000) for 2 hours at room temperature. As an internal control, a mouse monoclonal anti-alpha-tubulin antibody was used (DSHB University of Iowa, 12G10, 1:3000). For imaging of cathelicidin (CAMP) and α-tubulin protein, the membrane was incubated with IRDye 800CW goat anti-mouse IgG (H+L) (Li-Cor Biosciences, P/N 926–32210, 1:15000) for 1 hour at room temperature. Afterward, the membrane was processed with the Odyssey CLx Imaging system (Li-Cor Biosciences). Densitometric analysis of bands was performed using Image Studio software (Li-Cor Biosciences).
Cytotoxicity assay
THP-1, BEAS-2B, and primary bronchial epithelial cells were cultured in a flat-bottom, 96-well plate compatible with spectrophotometry. The culture media contained testing compounds: medium only, medium supplemented with 70-mM ethanol, medium supplemented with 10-mM DADS, and medium supplemented with a mixture of 70-mM ethanol and 10-mM DADS. Cells were cultured in the designated medium overnight under controlled conditions (37 °C, 5% CO2). Then the assay was conducted following instruction of the Piere LDH cytotoxicity assay kit (ThermoFisher-Scientific, catalog number 88953).
Statistical analysis
All quantitative experiments were performed independently and in triplicate. Experimental data were analyzed using Graph Pad Prism version 5.00 for Windows (Graph Pad Software, San Diego, California, United States). Non-linear regression was used for each experiment, data obtained were used to approximate using nonlinear regression. The means and standard error (SE) were compared to control and treated groups of cell mixture. When comparing three or more groups, a repeated-measure analysis of variance (ANOVA) (p < 0.05) followed by Bonferroni’s post hoc test was used to control for multiple comparisons. We used one-tailed Student’s t test to identify any difference in the means between two groups. All results were graphed to visually represent gradual measurement changes within each group and between each group.
A p value of <0.05 was considered as statistically significant.
Results
DADS restored inactive vitamin D [25(OH)D3] levels in ethanol-exposed epithelial cells
When compared to the control group, the levels of 25(OH)D3 protein were reduced significantly (~30% decrease) in BEAS-2B cells exposed to 700-mM ethanol for 24 hours. Concomitant exposure of DADS to ethanol-treated cells reversed and recovered levels of 25(OH)D3 protein to within control levels (Fig. 2A). Moreover, this trend of response was observed in primary alveolar epithelial cells. The levels of 25(OH)D3 protein were significantly reduced (~35% decrease) in primary alveolar epithelial cells exposed to 70-mM ethanol for 24 hours. Additional treatment with 10-μM DADS to ethanol-treated cells restored the 25(OH)D3 protein levels to ~50 ng/mL/mg of total protein, and this level was significantly higher than the levels of the ethanol-treated group at ~25 ng/mL/mg of total protein. This increase was also relatively comparable to control levels at ~57 ng/mL/mg of total protein (Fig. 2B).
Fig. 2. 25(OH)D3 levels in cellular lysate of DADS-treated ethanol-exposed BEAS-2B and primary alveolar epithelial cells.

(A) In BEAS-2B cells, 24-hour exposure to 70-mM ethanol reduced 25(OH)D3 levels when compared with untreated controls (approximately 25% decrease). Treatment of ethanol-exposed cells with 10-μM DADS restored 25(OH)D3 levels to above control levels. The increase in 25(OH)D3 level was significant when compared with untreated control (16%) and ethanol-only-exposed group (~40% increase). (B) Comparable phenomenon observed in primary alveolar epithelial cells. Ethanol exposure decreased 25(OH)D3 by 50% when compared to controls.
* indicates statistically significant difference from the untreated control, p value < 0.05.
# indicates statistically significant difference from the untreated ethanol-exposed group, p value < 0.05.
DADS restored active vitamin D [1,25(OH)2D3] levels in ethanol-exposed epithelial cells
Exposure to 70-mM ethanol for 24 hours did not show a significant reduction in 1,25(OH)2D3 in BEAS-2B cells (~ 25% decrease). However, treatment with 10-μM DADS to ethanol-treated cells increased 1, 25(OH)2D3 levels to ~240 ng/mL/mg of total protein, a demonstrable increase (>100%) in comparison to the ethanol-treated group at ~6.1 ng/mL/mg of total protein (Fig. 3A). Parallels to this increasing trend were also observed in primary alveolar epithelial cells. When primary alveolar epithelial cells were treated with 70-mM ethanol for 24 hours, 1, 25(OH)2D3 levels were reduced to 0.6 ng/mL/mg of total protein (60% decrease) as compared to (untreated) control levels. Co-exposure to 10-μM DADS recovered 1, 25(OH)2D3 protein levels to within the standard error of the mean of the control (Fig. 3B).
Fig. 3. 1,25(OH)2D3 levels in cellular lysate of DADS-treated ethanol-exposed BEAS-2B and primary alveolar epithelial cells.

(A) 24-hour exposure of BEAS-2B cells to 70-mM ethanol slightly reduced (non-significantly) 1,25(OH)2D3 protein levels (~25%) when compared with untreated controls. Treatment of ethanol-exposed cells with 10-μM DADS significantly restored 1,25(OH)2D3 levels to above control levels. This increase was significant when compared with the ethanol-only-exposed group. (B) Similar response observed in the primary alveolar epithelial cells. The ethanol-only-exposed group had a significant reduction (~55%) in levels of 1,25(OH)2D3 protein when compared with untreated controls. Additional treatment of ethanol-exposed cells with DADS restored 1,25(OH)2D3 protein levels to above control levels (24% increase). This increase was over 100% when compared with the untreated, ethanol-only-exposed group.
DADS restored cathelicidin/LL-37 levels in ethanol-exposed pulmonary cells
Ethanol-treated BEAS-2B cells (24-hour exposure) had significantly lower cathelicidin/LL-37 levels when compared to untreated control. Cathelicidin/LL-37 protein levels of the ethanol-treated group were reduced from ~1.8 ng/mL/mg of total protein observed in control to ~1.0 ng/mL/mg of total protein, a reduction of 45%. Concomitant treatment with 10μM DADS of the ethanol-treated group restored cathelicidin/LL-37 levels to ~1.4 ng/mL/mg of total protein, a ~40% increase when compared to the ethanol-treated group (Fig. 4A), and this comparable pattern was also observed in cathelicidin (CAMP) protein expression in western blot analysis. The protein expression in the western blot analysis shows significant CAMP reduction when BEAS-2B cells have been exposed to ethanol, as compared to control. Treatment with 10μM DADS of the ethanol-treated group increased the CAMP protein expression when compared to the ethanol-treated group (Fig. 5). Similar reductions in cathelicidin/LL-37 were also observed in alveolar epithelial cells when treated with 70-mM ethanol for 24 hours (~0.02 ng/mL/mg of total protein; 80% decrease), displaying a complete recovery of cathelicidin/LL-37 in the co-exposed treatment of 10-μM DADS as compared to levels of untreated control (Fig. 4B).
Fig. 4. Cathelicidin/LL-37 levels in cellular lysate of DADS-treated ethanol-exposed BEAS-2B and primary alveolar epithelial cells.

(A) When BEAS-2B cells were treated with 70-mM ethanol for 24 hours, a reduction in cathelicidin/LL-37 levels, when compared with untreated controls, was observed (~45% reduction). Treatment of ethanol-only-exposed cells with 10-μM DADS restored cathelicidin/LL-37 levels to 1.4 ng/mL/mg, a 29% increase above that which was observed when compared with the untreated ethanol-exposed group. (B) Similar pattern of response observed in primary alveolar epithelial cells. Significant reduction of cathelicidin/LL-37 (~80%) protein levels was observed in primary alveolar epithelial cells that were exposed to ethanol. The effect was reversed by treatment of ethanol-only-exposed cells with DADS, with final levels at a higher value than observed with untreated controls.
* indicates statistically significant difference from the untreated control, p value < 0.05.
# indicates statistically significant difference from the untreated ethanol-exposed group, p value < 0.05.
Fig. 5. Modulation of cathelicidin (CAMP) protein expression in cellular lysate of DADS-treated, ethanol-exposed BEAS-2B cells.

When BEAS-2B cells were treated with 70-mM ethanol for 24 hours, CAMP protein (19 kDa) was statistically diminished, compared to control. CAMP protein was significantly more abundant when the ethanol-exposed group was treated with 10-μM DADS. α-tubulin (49.5 kDa) was used as an internal control to normalize density of CAMP protein.
* indicates statistically significant difference from the untreated control, p value < 0.05.
# indicates statistically significant difference from the untreated ethanol-exposed group, p value < 0.05.
Studies presented in this manuscript led the authors to select 24 hours as a concluding time line for experiments on dose-response assessment. When BEAS-2B cells were treated with different concentrations of calcidiol/25(OH)D3 for 24 hours, increases in levels of cathelicidin/LL-37 protein were observed when compared to untreated control (no calcidiol treatment) at 1.0 ng/mL/mg of total protein. The cathelicidin/LL-37 protein levels were significantly increased to ~1.4 ng/mL/mg of total protein (40% increase) and to ~1.25 ng/mL/mg of total protein (25% increase) when treated with 5 ng/mL and 20 ng/mL of calcidiol, respectively. The most effective calcidiol/25(OH)D3 concentration in BEAS-2B cells as measured by LL-37 production was 50 ng/mL, which elicited an 80% increase in LL-37 levels as compared to the control. A significant reduction in the levels of cathelicidin/LL-37 (~50% as compared to control) was observed at calcidiol/25(OH)D3 concentration of 100 ng/mL, signifying an upper limit of positive calcidiol LL-37 protein expression modulation (Fig. 6).
Fig. 6. Dose-response relationship between cathelicidin/LL-37 levels in BEAS-2B cells and treatment with calcidiol or 25(OH)D3.

After 24-hour exposure, a different dose treatment from 5 ng/mL to 20 ng/mL of calcidiol increased cathelicidin/LL-37 levels at relatively comparable levels to those of untreated controls. The percentage increases in cathelicidin/LL-37 levels were ~23% and ~21% when BEAS-2B cells were treated with 5 ng/mL and 20 ng/mL of calcidiol, respectively. The highest cathelicidin/LL-37 protein level increase was observed when BEAS-2B cells were treated with 50 ng/mL (approximately 80% increase). However, treatment with 100 ng/mL calcidiol is probably cytotoxic, as it significantly reduced cathelicidin/LL-37 protein levels when compared with untreated controls and other groups treated with smaller doses.
* indicates statistically significant difference compared with the untreated control, p value < 0.002.
# indicates statistically significant difference compared with the untreated control, p value < 0.0001.
After optimal treatment concentration of calcidiol was justified, time-course experiments of calcidiol and calcitriol were performed using optimal treatment concentration of 50 ng/mL. Treatment of 25(OH)D3 or calcidiol to BEAS-2B cells increased cathelicidin/LL-37 levels after a 6-hour exposure. Increase in cathelicidin/LL-37 reached its highest peak of ~0.45 ng/mL/mg of total protein at 10 to 12 hours after treatment. Following this exposure, cathelicidin/LL-37 levels gradually declined to ~0.3 ng/mL/mg of total protein after 24 hours of treatment. Furthermore, levels of the protein remained relatively stable until the 48 hours after treatment. This comparable trend was also observed when BEAS-2B cells were treated with 50 ng/mL of calcitriol/1, 25(OH)2D3. Cathelicidin/LL-37 levels were increased after a 6-hour exposure and reached highest peak response at ~0.44 ng/mL/mg of total protein at 10 to 12 hours after treatment. The levels of protein were slightly declined to ~0.42 ng/mL/mg of total protein at 24 hours and remained constant after 48 hours of exposure/treatment (Fig. 7). This experiment suggests the optimal duration to determine cathelicidin/LL-37 response is 10 to 24 hours after exposure. Therefore, exposure duration for additional experiments investigating the effect of exposure to ethanol on cathelicidin/LL-37 levels will be determined within this range of exposure.
Fig. 7. Time course experiment of calcidiol [25(OH)D3] and calcitriol [1, 25(OH)2D3] treatment in BEAS-2B cells.
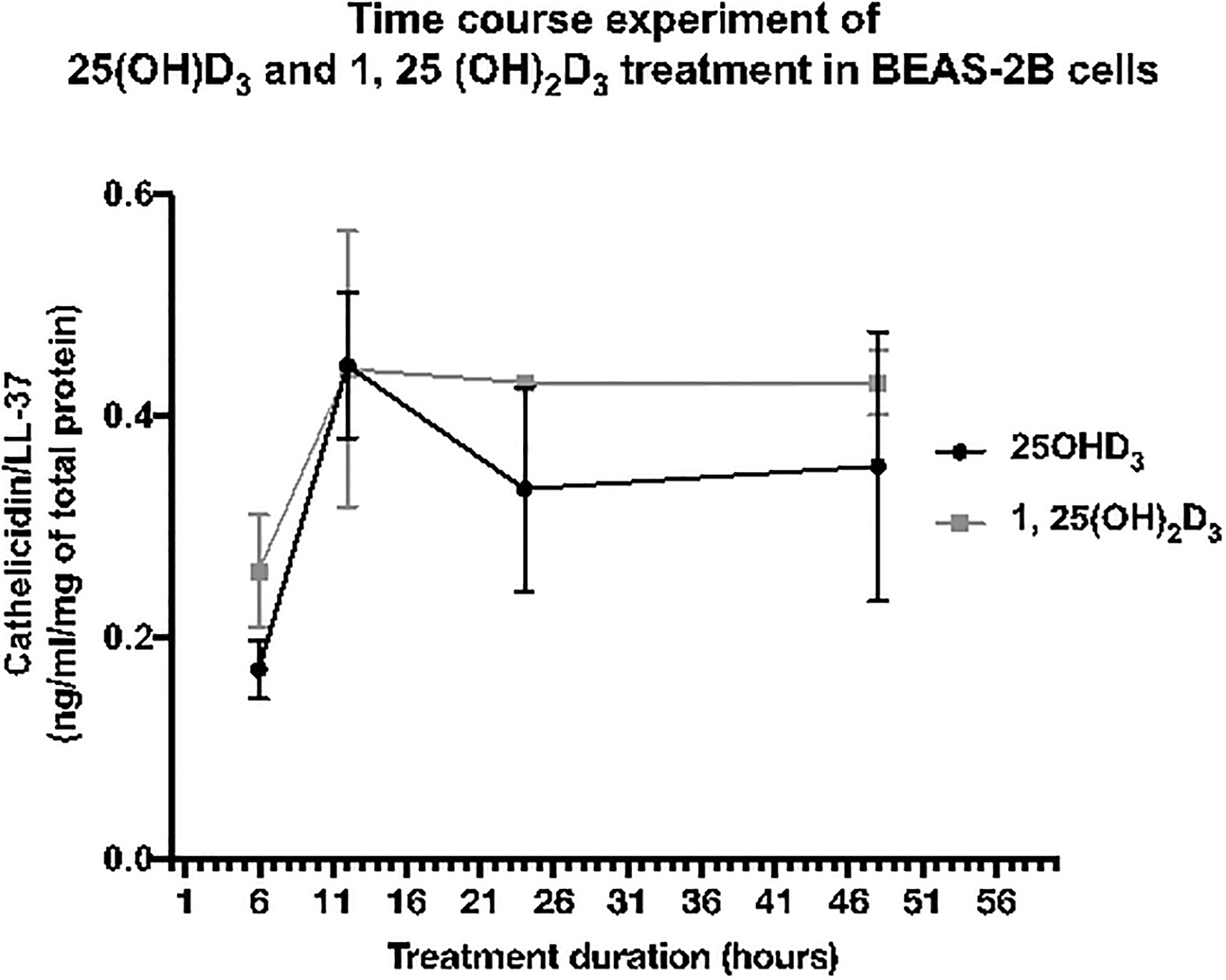
Treatment with 50-ng/mL calcidiol increased cathelicidin/LL-37 levels after exposure for 6 hours, and reached the highest response at 10 to 12 hours post-treatment. After 10 to 12 hours of exposure to 50-ng/mL calcidiol, cathelicidin/LL-37 levels gradually declined to ~0.3 ng/mL/mg of total protein and remained relatively stable for up to 48 hours post-treatment. This trend was also observed in calcitriol (0.05 ng/mL) treatment. The highest peak of cathelicidin/LL-37 response to calcidiol and calcitriol was observed when BEAS-2B cells were treated for 12 hours, and this response remained relatively stable after 24 hours of exposure. Therefore, exposure duration for further experiments in this study was determined to be 10-hour and 24-hour exposure.
To determine whether the observations from our experiments reflected true effects from the treated samples, and were not influenced by a toxicity-mediated cell death, we performed a cytotoxicity assay. Fig. 8A, B, and C displays the percent of cytotoxicity in THP-1 cells, BEAS-2B, and primary bronchial epithelial cells elicited by exposure to experimental treatments. When compared to untreated control, treatment with 70-mM ethanol, 10-μM DADS, and treatment mixture did not show a significant difference in cytotoxicity. These results potentially confirm that treatment of samples with ethanol and DADS at this concentration (70 mM and 10 μM, respectively) for 24 hours did not cause treatment-specific cell death or any other off-target effects, and the response observed from the experiment was in congruence with our null hypothesis.
Fig. 8. Cytotoxicity levels after 24-hour exposure of THP-1, BEAS-2B, and primary bronchial epithelial cells to ethanol and DADS.

(A) The cytotoxicity of 70-mM ethanol and 10-μM DADS in THP-1 cells after 24-hour exposure was expressed as % cytotoxicity. When compared with control levels, treatment with ethanol and DADS alone did not show any significant difference in % cytotoxicity. (B) Treatment with ethanol did not show any significant difference in % cytotoxicity in BEAS-2B, when compared to controls. However, treatment with DADS alone and combination treatment of ethanol and DADS slightly increased (non-significantly) levels of % cytotoxicity when compared with the control. (C) Combination treatment with ethanol and DADS in primary bronchial epithelial cells did not show any significant difference in cytotoxicity. However, treatment with either ethanol or DADS alone slightly increased levels of cytotoxicity but not to a statistically significant degree.
When compared to untreated control, cathelicidin/LL-37 levels in THP-1 cells treated with 70-mM ethanol for 10 hours were significantly reduced to ~3.0 ng/mL/mg of total protein or ~50% decrease as compared to ~6.5 ng/mL/mg of total protein of untreated control. The cathelicidin/LL-37 levels were significantly increased to 5.6 ng/mL/mg of total protein when the ethanol-treated group was treated with 10-μM DADS (Fig. 9A). However, the ethanol exposure for 10 hours did not produce a statistically significant reduction in cathelicidin/LL-37 levels (0.08 to 0.02 ng/mL/mg of total protein or 75% increase, approximately) when compared to untreated control. Additional treatment with 10-μM DADS to the ethanol-treated group increased cathelicidin/LL-37 levels ~60% to 0.03 ng/mL/mg of total protein (Fig. 9B). A similar trend of cathelicidin/LL-37 response was also observed in experiments involving primary bronchial epithelial cells. Ten-hour exposure to ethanol decreased cathelicidin/LL-37 levels to ~0.3 ng/mL/mg of total protein or ~40% reduction when compared to the levels of untreated control at ~0.5 ng/mL/mg of total protein. A 0.45 ng/mL/mg of total protein or about a 40% increase in cathelicidin/LL-37 was observed when 10-μM DADS concomitantly exposed to the ethanol-treated group (Fig. 9C).
Fig. 9. Cathelicidin/LL-37 levels in cellular lysate of DADS-treated ethanol-exposed THP-1, primary alveolar epithelial, and primary bronchial epithelial cells.
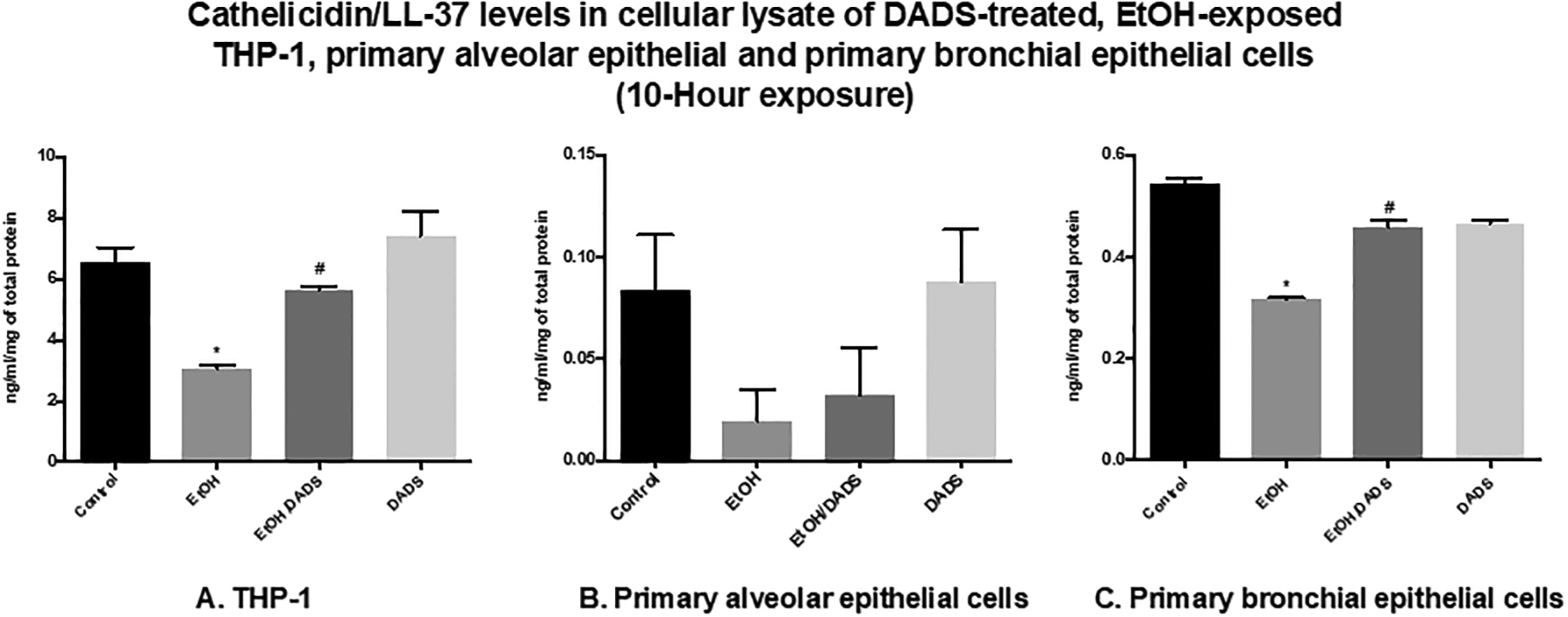
(A) 10-hour exposure to 70-mM ethanol reduced cathelicidin/LL-37 levels in THP-1 cells compared with controls (~50% reduction). Additional treatment of ethanol-exposed cells with 10-μM DADS restored cathelicidin/LL-37 levels to those of controls. This increase was statistically significant (over ~80% when compared with the untreated ethanol-exposed group). (B), (C) Comparable response observed in primary alveolar epithelial and primary bronchial epithelial cells. Exposure to ethanol decreased cathelicidin/LL-37 levels in primary alveolar epithelial and primary bronchial epithelial cells (75% and 40% reduction, respectively). Additional treatment of ethanol-exposed cells with DADS restored cathelicidin/LL-37 levels, which was statistically significant when compared to untreated ethanol-exposed group (60% and 45% increase, respectively).
* indicates statistically significant difference from the untreated control, p value < 0.05.
# indicates statistically significant difference from the untreated ethanol-exposed group, p value < 0.05.
Similar modules of treatment were also applied to the next set of cell lines and treatment groups, but exposure duration was changed to 24 hours. When compared to untreated control, cathelicidin/LL-37 levels in THP-1 cells treated with 70-mM ethanol for 24 hours were significantly reduced to approximately 2.7 ng/mL/mg of total protein, or a 65% decrease as compared to ~8.4 ng/mL/mg of total protein level observed in the untreated control. Additional treatment with 10-μM DADS to the ethanol-treated group significantly reversed cathelicidin/LL-37 levels to ~9.3 ng/mL/mg of total protein, which was above levels in untreated control, and it was a greater than 100% increase when compared to the ethanol-treated group (Fig. 10A). This trend was also observed in primary alveolar epithelial cells treated with 70-mM ethanol for 24 hours. Cathelicidin/LL-37 levels were significantly decreased to ~0.02 ng/mL/mg of total protein or an 80% reduction when compared to levels of untreated control at ~0.10 ng/mL/mg of total protein. The addition of 10-μM DADS to the ethanol-treated group increased cathelicidin/LL-37 levels to ~0.15 ng/mL/mg of total protein or 80% as compared to the ethanol-treated group (Fig. 10B). However, 24-hour exposure to 70-mM ethanol did not produce a statistically significant decrease in cathelicidin/LL-37 levels when compared to untreated control. It reduced cathelicidin/LL-37 from ~3.1 to ~1.2 ng/mL/mg of total protein, or approximately a 60% reduction as compared to control. Additional treatment with 10-μM DADS to the ethanol-treated group restored cathelicidin/LL-37 levels to 2.6 ng/mL/mg of total protein, or a greater than 100% increase when compared to the ethanol-treated group (Fig. 10C).
Fig. 10. Cathelicidin/LL-37 levels in cellular lysate of DADS-treated ethanol-exposed THP-1, primary alveolar epithelial, and primary bronchial epithelial cells.

(A) 24-hour exposure to 70-mM ethanol reduced cathelicidin/LL-37 levels in THP-1 cells when compared with controls (~65% reduction). Additional treatment of ethanol-exposed cells with 10-μM DADS restored cathelicidin/LL-37 levels to above control levels, a statistically significance increase of over 100% when compared with untreated ethanol-exposed cells. (B), (C) Similar response pattern observed in primary alveolar epithelial and primary bronchial epithelial cells. Cathelicidin/LL-37 levels were significantly reduced when cells were treated with ethanol (80% and 60% in primary alveolar and bronchial epithelial cells, respectively). Additional treatment of ethanol-exposed cells with DADS increased cathelicidin/LL-37 levels to above control levels, an increase of over 100% when compared to untreated ethanol-exposed cells.
* indicates statistically significant difference from the untreated control, p value < 0.05.
# indicates statistically significant difference from the untreated ethanol-exposed group, p value < 0.05.
DADS attenuated metabolizing enzyme CYP2E1 levels and restored vitamin D-activating enzyme CYP27B1 levels in ethanol-exposed epithelial cells
Exposure to 70-mM of ethanol for 24 hours noticeably increased CYP2E1 levels in BEAS-2B cells to ~1.9 ng/mL/mg of total protein, a 200% increase when compared to untreated control. The increase in CYP2E1 levels was drastically attenuated to 0.3 ng/mL/mg of total protein when the ethanol-treated group was treated with 10-μM DADS. In this experiment, CYP2E1 levels were less than observed levels in untreated control at ~0.5 ng/mL/mg of total protein, or ~80% reduction when compared to the ethanol-treated group (Fig. 11A). The reverse response was observed in the CYP27B1 experiment. Twenty-four-hour exposure to 70-mM ethanol decreased CYP27B1 levels to ~0.03 ng/mL/mg of total protein, or an approximately 90% reduction when compared to ~0.4 ng/mL/mg of total protein, the level observed in the untreated control. Additional treatment with 10-μM DADS on the ethanol-treated group restored CYP27B1 levels to ~0.3 ng/mL/mg of total protein (Fig. 11B).
Fig. 11. Alcohol-metabolizing enzyme CYP2E1 and vitamin D-activating enzyme CYP27B1 in cellular lysate of DADS-treated ethanol-exposed BEAS-2B cells.

(A) Exposure to 70-mM ethanol for 24 hours increased CYP2E1 levels when compared with untreated controls. Additional treatment of ethanol-exposed cells with 10-μM DADS attenuated the effect, reducing CYP2E1 to levels comparable to those of untreated controls. (B) Opposite response observed in CYP27B1 levels. Exposure to ethanol significantly reduced CYP27B1 levels when compared with untreated controls. Additional treatment of ethanol-exposed cells with DADS restored CYP27B1 to levels comparable with those of untreated controls.
* indicates statistically significant difference from the untreated control, p value < 0.05.
# indicates statistically significant difference from the untreated ethanol-exposed group, p value < 0.05.
Discussion
In this study, various pulmonary cell lines were selected based on their distinct characteristics to determine their individual susceptibility to excessive ethanol exposure as measured by 25(OH)D3, 1,25(OH)2D3, and cathelicidin levels. This study has affirmed that vitamin D metabolism and subsequent activation of antimicrobial peptide cathelicidin/LL-37 in human pulmonary epithelial cells and monocytes can be significantly disrupted by exposure to physiologically relevant levels of ethanol. The disruption in vitamin D metabolism by exposure to excessive ethanol is evident by the statistically significant reductions in intracellular levels of 25(OH)D3, 1, 25(OH)2D3, and LL-37 protein expression in pulmonary epithelial and monocyte cells. Concomitant treatment with DADS elicited a significant recovery of 25(OH)D3, 1,25(OH)2D3, and LL-37 in each experimental cell line. Even though inactive precursor vitamin D-25(OH)D3, as well as enzyme CYP27B1, were significantly decreased, 1,25(OH)2D3 levels in BEAS-2B cells were not statistically reduced accordingly when exposed to high levels of ethanol. The authors hypothesize that due to the highly regulated nature of 1,25(OH)2D3 in mammalian systems, it is possible that ethanol-mediated perturbations in 1,25(OH)2D3 levels occurred quickly and the cellular model was nearly in full recovery before our elected time point. However, the downstream effect of ethanol-mediated 1,25(OH)2D3 inhibition was still observed, as reductions in cathelicidin were significant in treated BEAS-2B cells. Data from these in vitro model experiments aligned with published reports of ethanol-induced reductions and DADS-mediated recovery in 25(OH)D3, 1,25(OH)2D3 assayed from whole lung tissue homogenate harvested from C57BL/6 mice that had been chronically fed ethanol and DADS (McCaskill et al., 2015).
Acknowledging the risks and clear limitations of attempting to translate in vitro data directly to human experiences, the authors were encouraged by the observation that the most effective concentration of calcidiol treatment for producing LL-37 in BEAS-2B cells falls within the published optimal range for immunomodulatory benefits in previous epidemiological vitamin D studies (Dawson-Hughes et al., 2005).
When exposed to physiologically relevant levels of calcidiol (50 ng/mL), constitutive LL-37 levels in primary human bronchial epithelial cells, primary type I/II alveolar epithelial cells, and ThP-1 monocyte cells varied by nearly 2 orders of magnitude (6.4 ng/mL/mg total protein to 0.075 ng/mL/mg total protein). THP-1 cells displayed the highest levels of constitutive LL-37 protein (~6.4 ng/mL/mg total protein) followed by HBE cells (0.53 ng/mL/mg total protein), while the lowest levels displayed were in the HPAEpi cells (0.073 ng/mL/mg total protein). Due to the fundamental cellular function of monocytes, increased intracellular stores of LL-37 seemed appropriate. Unexpectedly, after 24 hours of calcidiol treatment, HBE cells displayed the most robust (40-fold) increase in LL-37 protein expression. Although THP-1 cells displayed the most substantial calcidiol-mediated LL-37 protein expression overall, HBE cells are vitamin D-responsive and possibly the most susceptible to fluctuating levels of calcidiol availability as measured by intra-cellular LL-37.
All three cell types displayed ethanol-mediated significant reductions in LL-37 at the 10-and 24-hour time points.
LL-37 levels in HPAEpis, THP-1, and primary bronchial epithelial ethanol-exposed cells were 75%, 50%, and 40% of control, respectively. From these results, we may be able to conclude that the most susceptible cell type to excessive ethanol exposure in vitro in descending order was alveolar epithelium, monocyte, and bronchial epithelium. HPAEpi cells constitutively expressed very low levels of calcidiol-induced LL-37 at both time points, and ethanol-induced LL-37 reductions were particularly extreme in the 24-hour exposure group, suggesting that alveolar epithelial cells may be selectively at risk of ethanol-induced vitamin D dysregulation.
Alveolar epithelium may be more susceptible to exposure to ethanol than bronchial epithelium, possibly due to its metabolic and physiological characteristics. The lung alveolar epithelial surface is mostly covered by type I cells in vivo; type I cells are transformed to type II during cell culture. Although type II cells are more metabolically active and have more complex cytoplasmic fluid containing endoplasmic reticulum and CYP450 detoxifying enzymes, the alveolar epithelium is more susceptible to xenobiotic than bronchial epithelium or Clara cells. Non-ciliated bronchial epithelial (Clara) cells are more cytologically and enzymatically heterogenous. As a result, they will contain more smooth endoplasmic reticulum and CYP450 enzymes.
This observation was further confirmed by more extended exposure duration (24 hours), where reduction of cathelicidin/LL-37 levels in the ethanol-treated THP-1, HPAEpiC, and primary bronchial epithelial cells were 65%, 80%, and 60%, respectively, and the observed reduction was more severe when compared to 10-hour exposure. According to our results, ethanol-induced statistically significant reductions in intracellular THP-1 LL-37 provide insight into the effects of chronic alcohol binge exposure in the immune response. Pulmonary monocytes and macrophages play integral roles in innate host defense and in activation of cathelicidin/LL-37, an important anti-microbial peptide with significant protective functions in the human immune system (Baines, 1978; Hansdottir et al., 2008).
These results provide a potential explanation of why a subject with AUD might present with more severe manifestation and frequent respiratory infections due to low levels of vitamin D. Not only is cathelicidin/LL-37 needed for neutralizing endotoxins in the human system (Engs & Aldo-Benson, 1995; Gombart, 2009), but it also serves as a chemoattractant for activation of neutrophils and mononuclear cells (Dorschner et al., 2001; Frohm et al., 1997; Gombart, 2009; Hansdottir et al., 2008; Ong et al., 2002).
CYP2E1 has been established to play a role in the metabolism of many diverse xenobiotics, specifically, its inducible degradative role in ethanol metabolism (Lieber, 1997; Lu & Cederbaum, 2008). We observed significantly higher levels of CYP2E1 protein expression in cell lines when treated with ethanol concentrations. When ethanol-treated BEAS-2B cells were treated with 10-μM DADS, CYP2E1 protein concentration levels were reduced by approximately 80% of ethanol-treated BEAS-2B cells. This pattern highly suggested potential inhibitory effects of DADS on CYP2E1 (Lu & Cederbaum, 2008). In a different in vitro experiment, our laboratory observed that DADS is at least as effective at inhibiting CYP2E1 as 4-methylpyrazole-mediated inhibition, (potent CYP2E1 substrate inhibitor; data not shown). Moreover, the observed ethanol-mediated modulation of CYP2E1 and CYP27B1 protein expression in BEAS-2B, HBE, HPAE, and THP-1 cells confirms the metabolic competency necessary for vitamin D activation and ethanol metabolism. Additionally, concomitant treatment with 10-μM DADS attenuated the induction of ethanol-mediated CYP2E1 and recovered CYP27B1 proteins in each of this study’s cell types.
When similar studies were conducted in a murine model, a significant reduction in the levels of CYP27B1 proteins was observed in the ethanol-treated mice when compared to untreated control (McCaskill et al., 2015). These observations provide fundamental insight into the chemopreventive properties of DADS applied to the inhibition of ethanol-induced vitamin D and cathelicidin/LL-37 dysmetabolism.
Conclusion
Findings from our research showed statistically reduced levels of 25(OH)D3 and 1, 25(OH)2D3 protein in bronchial and alveolar epithelial cells when exposed to physiologically relevant levels of ethanol, and the effect was reversed by treatment with 10-μM DADS. High levels of ethanol exposure to pulmonary cells via the bronchial circulation have the potential to disrupt the metabolism of vitamin D and indirectly downregulate activation of cathelicidin/LL-37. This dysregulation of vitamin D-mediated LL-37 may be clinically relevant because this protein helps to protect the human pulmonary system from infections directly and via the activation of other anti-microbial peptides. Results from this study have the potential to improve clinical management of AUD by increasing our knowledge and understanding of the mechanism of excessive ethanol exposure on vitamin D metabolism in pulmonary cells. The chemopreventive attributes of DADS as an ethanol inhibitor can also be potentially leveraged in the development of a more effective therapeutic intervention for infection prevention in high-risk patients with alcohol use disorders.
Highlights.
High exposure to ethanol reduces lung intracellular 25(OH)D3 and 1,25(OH)2D3 protein.
Lung LL-37 protein helps to protect the human pulmonary system from infections.
High exposure to ethanol reduces essential intracellular lung antimicrobial LL-37.
Diallyl disulfide treatment reverses ethanol-induced reduction of vitamin D and LL-37.
Abbreviations
- ANOVA
analysis of variance
- AUD
alcohol use disorder
- BEAS-2B
human bronchoepithelial cells
- CYP2E1
cytochrome P450 (2E1)
- CYP24A1
cytochrome P450 (24A1)
- CYP27B1
cytochrome P450 (27B1)
- DADS
diallyl disulfide
- ELISA
enzyme-linked immunosorbent assay
- LL-37
cathelicidin/LL-37
- NIAAA
National Institute on Alcohol Abuse and Alcoholism
- PELE
pulmonary epithelium lining environment
- TLR2/1
toll-like receptor family (receptors of the innate immune system)
- URTIs
upper respiratory tract infections
- VDR
vitamin D receptors
- VDRE
vitamin D response elements
- 1, 25(OH)2 D3
1, 25 dihydroxycholecalciferols
- 25(OH) D3
25, hydroxyergocalciferols
References
- Baines M (1978). Detection and incidence of B and C vitamin deficiency in alcohol-related illness. Annals of Clinical Biochemistry, 15, 307–312. doi: 10.1177/000456327801500173 [DOI] [PubMed] [Google Scholar]
- Bouillon R, De Groot LJ, & Jameson JL (2001). Vitamin D: From photosynthesis, metabolism, and action to clinical applications. Saunders; Retrieved from https://clinicalgate.com/vitamin-d-from-photosynthesis-metabolism-and-action-to-clinical-applications/ [Google Scholar]
- Cesur Y, Caksen H, Gündem A, Kirimi E, & Odabaş D (2003). Comparison of low and high dose of vitamin D treatment in nutritional vitamin D deficiency rickets. Journal of Pediatric Endocrinology & Metabolism, 16, 1105–1109. [DOI] [PubMed] [Google Scholar]
- Cooke NE, & Haddad JG (1989). Vitamin D binding protein (Gc-globulin). Endocrine Reviews, 10, 294–307. doi: 10.1210/edrv-10-3-294 [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, & Vieth R (2005). Estimates of optimal vitamin D status. Osteoporosis International, 16, 713–716. doi: 10.1007/s00198-005-1867-7 [DOI] [PubMed] [Google Scholar]
- Dolganiuc A, & Szabo G (2009). In vitro and in vivo models of acute alcohol exposure. World Journal of Gastroenterology, 15, 1168–1177. doi: 10.3748/wjg.15.1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, et al. (2001). Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A streptococcus. The Journal of Investigative Dermatology, 117, 91–97. doi: 10.1046/j.1523-1747.2001.01340.x [DOI] [PubMed] [Google Scholar]
- Engs RC, & Aldo-Benson M (1995). The association of alcohol consumption with self-reported illness in university students. Psychological Reports, 76, 727–736. doi: 10.2466/pr0.1995.76.3.727 [DOI] [PubMed] [Google Scholar]
- Eysseric H, Gonthier B, Soubeyran A, Bessard G, Saxod R, & Barret L (1997). There is no simple method to maintain a constant ethanol concentration in long-term cell culture: keys to a solution applied to the survey of astrocytic ethanol absorption. Alcohol, 14, 111–115. [DOI] [PubMed] [Google Scholar]
- Frohm M, Agerberth B, Ahangari G, Stâhle-Bäckdahl M, Lidén S, Wigzell H, et al. (1997). The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. The Journal of Biological Chemistry, 272, 15258–15263. doi: 10.1074/jbc.272.24.15258 [DOI] [PubMed] [Google Scholar]
- Gao C, Jiang X, Wang H, Zhao Z, & Wang W (2013). Drug metabolism and pharmacokinetics of organosulfur compounds from garlic. Journal of Drug Metabolism and Toxicology, 4, 159. doi: 10.4172/2157-7609.1000159 [DOI] [Google Scholar]
- Goldsmith RH, Iber FL, & Miller PA (1983). Nutritional status of alcoholics of different socioeconomic class. Journal of the American College of Nutrition, 2, 215–220. [DOI] [PubMed] [Google Scholar]
- Gombart AF (2009). The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiology, 4, 1151–1165. doi: 10.2217/fmb.09.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield EA (2018). Protein Quantitation. Cold Spring Harbor Protocols, 2018 pdb.prot098202. doi: 10.1101/pdb.prot098202 [DOI] [PubMed] [Google Scholar]
- Haddad JG, Matsuoka LY, Hollis BW, Hu YZ, & Wortsman J (1993). Human plasma transport of vitamin D after its endogenous synthesis. The Journal of Clinical Investigation, 91, 2552–2555. doi: 10.1172/JCI116492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, & Hunninghake GW (2008). Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. Journal of Immunology, 181, 7090–7099. doi: 10.4049/jimmunol.181.10.7090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlastala MP (1998). The alcohol breath test--a review. Journal of Applied Physiology, 84, 401–408. doi: 10.1152/jappl.1998.84.2.401 [DOI] [PubMed] [Google Scholar]
- Holick MF (2007). Vitamin D deficiency. The New England Journal of Medicine, 357, 266–281. doi: 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- Huber GL, First MW, & Grubner O (1991). Marijuana and tobacco smoke gas-phase cytotoxins. Pharmacology, Biochemistry, and Behavior, 40, 629–636. [DOI] [PubMed] [Google Scholar]
- Hughes DA, & Norton R (2009). Vitamin D and respiratory health. Clinical and Experimental Immunology, 158, 20–25. doi: 10.1111/j.1365-2249.2009.04001.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs ET, Alberts DS, Foote JA, Green SB, Hollis BW, Yu Z, et al. (2008). Vitamin D insufficiency in southern Arizona. The American Journal of Clinical Nutrition, 87, 608–613. doi: 10.1093/ajcn/87.3.608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen-Heininger Y, Poynter ME, & Baeuerle PA (2000). Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radical Biology & Medicine, 28, 1317–1327. [DOI] [PubMed] [Google Scholar]
- Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, et al. (2010). Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax, 65, 215–220. doi: 10.1136/thx.2009.120659 [DOI] [PubMed] [Google Scholar]
- Jerrells TR, Pavlik JA, DeVasure J, Vidlak D, Costello A, Strachota JM, et al. (2007). Association of chronic alcohol consumption and increased susceptibility to and pathogenic effects of pulmonary infection with respiratory syncytial virus in mice. Alcohol, 41, 357–369. doi: 10.1016/j.alcohol.2007.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, & Baillie TA (1997). Metabolism of the chemoprotective agent diallyl sulfide to glutathione conjugates in rats. Chemical Research in Toxicology, 10, 318–327. doi: 10.1021/tx9601768 [DOI] [PubMed] [Google Scholar]
- Johns Hopkins Medical Institutions. (2011). Low vitamin D in kids may play a role in anemia. Retrieved from https://www.sciencedaily.com/releases/2011/05/110501195148.htm
- Jones G (2008). Pharmacokinetics of vitamin D toxicity. The American Journal of Clinical Nutrition, 88, 582S–586S. doi: 10.1093/ajcn/88.2.582S [DOI] [PubMed] [Google Scholar]
- Kasama T, Strieter RM, Standiford TJ, Burdick MD, & Kunkel SL (1993). Expression and regulation of human neutrophil-derived macrophage inflammatory protein 1alpha. The Journal of Experimental Medicine, 178, 63–72. doi: 10.1084/jem.178.1.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns MD, Binongo J, Watson D, Alvarez JA, Lodin D, Ziegler TR, et al. (2014). The effect of a single, large bolus of vitamin D in healthy adults over the winter and following year: a randomized, double-blind, placebo-controlled trial. European Journal of Clinical Nutrition, 69, 193–197. doi: 10.1038/ejcn.2014.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent JC, Devlin RD, Gutteridge DH, & Retallack RW (1979). Effect of alcohol on renal vitamin D metabolism in chickens. Biochemical and Biophysical Research Communications, 89, 155–161. doi: 10.1016/0006-291X(79)90957-4 [DOI] [PubMed] [Google Scholar]
- Lamminpaa A, & Vilska J (1991). Acid-base balance in alcohol users seen in an emergency room. Veterinary and Human Toxicology, 33, 482–485. [PubMed] [Google Scholar]
- Lee HY, Andalibi A, Webster P, Moon SK, Teufert K, Kang SH, et al. (2004). Antimicrobial activity of innate immune molecules against streptococcus pneumoniae, Moraxella catarrhalis and nontypeable Haemophilus influenzae. BMC Infectious Diseases, 4, 12. doi: 10.1186/1471-2334-4-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS (1997). Cytochrome P-4502E1: its physiological and pathological role. Physiological Reviews, 77, 517–544. doi: 10.1152/physrev.1997.77.2.517 [DOI] [PubMed] [Google Scholar]
- Litonjua AA, & Weiss ST (2007). Is vitamin D deficiency to blame for the asthma epidemic? The Journal of Allergy and Clinical Immunology, 120, 1031–1035. doi: 10.1016/j.jaci.2007.08.028 [DOI] [PubMed] [Google Scholar]
- Lo CW, Paris PW, & Holick MF (1986). Indian and Pakistani immigrants have the same capacity as Caucasians to produce vitamin D in response to ultraviolet irradiation. The American Journal of Clinical Nutrition, 44, 683–685. doi: 10.1093/ajcn/44.5.683 [DOI] [PubMed] [Google Scholar]
- Lowenthal A, & Levy R (1999). Essential requirement of cytosolic phospholipase A(2) for activation of the H(+) channel in phagocyte-like cells. The Journal of Biological Chemistry, 274, 21603–21608. doi: 10.1074/jbc.274.31.21603 [DOI] [PubMed] [Google Scholar]
- Lu Y, & Cederbaum AI (2008). CYP2E1 and oxidative liver injury by alcohol. Free Radical Biology & Medicine, 44, 723–738. doi: 10.1016/j.freeradbiomed.2007.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaskill ML, Hottor HT, Sapkota M, & Wyatt TA (2015). Dietary diallyl disulfide supplementation attenuates ethanol-mediated pulmonary vitamin D speciate depletion in C57Bl/6 mice. BMC Nutrition, 1, pii:18. Epub 2015 Aug 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald PP, Bald A, & Cassatella MA (1997). Activation of the NF-kappaB pathway by inflammatory stimuli in human neutrophils. Blood, 89, 3421–3433. [PubMed] [Google Scholar]
- Mehls O, Wolf H, & Wille L (1989). Vitamin D requirements and vitamin D intoxication in infancy. International Journal for Vitamin and Nutrition Research. Supplement, 30, 87–94. [PubMed] [Google Scholar]
- Olson KN, Smith SW, Kloss JS, Ho JD, & Apple FS (2013). Relationship between blood alcohol concentration and observable symptoms of intoxication in patients presenting to an emergency department. Alcohol and Alcoholism, 48, 386–389. doi: 10.1093/alcalc/agt042 [DOI] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. (2002). Endogenous antimicrobial peptides and skin infections in atopic dermatitis. The New England Journal of Medicine, 347, 1151–1160. doi: 10.1056/NEJMoa021481 [DOI] [PubMed] [Google Scholar]
- Pelissier F, Lauque D, Charpentier S, & Franchitto N (2014). Blood alcohol concentration in intoxicated patients seen in the emergency department: does it influence discharge decisions? Journal of Studies on Alcohol and Drugs, 75, 937–944. doi: 10.15288/jsad.2014.75.937 [DOI] [PubMed] [Google Scholar]
- Schwalfenberg GK (2011). A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Molecular Nutrition & Food Research, 55, 96–108. doi: 10.1002/mnfr.201000174 [DOI] [PubMed] [Google Scholar]
- Sisson JH (2007). Alcohol and airways function in health and disease. Alcohol, 41, 293–307. doi: 10.1016/j.alcohol.2007.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urso T, Gavaler JS, & Van Thiel DH (1981). Blood ethanol levels in sober alcohol users seen in an emergency room. Life Sciences, 28, 1053–1056. doi: 10.1016/0024-3205(81)90752-9 [DOI] [PubMed] [Google Scholar]
- Wang Y, Walter G, Herting E, Agerberth B, & Johansson J (2004). Antibacterial activities of the cathelicidins prophenin (residues 62 to 79) and LL-37 in the presence of a lung surfactant preparation. Antimicrobial Agents and Chemotherapy, 48, 2097–2100. doi: 10.1128/AAC.48.6.2097-2100.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JH (2010). Vitamin D as an inducer of cathelicidin antimicrobial peptide expression: past, present and future. Journal of Steroid Biochemistry and Molecular Biology, 121, 234–238. doi: 10.1016/j.jsbmb.2010.03.034 [DOI] [PubMed] [Google Scholar]


