Abstract
Multiple Myeloma (MM) is highly sensitive to disruptions in cellular protein homeostasis. Proteasome inhibitors (PIs) are initially effective in the treatment of MM, although cures are not achievable and the emergence of resistance limits the durability of responses. New therapies are needed for refractory patients, and those that combat resistance to standard of care agents would be particularly valuable. Screening of multiple chemical libraries for PI re-sensitizing compounds identified E61 as a potent enhancer of multiple PIs and MM specific activity. Using a tandem approach of click chemistry and peptide mass fingerprinting, we identified multiple protein disulfide isomerase (PDI) family members as the primary molecular targets of E61. PDIs mediate oxidative protein folding, and E61 treatment induced robust ER and oxidative stress responses as well as the accumulation of ubiquitinylated proteins. A chemical optimization program led to a new structural class of indene (exemplified by lead E64FC26), which are highly potent pan-style inhibitors of PDIs. In mice with MM, E64FC26 improved survival and enhanced the activity of bortezomib without any adverse effects. This work demonstrates the potential of E64FC26 as an early drug candidate and the strategy of targeting multiple PDI isoforms for the treatment of refractory MM and beyond.
Introduction
Multiple myeloma (MM) is an incurable hematological malignancy characterized by the accumulation of clonal plasma cells within the bone marrow. The plasma cell is naturally designed for the mass production of immunoglobulin (Ig) proteins and is capable of producing thousands of Igs per second [1]. This specialized production and secretory capacity is remarkable; however, it comes at a cost to the cell. High rates of protein synthesis and folding are physiologically taxing, and plasma cells are characterized by elevated levels of cellular stress that include activation of the unfolded protein response (UPR) and high levels of oxidative stress, as protein disulfide bond formation generates equimolar quantities of reactive oxygen species (ROS) [2–5]. Therefore, the natural biology of the plasma cell makes MM uniquely sensitive to disruptions in protein homeostasis, a vulnerability that can be exploited therapeutically [6]. The first evidence of this therapeutic opportunity was apparent in early phase clinical trials of bortezomib/VELCADE® (Btz) where measurable responses were observed in nearly all MM patients [7, 8]. The magnitude and rate of response to Btz are often dramatic, although most patients eventually progress to a stage of resistance. Next-generation PIs, such as carfilzomib/ KYPROLIS® (Crflz), offer hope to Btz refractory patients, however a large percentage of these patients are unresponsive to Crflz as well [9, 10].
Targeting protein folding as a strategy for the treatment of cancer has been proposed and includes the inhibition of molecular chaperones like HSP90 [11, 12], and more recently the inhibition of protein disulfide isomerase (PDI) [13–16]. PDIs are a family of more than 20 ER resident oxidoreductase enzymes [17]. They primarily ensure proper folding of nascent polypeptides by forming disulfide bonds between cysteine residues. PDI catalytic activity is redox dependent, involving the oxidation of thiols on un/misfolded protein substrates. PDI activity and its role in the control of protein folding has been implicated in the pathogenesis of multiple diseases, including neurodegenerative disorders like Huntington’s [18], Alzheimer’s [19, 20], Parkinson’s disease [21], thrombosis [22, 23], HIV infection [24, 25], and cancer [26]. PDIs are overexpressed in a variety of tumor types including MM [26–28], and experimental PDI inhibitors have been reported [13–16]. However, to date, none of these molecules have advanced to the clinical stage of development despite a well-supported therapeutic rationale for targeting PDIs in oncology. Factors that have impeded PDI inhibitor discovery programs include the assays used for drug screening [29], and a limited focus on inhibitors of PDI (i.e., the PDIA1 isoform), which is just one isoform in a family of more than 20 with varying degrees of overlapping function [30].
Here, we report results from a discovery program that identified a new chemical class of PDI family inhibitor that sensitizes MM cells to PIs. The lead molecules we discuss rapidly induce the accumulation of misfolded poly-ubiquitinylated proteins and the induction of ER and oxidative stress responses. The medicinal chemistry phase of the program delivered clear structure activity relationships (SAR) and led to the discovery of optimized chemical lead E64FC26. E64FC26 outperforms other PDI inhibitors that have been reported in the literature in terms of target potency, broad-spectrum activity against multiple PDI isoforms, and anti-MM activity in cell culture and mouse models of MM.
Materials and methods
Cell lines and reagents
PI-resistant MM.1S BzR and U266 BzR were a generous gift from Dr. Brian Van Ness of the University of Minnesota. All cell lines were cultured in standard conditions. Purity and chemical composition of all synthesized compounds reported were determined by NMR and HPLC-MS. Each derivative synthesized was at least 95% pure.
Cell viability and apoptosis assays
Cell viability and apoptosis were measured in 96-well cell culture plates (3 × 104 cells/well) using the Cell Titer-Glo Luminescent Cell Viability Assay (Promega) and the Caspase-Glo 3/7 Assay (Promega), respectively, according to the manufacturers protocol. Luminescence was recorded on a SpectraMax L Microplate Reader (Molecular Devices, Sunnyvale, CA, USA) at 470 nm with a 1-second integration time.
Labeling of cells with E61-Y
Cells were treated with 5 μM E61 or 5 μM E61-Y for 2 h. Cells were then washed with DPBS, harvested by centrifugation, and stored at −80 °C overnight. The next day, cells were lysed in click chemistry lysis buffer (10 mM NaPO4, pH 7.4, 1% (v/v) Triton X-100, 0.1% (w/v) CHAPS, 1x EDTA free Sigma Protease Inhibitor, and 1x Sigma Phosphatase Inhibitor). Lysate was clarified by centrifugation and protein concentrations were standardized between treated samples as determined by DC protein assay (Bio-Rad).
Click chemistry
All click reactions were performed in click chemistry lysis buffer as previously described with some minor modifications (for details see Supplemental Methods) [31].
Identification of E61 Targets
Protein bands from gels containing the contents of the click pull-downs were excised, digested, and analyzed by LC/MS/MS as previously described (for details see Supplemental Methods) [32–34].
Insulin reduction assays
The reductase activities of the PDI family members were determined by measuring the aggregation of insulin as previously described with some minor modifications (for details see Supplemental Methods) [35].
In vivo studies
All xenotransplant studies with NSG mice were conducted under the approval of the Institutional Animal Care and Use Committee (IACUC) of the Medical University of South Carolina (MUSC). Experiments involving Vk*MYC transgenic mouse models were performed under approval of The Mayo Foundation Institutional Animal Care and Use Committee, and conformed to all the regulatory Environmental Safety standards.
Results
Discovery of PI re-sensitizing small molecules
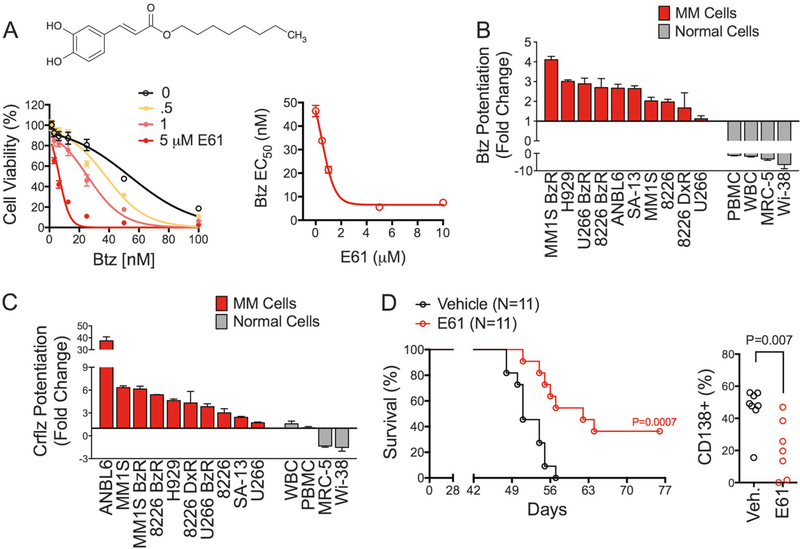
A cell-based drug-screening method was used to identify small molecules that restore sensitivity to PIs in resistant MM cells as described previously [36]. Our lab has previously shown that these PI-resistant cell models lack PSMB5 mutations or drug efflux transporters [37], suggesting that resistance is due to protective cellular adaptations rather than mutations to the proteasome or multidrug resistance transporters. We screened approximately 20,000 compounds spanning multiple chemical libraries (NCI Diversity Set II, NCI Approved Oncology Set, Chembridge DIVERSet, ENZO SCREEN-WELL Redox Library). Compound E61 (n-octyl caffeate, C17H24O4, Fig. 1a) was a striking hit, showing > 6-fold enhancement of Btz cytotoxicity and PI re-sensitizing activity at low micromolar concentrations (Fig. 1a, b). The synergistic effects of E61 on PI-induced cell death was at least in part due to enhanced apoptosis, as E61 increased caspase-8, −9, −3, cleaved PARP, and caspase-3/7 activity (Fig. S1A–B), and pretreatment with the pan-caspase inhibitor Z-VAD-FMK significantly reduced the cytotoxic effects of the E61/Btz combinations (Fig. S1C). These effects were also evident in combination with next-generation PIs, including carfilzomib (Crflz), ixazomib (Ixaz), and oprozomib (Oprz) in both PI sensitive and resistant MM cell lines (Fig. S1D–F). The synergistic effects of E61 showed specificity for PIs, as E61 had no effect on dexamethasone activity in dexamethasone resistant RPMI-8226 cells (Fig. S2A). Also, E61 did not affect either lenalidomide or doxorubicin cytotoxicity in MM.1S BzR cells (Fig. S2B–C). E61 also did not inhibit the chymotrypsin-like activity of the 20S proteasome (Fig. S2D). This suggests that E61 works through a proteasome-independent specific mechanism rather than through a non-specific mechanism that generally enhances cell death.
Fig. 1.
E61 sensitizes MM to PIs and is active in vivo. a MM.1S BzR cells were treated for 24 h with a dose range of Btz in the presence of the indicated concentrations of E61 (bottom left panel). Viability was normalized to RLU values in the absence of Btz to account for cell death induced by E61 alone. Thus any separation of the curves is indicative of a synergistic drug interaction. Btz EC50 values from the left panel were plotted as a function of E61 concentration (bottom right panel). b Waterfall plot showing the change in Btz sensitivity in the presence of 5 μM E61 for the indicated MM (red bars) and normal (gray bars) cell lines. Values greater than 1 indicate sensitization to Btz cytotoxicity and values less than 1 indicate protection from Btz cytotoxicity. Data were extrapolated from individual 8-dose Btz response curves. Fold change was determined by dividing the Btz EC50 in the absence of E61 by the EC50 in the presence of E61. c Waterfall plot showing the change in sensitivity to Crflz in the presence of 5 μM E61. Data were analyzed as described for Fig. 1b. d Kaplan–Meier survival curves (left panel) are shown for NSG mice that were xenotransplanted (i.v.) with MM.1S BzR cells. Mice received daily dosing of vehicle or 50 mg/kg E61 i.p. (P = 0.0007, N = 11) starting 21 days post-xenotransplantation. The percentage of CD138 + cells in the bone marrow is shown (right panel) for mice treated daily with vehicle (N = 8) or 50 mg/kg i.p. E61 (N = 7). Statistical significance was determined by t-test (P = 0.007)
Selective anti-MM activity of hit stage compound E61
We evaluated the activity of E61 in a panel of normal human primary cells in order to rule out non-MM specific PI sensitization. In stark contrast to MM cells, E61 failed to enhance the cytotoxic effects of PIs in normal cells. We tested normal human peripheral blood mononuclear cells (PBMCs) and lymphocytes (WBCs), and also evaluated a number of normal human lung (MRC-5, Wi-38), human foreskin (HFF), and mouse (MEF) fibroblast cell lines. In all cases, E61 either failed to increase PI sensitivity, or provided protection from the cytotoxic effects of Btz and Crflz (Fig. 1b, c). E61 also showed selective anti-MM activity in a panel of genetically diverse MM cell lines as a single agent (Fig. S3). These findings suggest that E61 targets a mechanism that is selectively toxic to MM cells, thereby possibly giving it a wide therapeutic index in vivo.
In vivo anti-MM efficacy of compound E61
We next investigated the anti-MM activity and tolerability of E61 in vivo using a NOD-SCID IL2Rγ−/− (NSG) mouse model (Fig. S4A). This xenotransplant model provides multiple endpoints, including survival and the direct quantification of MM tumor burden in the mouse bone marrow (Fig. S4B–C). E61, at a dose of 50 mg/kg/day (i.p., continuous dosing), showed a clear effect, prolonging median survival by 11 days (51 vs. 62 days, Fig. 1d, P= 0.0007, N= 11). Notably, 4 of the 11 E61-treated mice never reached the survival endpoint, suggesting that E61 induced a durable response in ~1/3 of mice. In a parallel experiment, mice were sacrificed on day 49 for quantitative analysis of human MM cells in bone marrow biopsies. The results mirrored the survival study, as E61-treated mice exhibited a significant reduction in the number of CD138 + MM cells (Fig. 1d, P= 0.007, N= 7–8), with two mice achieving an apparent complete response. E61 was well tolerated after continuous dosing for 40+ days as mice showed no overt signs of distress, nor loss of body weight (Fig. S4D). In summary, these results demonstrate the potential of E61 to enhance the activity of PIs and selectively target MM. Furthermore, the in vivo activity of E61 was strong for a hit stage compound that had not yet undergone chemical optimization. We therefore set out to understand the molecular mechanism of action and to optimize the chemical structure to further improve potency and drug suitability properties.
E61 inhibits PDI family members
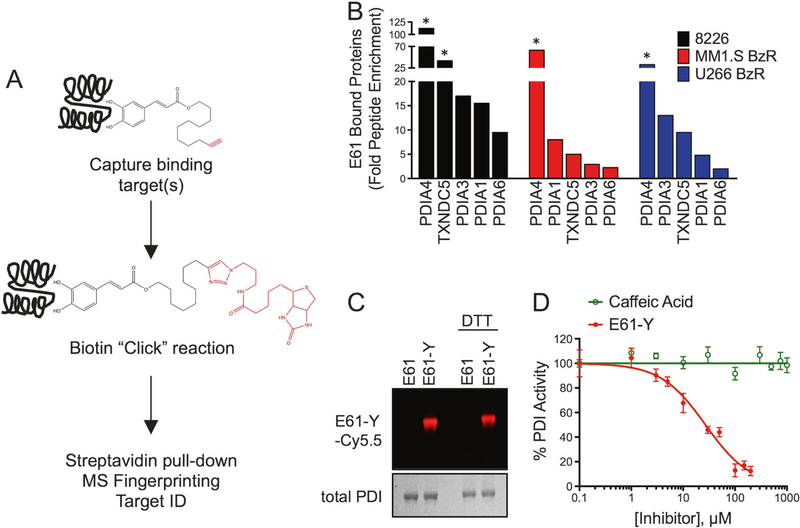
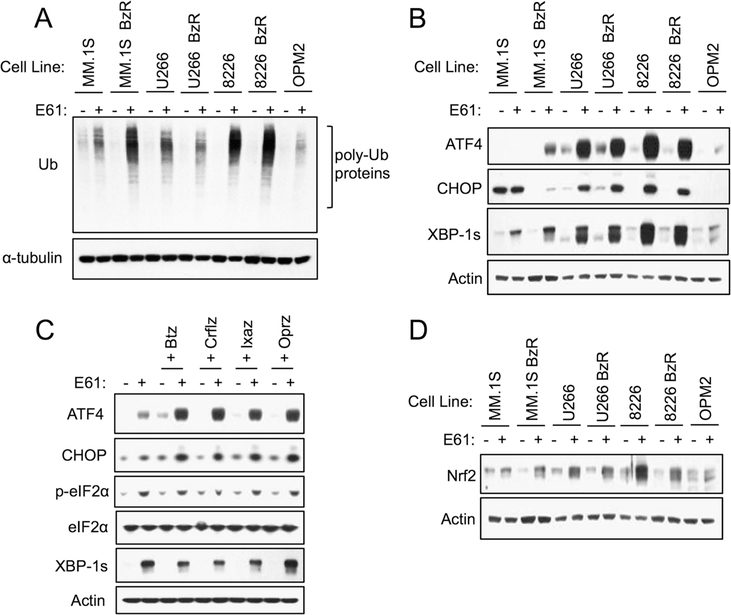
The molecular binding target of E61 was identified using a tandem Cu(I)-catalyzed azide-alkyne cycloaddition (click) chemistry and proteomics approach (Fig. 2a) [31, 38]. We added a terminal alkyne group to E61, a modification that required the addition of three extra carbons to the aliphatic chain in order to preserve the PI sensitizing activity of the molecule (Fig. S5A–B). The alkyne derivative of E61 (E61-Y) was then used to “click” the molecule to labeling and immobilizing agents. We first conducted an evaluation of E61-Y binding to the proteome of MM cells by incubating E61-Y with clarified cell lysates and then labeling drug: protein complexes with Cy5.5-azide. E61-Y was observed to bind to a relatively small group of proteins (Fig. S5C). To determine the identity of E61-Y targets, we then immobilized E61-Y-bound proteins by click chemistry to biotinazide followed by streptavidin pull-down. Mass spectrometry (MS)-based peptide fingerprinting was then used to identify E61 bound proteins. Using three different cell lines, the top hits in all cases were PDI family members (PDIA1, PDIA3, PDIA4, PDIA6, and TXNDC5; Fig. 2b). The MS determinations of target proteins were all high confidence, as defined by observation of > 100 peptides unique to PDI family members. PDI family members were identified in > 10-fold higher abundance in E61-Y pull-downs compared to control pull-downs that were conducted using an unlabeled E61. The enrichment of PDI family members, but not other proteins involved in protein folding (i.e., Grp78/BiP), was confirmed in E61-Y pull-down samples by western blotting (Fig. S5D). We then confirmed binding of E61-Y to PDI in vitro using recombinant PDIA1 (Fig. 2c), further validating PDIs as binding targets. In functional studies, E61 inhibited PDI reductase activity in vitro (Fig. 2d and S5E), while caffeic acid, which has structural similarity to E61, showed no activity as a PDI inhibitor. Caffeic acid also failed to enhance the sensitivity of MM cells to PIs (Fig. S5F). Importantly, this observation correlates activity at the molecular target in vitro and anti-MM function in cells. Further showing the importance of PDIs to MM homeostasis, knockdown of PDIA1, PDIA3, and PDIA4 in MM.1S BzR cells led to growth inhibition (Fig. S6A–C). Given the role of PDI in normal protein folding, we predicted that ER stress and induction of the UPR would be a downstream effect of E61 treatment. In support, E61 treatment led to the accumulation of poly-ubiquitinylated proteins (Fig. 3a), and induced a robust ER stress response characterized by the upregulation of canonical ER stress markers including ATF4, CHOP, and XBP-1s in a variety of PI sensitive and resistant MM cell lines (Fig. 3b). In combination with multiple PIs, E61 enhanced the accumulation of ubiquitinylated proteins and ER stress biomarkers (Fig. 3c and S7).
Fig. 2.
PDI family members are the molecular target of E61. a Schematic outlining the workflow of identification of target proteins using an alkyne labeled E61 derivative (E61-Y) as a probe. b Waterfall plots show the top enriched proteins identified by peptide MS fingerprinting after pull-down with E61-Y in 8226, MM.1S BzR, and U266 BzR cell lines. Enrichment was calculated by dividing the number of peptides identified for each protein in the E61-Y pull-down by the number of peptides identified for the same protein in the unlabeled E61 pull-down control. Proteins denoted with a star indicate proteins that were unique to the E61-Y pull-down and absent in the E61 pull-down. c Recombinant PDIA1 was incubated with E61 or E61-Y (5 μM) in the absence and presence of DTT. Cy5.5 was then conjugated to E61-Y by Cu(I)-catalyzed azide-alkyne cycloaddition chemistry. Drug bound complexes were then separated by SDS-PAGE and imaged using a fluorescence imager. A Coomassie stain of the gel is shown to demonstrate equal loading of PDI protein. d Inhibition of PDI activity by E61-Y and caffeic acid
Fig. 3.
E61 induces ER and oxidative stress in MM cells. a The indicated cell lines were treated with 5 μM E61 for 8 h. Western blots for ubiquitin in whole cell lysates are shown. b The indicated cell lines were treated with 5 μM E61 for 8 h. Western blots for ER stress biomarkers are shown. c MM.1S BzR cells were treated for 8 h ±5 μM E61 and in combination with indicated PIs (25 nM Btz, 25 nM Crflz, 250 nM Ixaz, and 250 nM Oprz). Western blots are shown. d The indicated cell lines were treated with 5 μM E61 for 8 h. Western blots for the cellular antioxidant response marker, Nrf2, are shown
PDIs facilitate proper folding of newly synthesized proteins by forming and isomerizing disulfide bonds by a process that is dependent on redox chemistry [39]. E61 showed a pro-oxidant phenotype in MM cells, inducing ROS as a single agent (Fig. S8A) and enhancing Btz-induced ROS generation (Fig. S8B). Likewise, E61 treatment, alone and in combination with PIs, induced the stabilization of Nrf2, a master regulator of the antioxidant cellular response (Fig. 3d and S8C) [40]. The combination of E61 and Btz led to the oxidation of protein thiols, suggesting that the combination of E61 and PIs, increases oxidative damage to proteins in cells (Fig. S8D). Oxidative stress was a critical effector of the E61 induced anti-MM phenotype, as co-incubation with ROS scavengers abrogated the single-agent cytotoxic effects of E61 (Fig. S8E), and the combination with PIs (Fig. S8F, P < 0.001). These results demonstrate that, in addition to ER stress, E61 induces an oxidative stress response and enhances the activation of both pathways in response to PIs.
Medicinal chemistry optimization and discovery of lead E64FC26
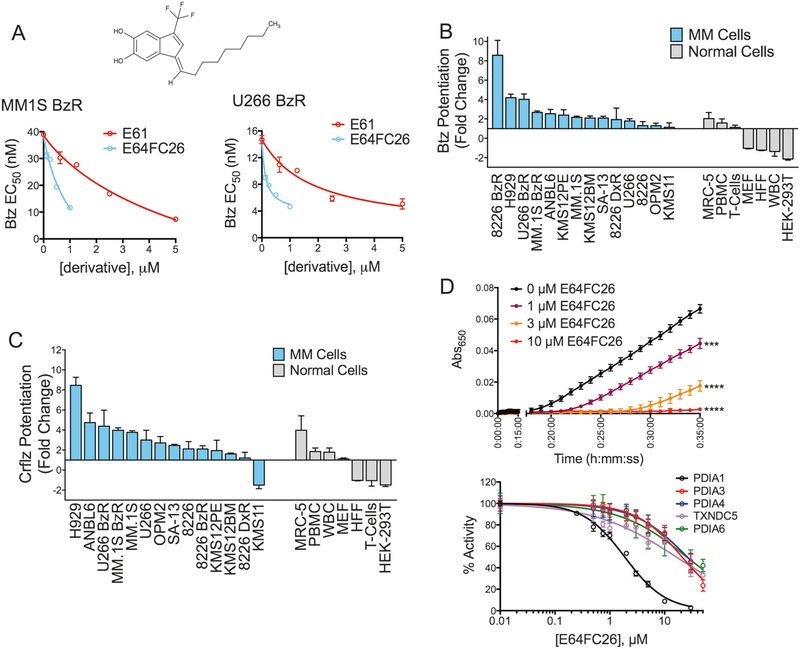
Next, we began a medicinal chemistry and SAR program, synthesizing and evaluating the activities of structural analogs of E61 using cellular models of PI sensitization and PDI inhibition in vitro as screening outputs. Approximately 150 E61 derivatives were synthesized and evaluated. Most notably, we prepared a series of highly potent alkenyl indenes that formed upon cyclization and dehydration of the ketone analog of E61. This led to the discovery of a very active and distinct structural class in our program, exemplified by lead candidate E64FC26 (Fig. 4a). E64FC26 was highly synergistic with PIs at concentrations as low as 200 nM, which is >10 times more potent than E61 in cellular models (Fig. 4a). Likewise, the single-agent anti-MM activity of E64FC26 was significantly greater than E61. After 24 h of exposure, E64FC26 had an EC50 of 0.59 μM compared to 18.3 μM for E61 (Fig. S9). Sub-micromolar concentrations of E64FC26 sensitized a genetically diverse panel of MM cell lines to both Btz and Crflz, whereas non-malignant cell lines were either unaffected or protected from the cytotoxic effects of PIs (Fig. 4b, c). Also, E64FC26 as a single agent was preferentially cytotoxic to MM cell lines over normal cell types, suggesting a wide therapeutic index. As an inhibitor of PDI activity in vitro, E64FC26 was >50 times more potent than E61, with an IC50 of 1.9 ± 0.1 μM against PDIA1 (Fig. 4d). Lead E64FC26 also inhibited all other members of the PDI family tested, including PDIA3, PDIA4, TXNDC5, and PDIA6 (Fig. 4d), demonstrating a pan style of inhibition.
Fig. 4.
E64FC26 is an inhibitor of PDI family members. a Structure of compound E64FC26 and PI sensitizing activity of E64FC26 compared to E61 is shown in MM.1S BzR (left panel) and U266 BzR (right panel) cells. Independent 8-dose Btz kill curves were conducted in the presence of varying concentrations of E64FC26 and E61. Btz EC50 values were extrapolated and plotted against the concentration of E64FC26 or E61. b Waterfall plot showing the change in Btz sensitivity in the presence of 0.5 μM E64FC26 for the indicated MM (blue bars) and normal (gray bars) cell lines. Values greater than 1 indicate sensitization to Btz cytotoxicity and values less than 1 indicate protection from Btz cytotoxicity. Data were extrapolated from individual 8-dose Btz response curves. Fold change was determined by dividing the Btz EC50 in the absence of E64FC26 by the EC50 in the presence of E61. c Waterfall plot showing the change in sensitivity to Crflz in the presence of 0.5 μM E64FC26. Data were analyzed as described for Fig. 4b. d Kinetic traces of PDI activity are shown in the insulin turbidity assay in the presence of the indicated concentrations of E64FC26 (top panel). The in vitro activity of the indicated recombinant PDI isoforms is shown in the presence of a dose range of E64FC26 (bottom)
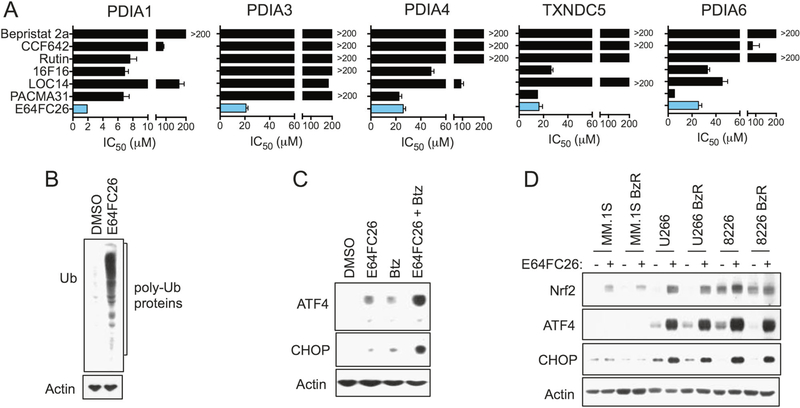
Previous studies have identified PDI as a promising oncology drug target, and developmental inhibitors have been reported by others [13, 15, 16, 18, 22, 41–44]. However, in head-to-head comparisons, E64FC26 showed superior in vitro potency against PDIA1 and the other PDI isoforms that were tested (Fig. 5a, S10, S11A–B, and Table S1). Furthermore, E64FC26 was the only compound to sensitize MM cells to PIs (Fig. S12A and Table S1), with an average increase in PI sensitivity ranging from 6–7 fold. In these experiments, cells were treated with an empirically determined EC10–20 value for each PDI inhibitor in order to normalize for differential cytotoxic effects of each drug (Fig. S11A). E64FC26 was also more cytotoxic against a genetically diverse panel of MM cell lines when compared to non-malignant cells (Fig. S12B). As expected, E64FC26 treatment induced a robust ER stress response in MM cells. Within eight hours of treatment, we observed the accumulation of poly-ubiquitinylated proteins of varying molecular weights (Fig. 5b). E64FC26 induced the expression of ER stress markers ATF4 and CHOP as a single agent and synergistically enhanced the induction of these markers with Btz (Fig. 5c). E64FC26 was superior to the other PDI inhibitors we tested in terms of activating ER stress (Fig. S12C), a result that mirrored the ability of the different inhibitors to synergize with PIs. In addition to the prominent ER stress response, E64FC26 induced an oxidative stress response in a panel of heterogeneous PI sensitive and resistant cell lines that was characterized by the induction of Nrf2 (Fig. 5d). Furthermore, the cytotoxicity of E64FC26 was at least partially dependent on oxidative stress, as co-treatment with the ROS scavenger N-acetyl cysteine significantly reduced effects on cell death (Fig. S12D). To summarize, our SAR program delivered lead E64FC26, which is a potent inhibitor of multiple PDI family members that induces ER and oxidative stress in MM cells and synergistically enhances the anti-MM cytotoxic effects of PIs.
Fig. 5.
E64FC26 is a pan-PDI inhibitor and induces ER and oxidative stress in MM cells. a IC50 values for E64FC26 and other reported PDI inhibitors are shown for the indicated recombinant PDI family members as determined by the insulin reduction assay. Values were extrapolated from 10-dose curves similar to those shown in Fig. 4d. b MM.1S BzR cells were treated for 8 h with 500 nM E64FC26. Western blots indicating a global increase in poly-ubiquitinylated proteins are shown. c RPMI-8226 cells were treated with 500 nM E64FC26, 10 nM Btz, or a combination of the two agents for 8 h. Western blots for the ER stress markers ATF4 and CHOP are shown. d The indicated cell lines were treated with 500 nM E64FC26 for 8 h. Western blots for the cellular antioxidant response protein Nrf2 and ER stress markers ATF4 and CHOP are shown
In vivo anti-MM activity of E64FC26
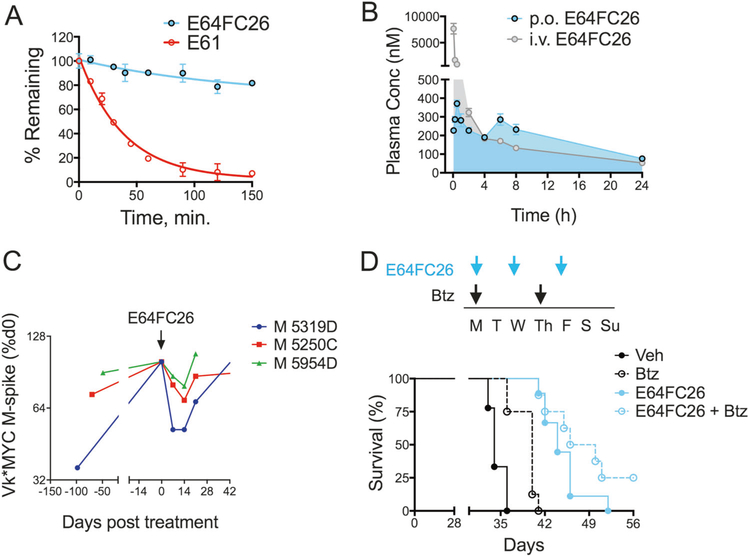
E64FC26 showed increased stability in serum, simulated gastric and intestinal fluids, and human liver microsomes compared to E61 (Fig. 6a and Fig. S13). The calculated intrinsic clearance of E64FC26 in human liver microsomes was 6.22 ± 0.98 μL/min/mg compared to 97.6 ± 6.0 μL/min/mg for E61. Furthermore, pharmacokinetic (PK) analysis in CD-1 mice demonstrated adequate oral bioavailabilty of 34% with systemic exposure approaching a maximum concentration (Cmax) of 400 nM after a single oral dose of 5 mg/kg with a terminal half-life of 9.5 h (Fig. 6b). We next evaluated the single-agent anti-MM efficacy of E64FC26 in vivo using Vk*MYC transgenic mice. Vk*MYC mice develop MM with biological and clinical features that closely resemble human MM. Furthermore, this model is predictive of both clinical activity and inactivity [45, 46], and offers serum M-protein as a quantitiatve biomarker of MM disease progression/burden. Three mice with progressing M-spike levels were treated intermittently with E64FC26 at a dose of 2 mg/kg (i.p.) on days 1, 3, and 5 of each week for 2 weeks. E64FC26 treatment induced an immediate anti-MM response, decreasing serum M-protein in all mice by an average of 33 ± 7.9% (P= 0.0135; Fig. 6c). Similar effects were observed in a human xenotransplant MM model. Mice were randomly assigned to four treatment groups that included vehicle, E64FC26 (2 mg/kg, i.p., 3 days/week), Btz (0.25 mg/kg, i.p., 2 days/week), and the combination (Fig. 6d). E64FC26 showed a clear anti-MM effect in this model also, increasing median survival by 2 weeks over vehicle treated mice, a 40% increase (Fig. 6d, N= 9, P < 0.0001). Highlighting this effect, on day 36, 0% (0/9) of vehicle treated mice were surviving whereas 100% (9/9) of E64FC26 were alive. Single-agent Btz also increased survival by 6 days (N= 8, P= 0.0007). The combination produced the greatest improvement in survival, extending the median survival by 20 days (N= 8, P < 0.0001). Two mice (25%) in the combination group never reached the survival endpoint at the termination of the study, suggesting a complete response was achieved in these mice. No overt toxicity or body weight fluctuations were observed for mice treated with either E64FC26 or the combination (Fig. S14). Toxicology experiments evaluating blood serum chemistry markers revealed mild thrombocytopenia and neutropenia after one week of E64FC26 treatment that resolved itself after a second week of treatment as levels returned to normal (Fig. S15). These results provide preclinical proof-of-concept for the strategy of targeting PDI with this new class of compound for the treatment of MM.
Fig. 6.
E64FC26 ADME-PK and in vivo anti-MM activity. a The stability of E64FC26 and E61 against oxidative metabolism by human liver microsomes. b PK of E64FC26 was measured in CD-1 mice. E64FC26 was administered i.v. (2 mg/kg; gray tracing) or p.o. (5 mg/kg; blue tracing) and plasma drug concentrations were measured over a 24 h period using an optimized LC-MS detection protocol. c Vk*MYC transgenic mice were treated with E64FC26 (2 mg/kg, i.p., 3 days/week) for two consecutive weeks. Blood serum M-protein levels were measured on days 0, 7, and 14 of treatment. M-spike data are shown as a percentage of day 0 for three individual mice. d NSG mice were xenotransplanted with MM.1S cells (1 × 106 cells, i.v.) and randomized to receive vehicle, Btz (0.25 mg/kg, i.p.), E64FC26 (2 mg/kg, i.p.), or combination treatment with both agents. The 7-day dosing schedule is shown (top panel). Treatment was initiated 7 days post-xenotransplantation. Kaplan–Meier survival curves (bottom panel) from NSG mice treated as described are shown. Statistical significance was determined by Wilcoxon rank-sum test (N = 10). Vehicle vs. Btz (P = 0.001), Vehicle vs. E64FC26 (p < 0.0001), Vehicle vs. E64FC26 + Btz (P < 0.0001)
Discussion
Protein homeostasis is critical to the survival of cancer cells given their high metabolic and proliferative demands. This is especially true for MM plasma cells as they continuously express and secrete mass amounts of Ig proteins. Therefore, disrupting the balance of new protein synthesis, folding, and breakdown is a logical therapeutic strategy in oncology, and the clinical success of PIs like bortezomib/VELCADE® and carfilzomib/KYPROLIS® supports this hypothesis. These agents are FDA-approved for the treatment of newly diagnosed and relapsed/refractory MM, respectively, although responses are rarely durable due to the emergence of therapeutic resistance. MM is incurable and refractory patients will benefit from new therapies that specifically target molecular vulnerabilities of resistant cells. E64FC26, the lead developmental small molecule discovered in this study, inhibits multiple PDI isoforms, resulting in global protein misfolding and a proteotoxic stress response that promotes apoptosis in MM cells and enhances the activity of PIs. E64FC26 is an ideal candidate for further development given its superior potency compared to other reported PDI inhibitors, apparent lack of metabolic liabilities or structural alerts common to pan assay interference compounds [47, 48], favorable ADME-PK properties, and in vivo efficacy in mouse models of MM.
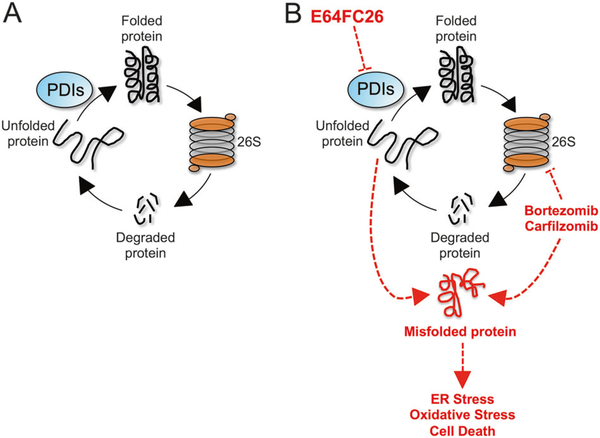
PDIs function at a critical step in protein folding in the ER where they catalyze the oxidative formation of disulfide bonds in nascent polypeptide chains [49]. MM plasma cells have high protein secretory rates that makes them uniquely vulnerable to disruptions in protein homeostasis. We propose a mechanistic model whereby inhibition of PDI isoforms by E64FC26 reduces fidelity of the protein folding process, promoting the accumulation of misfolded and poly-ubiquitinylated proteins, proteotoxic stress, and apoptosis in MM cells (Fig. 7). Simultaneous inhibition of the proteasome by PIs blocks the removal of toxic misfolded proteins and further enhances the proteotoxic/ER stress and apoptotic response. In addition to a robust ER stress response, E64FC26 induces a second critical blow with the induction of oxidative stress. Catalytic centers in PDIs donate and accept electrons from cysteine pairs in client proteins to form and isomerize disulfide bonds, and the induction of oxidative stress is likely the result of disturbing the cellular balance of redox chemistry by inhibition of PDI [39, 50].
Fig. 7.
Model of E64FC26 activity in MM. a Normal protein homeostasis is a cycle that consists of new protein synthesis, proper folding, function, and eventual breakdown by the 26S proteasome to liberate amino acids that allow the cycle to continue. PDI isoforms constitute essential machinery in the ER for the oxidative formation and isomerization of disulfide bonds and proper folding of newly synthesized polypeptides into functional proteins. b E64FC26 inhibits multiple members of the PDI family leading to the accumulation of misfolded proteins and induction of ER stress, oxidative stress, and apoptosis. The 26S proteasome alleviates some of the proteotoxic burden by degrading the misfolded proteins. However, in the presence of PIs, such as bortezomib and carfilzomib, the effects of E64FC26 are magnified, which accounts for the remarkable synergy observed between these two classes of drug
Pathways involved in protein folding have been implicated as potential therapeutic targets and include the HSP90 family and PDIs. HSP90 inhibitors have shown promise in preclinical models and in clinical trials of relapsed/refractory MM [12, 51, 52]. The molecular chaperone network is therefore a potentially viable target for the treatment of MM, and more work is needed in this area perhaps focused on the development of HSP90 isoform specific inhibitors [53–56]. Distinct from HSP90, but also related to protein folding, PDI has been proposed as a therapeutic target for cancer, as well as neurodegenerative diseases, thrombosis, and HIV infection [14, 18, 41, 42, 44, 57]. In cancer, evidence suggests that PDI expression is upregulated in a variety of tumor types and associated with clinical outcomes [26]. However, there are no FDA-approved or clinical stage developmental PDI inhibitors in oncology. Several factors may be contributing to that absence. First, there has been a specific focus on PDI (i.e., PDIA1, P4HB) and not the other ~20 isoforms in the family. It is likely that the other isoforms can compensate for lost PDI activity given that they mediate the same general process of oxidative protein folding [30]. Our data support this hypothesis where nearly all of the previously reported PDI inhibitors show selectivity for PDIA1 [42, 44, 57]. E64FC26, on the other hand, inhibits multiple PDI family members including PDIA1, PDIA3, PDIA4, TXNDC5, and PDIA6. PACMA 31 was the only other compound that inhibited PDIA4, TXNDC5, and PDIA6 similar to E64FC26, but failed to inhibit activity of PDIA3. Moreover, of all the inhibitors evaluated in our study, E64FC26 was the only one able to induce ER stress and sensitize MM cells to Btz and other PIs in a synergistic manner. These findings demonstrate the uniqueness of E64FC26 over other developmental inhibitors and suggest that targeting multiple PDI isoforms is critical therapeutically. Another recognized challenge in the discovery of PDI inhibitors includes the in vitro assay systems that are used to measure PDI activity [29]. One limitation is that these assays require the inclusion of reducing equivalents such as DTT or β-ME, which can interfere with the activity of potential screening hits, especially molecules that are dependent on redox chemistry. The activity of E61, for example, is mitigated in the presence of reducing agents, meaning that E61 would likely have been overlooked in a PDI target-based screen using the available in vitro assays. Our cell-based screening approach, by comparison, was advantageous as it selected for compounds that met the specific phenotypic criteria of synergy with PIs, and PDI was only later discovered to be the target using our click chemistry and peptide mass fingerprinting approach. In fact, the majority of PDI inhibitors reported to date have been discovered by a similar screening sequence rather than by a target-based forward screen for PDI inhibitors [13–15, 18, 41]. A final limitation of existing PDI assays is their low sensitivity. As an example, E61 induces biomarkers of PDI inhibition in cells at low micromolar concentrations, but shows a high micromolar IC50 in vitro. The same trend was observed for E64FC26 and the other PDI inhibitors we tested, demonstrating that the available in vitro assays grossly underestimate the potency of candidate inhibitors.
MM remains incurable for most patients. The treatment refractory population is most in need of new therapies, and a variety of new agents are in development. These include new proteasome inhibitors and other targeted small molecules, monoclonal antibodies, and adoptive cell therapy with chimeric antigen receptor (CAR) T cells. From our perspective, the goal of curability in MM is achievable, but will likely require combinations of the current standard of care plus new approaches. Our findings support the use of PDI inhibitors in the clinical context of refractory MM in combination with standard of care agents. Future studies will reveal whether PDI inhibition enhances the anti-MM activity of newer agents, findings that would establish the rationale for additional combinations. Most notably, our work discovered a new class of PDI inhibitor and delivered a lead development candidate with E64FC26. E64FC26 has several advantages over previously reported PDI inhibitors including superior potency and a pan-style mode of inhibition, optimized ADME-PK properties, and anti-MM activity in cellular and mouse models of MM.
Supplementary Material
Acknowledgements
N.G.D. is an investigator in the South Carolina Center of Biomedical Research Excellence (COBRE) in Oxidants, Redox Balance and Stress Signaling (P20GM103542) and is a Research Scholar of the American Cancer Society (RSG-14-156-01-CDD). This work was also supported by NIH/NCI (1R41CA213488-01), the South Carolina Clinical & Translational Research Institute with an academic home at the Medical University of South Carolina (MUSC; UL1 RR029882 and UL1 TR000062), shared resources of the MUSC Hollings Cancer Center (P30 CA138313), and by the Hollings Cancer Center T32 Ruth L. Kirschstein National Research Service Award Training Program T32 (CA193201). We would also like to thank Pablo Sobrado at Virginia Tech for the generous gifts of the pVP56K vector and the TEV-containing pVP67K vector.
Footnotes
Compliance with ethical standards
Conflict of interest RMR and NGD are inventors on patents. NGD has an ownership interest in Leukogene Therapeutics, Inc. The other authors declare that they have no conflict of interest.
Electronic supplementary material The online version of this article (https://doi.org/10.1038/s41375-018-0263-1) contains supplementary material, which is available to authorized users.
References
- 1.Shimizu Y, Hendershot LM. Oxidative folding: cellular strategies for dealing with the resultant equimolar production of reactive oxygen species. Antioxid Redox Signal. 2009;11:2317–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brewer JW, Hendershot LM. Building an antibody factory: a job for the unfolded protein response. Nat Immunol. 2005;6:23–9. [DOI] [PubMed] [Google Scholar]
- 3.Cenci S, Sitia R. Managing and exploiting stress in the antibody factory. FEBS Lett. 2007;581:3652–7. [DOI] [PubMed] [Google Scholar]
- 4.Bertolotti M, Yim SH, Garcia-Manteiga JM, Masciarelli S, Kim YJ, Kang MH, et al. B- to plasma-cell terminal differentiation entails oxidative stress and profound reshaping of the antioxidant responses. Antioxid Redox Signal. 2010;13:1133–44. [DOI] [PubMed] [Google Scholar]
- 5.Dolloff NG. Emerging therapeutic strategies for overcoming proteasome inhibitor resistance. Adv Cancer Res. 2015;127:191–226. [DOI] [PubMed] [Google Scholar]
- 6.Boise LH, Kaufman JL, Bahlis NJ, Lonial S, Lee KP. The tao of myeloma. Blood. 2014;124:1873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orlowski RZ, Stinchcombe TE, Mitchell BS, Shea TC, Baldwin AS, Stahl S, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002;20:4420–7. [DOI] [PubMed] [Google Scholar]
- 8.Aghajanian C, Soignet S, Dizon DS, Pien CS, Adams J, Elliott PJ, et al. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res. 2002;8:2505–11. [PubMed] [Google Scholar]
- 9.Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S, et al. A phase 2 study of single-agent carfilzomib (PX-171–003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vij R, Wang M, Kaufman JL, Lonial S, Jakubowiak AJ, Stewart AK, et al. An open-label, single-arm, phase 2 (PX-171–004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood. 2012;119:5661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Kung AL, Davies FE, et al. Antimyeloma activity of heat shock protein-90 inhibition. Blood. 2006;107:1092–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richardson PG, Badros AZ, Jagannath S, Tarantolo S, Wolf JL, Albitar M, et al. Tanespimycin with bortezomib: activity in relapsed/refractory patients with multiple myeloma. Br J Haematol. 2010;150:428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vatolin S, Phillips JG, Jha BK, Govindgari S, Hu J, Grabowski D, et al. Novel protein disulfide isomerase inhibitor with anticancer activity in multiple myeloma. Cancer Res. 2016;76:3340–50. [DOI] [PubMed] [Google Scholar]
- 14.Xu S, Butkevich AN, Yamada R, Zhou Y, Debnath B, Duncan R, et al. Discovery of an orally active small-molecule irreversible inhibitor of protein disulfide isomerase for ovarian cancer treatment. Proc Natl Acad Sci USA. 2012;109:16348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge J, Zhang CJ, Li L, Chong LM, Wu X, Hao P, et al. Small molecule probe suitable for in situ profiling and inhibition of protein disulfide isomerase. ACS Chem Biol. 2013;8:2577–85. [DOI] [PubMed] [Google Scholar]
- 16.Allimuthu D, Adams DJ. 2-Chloropropionamide as a low-reactivity electrophile for irreversible small-molecule probe identification. ACS Chem Biol. 2017;12:2124–31. [DOI] [PubMed] [Google Scholar]
- 17.Galligan JJ, Petersen DR. The human protein disulfide isomerase gene family. Hum Genom. 2012;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffstrom BG, Kaplan A, Letso R, Schmid RS, Turmel GJ, Lo DC, et al. Inhibitors of protein disulfide isomerase suppress apoptosis induced by misfolded proteins. Nat Chem Biol. 2010;6:900–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HT, Russell RL, Raina AK, Harris PL, Siedlak SL, Zhu X, et al. Protein disulfide isomerase in Alzheimer disease. Antioxid Redox Signal. 2000;2:485–9. [DOI] [PubMed] [Google Scholar]
- 20.Honjo Y, Ito H, Horibe T, Takahashi R, Kawakami K. Protein disulfide isomerase-immunopositive inclusions in patients with Alzheimer disease. Brain Res. 2010;1349:90–6. [DOI] [PubMed] [Google Scholar]
- 21.Conn KJ, Gao W, McKee A, Lan MS, Ullman MD, Eisenhauer PB, et al. Identification of the protein disulfide isomerase family member PDIp in experimental Parkinson’s disease and Lewy body pathology. Brain Res. 2004;1022:164–72. [DOI] [PubMed] [Google Scholar]
- 22.Jasuja R, Passam FH, Kennedy DR, Kim SH, van Hessem L, Lin L, et al. Protein disulfide isomerase inhibitors constitute a new class of antithrombotic agents. J Clin Invest. 2012;122:2104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho J, Furie BC, Coughlin SR, Furie B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J Clin Invest. 2008;118:1123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallina A, Hanley TM, Mandel R, Trahey M, Broder CC, Viglianti GA, et al. Inhibitors of protein-disulfide isomerase prevent cleavage of disulfide bonds in receptor-bound glycoprotein 120 and prevent HIV-1 entry. J Biol Chem. 2002;277:50579–88. [DOI] [PubMed] [Google Scholar]
- 25.Barbouche R, Miquelis R, Jones IM, Fenouillet E. Protein-disulfide isomerase-mediated reduction of two disulfide bonds of HIV envelope glycoprotein 120 occurs post-CXCR4 binding and is required for fusion. J Biol Chem. 2003;278:3131–6. [DOI] [PubMed] [Google Scholar]
- 26.Xu S, Sankar S, Neamati N. Protein disulfide isomerase: a promising target for cancer therapy. Drug Discov Today. 2014;19:222–40. [DOI] [PubMed] [Google Scholar]
- 27.Kurtoglu M, Philips K, Liu H, Boise LH, Lampidis TJ. High endoplasmic reticulum activity renders multiple myeloma cells hypersensitive to mitochondrial inhibitors. Cancer Chemother Pharmacol. 2010;66:129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horna-Terron E, Pradilla-Dieste A, Sanchez-de-Diego C, Osada J. TXNDC5, a newly discovered disulfide isomerase with a key role in cell physiology and pathology. Int J Mol Sci. 2014;15:23501–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe MM, Laurindo FR, Fernandes DC. Methods of measuring protein disulfide isomerase activity: a critical overview. Front Chem. 2014;2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rutkevich LA, Cohen-Doyle MF, Brockmeier U, Williams DB. Functional relationship between protein disulfide isomerase family members during the oxidative folding of human secretory proteins. Mol Biol Cell. 2010;21:3093–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Presolski SI, Hong VP, Finn MG. Copper-catalyzed azide-alkyne click chemistry for bioconjugation. Curr Protoc Chem Biol. 2011;3:153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. 2006;1:2856–60. [DOI] [PubMed] [Google Scholar]
- 33.Llarrull LI, Toth M, Champion MM, Mobashery S. Activation of BlaR1 protein of methicillin-resistant Staphylococcus aureus, its proteolytic processing, and recovery from induction of resistance. J Biol Chem. 2011;286:38148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren G, Champion MM, Huntley JF. Identification of disulfide bond isomerase substrates reveals bacterial virulence factors. Mol Microbiol. 2014;94:926–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmgren A Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J Biol Chem. 1979;254:9627–32. [PubMed] [Google Scholar]
- 36.Stessman HA, Lulla A, Xia T, Mitra A, Harding T, Mansoor A, et al. High-throughput drug screening identifies compounds and molecular strategies for targeting proteasome inhibitor-resistant multiple myeloma. Leukemia. 2014;28:2263–7. [DOI] [PubMed] [Google Scholar]
- 37.Thompson RM, Dytfeld D, Reyes L, Robinson RM, Smith B, Manevich Y, et al. Glutaminase inhibitor CB-839 synergizes with carfilzomib in resistant multiple myeloma cells. Oncotarget. 2017;8:35863–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Speers AE, Cravatt BF. Profiling enzyme activities in vivo using click chemistry methods. Chem Biol. 2004;11:535–46. [DOI] [PubMed] [Google Scholar]
- 39.Laurindo FR, Pescatore LA, Fernandes Dde C. Protein disulfide isomerase in redox cell signaling and homeostasis. Free Radic Biol Med. 2012;52:1954–69. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant respons eelement signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaplan A, Gaschler MM, Dunn DE, Colligan R, Brown LM, Palmer AG, et al. Small molecule-induced oxidation of protein disulfide isomerase is neuroprotective. Proc Natl Acad Sci USA. 2015;112:E2245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bekendam RH, Bendapudi PK, Lin L, Nag PP, Pu J, Kennedy DR, et al. A substrate-driven allosteric switch that enhances PDI catalytic activity. Nat Commun. 2016;7:12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banerjee R, Pace NJ, Brown DR, Weerapana E. 1,3,5-Triazine as a modular scaffold for covalent inhibitors with streamlined target identification. J Am Chem Soc. 2013;135:2497–500. [DOI] [PubMed] [Google Scholar]
- 44.Khan MM, Simizu S, Lai NS, Kawatani M, Shimizu T, Osada H. Discovery of a small molecule PDI inhibitor that inhibits reduction of HIV-1 envelope glycoprotein gp120. ACS Chem Biol. 2011;6:245–51. [DOI] [PubMed] [Google Scholar]
- 45.Chesi M, Robbiani DF, Sebag M, Chng WJ, Affer M, Tiedemann R, et al. AID-dependent activation of a MYC transgene induces multiple myeloma in a conditional mouse model of post-germinal center malignancies. Cancer Cell. 2008;13:167–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chesi M, Matthews GM, Garbitt VM, Palmer SE, Shortt J, Lefebure M, et al. Drug response in a genetically engineered mouse model of multiple myeloma is predictive of clinical efficacy. Blood. 2012;120:376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baell JB, Holloway GA. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem. 2010;53:2719–40. [DOI] [PubMed] [Google Scholar]
- 48.Baell J, Walters MA. Chemistry: chemical con artists foil drug discovery. Nature. 2014;513:481–3. [DOI] [PubMed] [Google Scholar]
- 49.Okumura M, Kadokura H, Inaba K. Structures and functions of protein disulfide isomerase family members involved in proteostasis in the endoplasmic reticulum. Free Radic Biol Med. 2015;83:314–22. [DOI] [PubMed] [Google Scholar]
- 50.Laboissiere MC, Sturley SL, Raines RT. The essential function of protein-disulfide isomerase is to unscramble non-native disulfide bonds. J Biol Chem. 1995;270:28006–9. [DOI] [PubMed] [Google Scholar]
- 51.Jurczyszyn A, Zebzda A, Czepiel J, Perucki W, Bazan-Socha S, Cibor D, et al. Geldanamycin and its derivatives inhibit the growth of myeloma cells and reduce the expression of the MET receptor. J Cancer. 2014;5:480–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stuhmer T, Zollinger A, Siegmund D, Chatterjee M, Grella E,Knop S, et al. Signalling profile and antitumour activity of the novel Hsp90 inhibitor NVP-AUY922 in multiple myeloma. Leukemia. 2008;22:1604–12. [DOI] [PubMed] [Google Scholar]
- 53.Lancet JE, Gojo I, Burton M, Quinn M, Tighe SM, Kersey K, et al. Phase I study of the heat shock protein 90 inhibitor alvespimycin (KOS-1022, 17-DMAG) administered intravenously twice weekly to patients with acute myeloid leukemia. Leukemia. 2010;24:699–705. [DOI] [PubMed] [Google Scholar]
- 54.Peterson LB, Eskew JD, Vielhauer GA, Blagg BS. The hERG channel is dependent upon the Hsp90alpha isoform for maturation and trafficking. Mol Pharm. 2012;9:1841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khandelwal A, Kent CN, Balch M, Peng S, Mishra SJ, Deng J, et al. Structure-guided design of an Hsp90beta N-terminal isoform-selective inhibitor. Nat Commun. 2018;9:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Que NLS, Crowley VM, Duerfeldt AS, Zhao J, Kent CN, Blagg BSJ. et al. Structure based design of a Grp94-selective inhibitor: exploiting a key residue in Grp94 to optimize paralog-selective binding. J Med Chem. 2018;61:2793–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin L, Gopal S, Sharda A, Passam F, Bowley SR, Stopa J, et al. Quercetin-3-rutinoside inhibits protein disulfide isomerase by binding to its b’x domain. J Biol Chem. 2015;290:23543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.