Abstract
Erectile dysfunction (ED) is a significant medical condition, with high impact on patient quality of life. Current treatments are minimally effective in prostatectomy, diabetic and aging patients due to injury to the cavernous nerve (CN); loss of innervation causes extensive smooth muscle (SM) apoptosis, increased collagen and ED. Sonic hedgehog (SHH) is a critical regulator of penile SM. We developed a self-assembling peptide amphiphile (PA) nanofiber hydrogel for extended release of SHH protein to the penis after CN injury, to suppress SM apoptosis. In this study we optimize the animal model, SHH concentration, duration of suppression, and location of delivery, to maximize SM preservation. SHH treatment suppressed apoptosis and preserved SM 48%. Increased SHH duration preserved SM 100%. Simultaneous penis/CN delivery increased SM 127%. Optimization of SHH PA delivery is essential for clinical translation to ED patients, and the PA vehicle has wide applicability as an in vivo delivery tool.
Keywords: cavernous nerve injury, smooth muscle regeneration, peptide amphiphile nanofiber hydrogel, Sonic hedgehog, penis, pelvic ganglia
Graphical Abstract
These studies show that Sonic hedgehog treatment of the penis and cavernous nerve by peptide amphiphile nanofiber hydrogel reduces apoptosis, preserves smooth muscle, and suppresses collagen induction that occurs in response to CN crush injury. Optimizing the method and duration of PA delivery improved smooth muscle preservation 80%, with implications towards preservation of erectile function.

Background
Erectile dysfunction (ED) is a significant health concern, that effects 50% of men aged 40 to 701 and 22% under 402. ED has high impact on men’s quality of life and health, and develops when the cavernous nerve (CN), which innervates penile tissues, is damaged at the time of radical prostatectomy surgery, and with peripheral neuropathy in aging and diabetic patients. Loss of innervation causes intensive, irreversible alterations to the architecture of the corpora cavernosa of the penis, including abundant apoptosis of smooth muscle, and increased collagen, resulting in ED3-6. Current treatments, such as PDE5i, are not effective in up to 69% of patients with ED and cavernous nerve injury7-8, including 82% of radical prostatectomy and 59% of diabetic patients9,8, so novel therapies are needed which target this difficult to treat population.
In order to prevent ED, treatments that prevent smooth muscle apoptosis, and accelerate CN regeneration, are essential. We’ve identified that the Sonic hedgehog (SHH) pathway is a key mediator of penile smooth muscle10-13,4, and CN regeneration. SHH is indispensable for organizing and preserving the smooth muscle architecture of the penis10, with blockade of SHH signaling causing smooth muscle apoptosis and ED10-11. Decreased SHH protein is common in corpora cavernosal tissue of ED patients4 and in prostatectomy11 and diabetic14 animal models. Introduction of exogenous SHH protein into the corpora cavernosa coincident with CN injury, suppresses the apoptotic response11-13, indicating significant potential to be advanced as an ED preventative strategy. SHH is also essential in maintaining the morphology of the CN and in its regeneration. SHH inhibition causes myelinated and non-myelinated fibers to undergo demyelination and axonal degeneration, resulting in loss of innervation to the penis, induction of penile apoptosis, and ED12,15. SHH protein abundance is lower in the PG/CN with injury5, and reintroduction of SHH protein curbs nerve damage, accelerates CN regeneration, improves erectile function, and quenches penile apoptosis, thus suppressing ED development15. Increasing SHH protein would be more effective for therapy development than RNA delivery, because we previously demonstrated blocked SHH protein synthesis from mRNA in CN injured rats11.
SHH protein has compelling clinical potential to be developed as a preventative ED strategy. Therefore it is essential to develop a non-invasive, biodegradable, extended release vehicle that can be used in vivo. We are developing nanoscale self-assembling peptide amphiphile (PA) hydrogels to deliver SHH protein to the penis and CN in order to accelerate CN regeneration, suppress apoptosis in the penis, and improve erectile function. The PAs were initially developed to resemble extracellular matrix16-18, for cell delivery19, and have been useful for bone20 spinal cord21-23, and blood vessel regeneration16. We have effectively utilized the PA technology in two distinct modalities: for delivery of SHH protein to the penis, where the PA forms in vivo within the sinusoidal spaces of the corpora caverosa and delivers SHH protein over an extended period as the PA breaks down. PA delivery of SHH protein was successful in lowering the apoptotic response in a CN resection radical prostatectomy model13,6. A second, separate configuration is made as a linear hydrogel that is placed adjacent to the CN for SHH protein delivery in vivo at the time of injury15,5,25-26. CN regeneration was accelerated and erectile function was improved ~60% by this methodology, indicating significant promise for clinical translation. These initial successful studies were performed with minimal optimization. We posit that these marked improvements in penile morphology can be significantly further enhanced with optimization of delivery conditions for SHH PA, which is vital for clinical translation. In this study we optimize the CN injury model to more effectively parallel the majority of radical prostatectomy patient injury, the concentration of SHH protein delivered, the duration of apoptosis suppression, and simultaneous distribution of SHH to the penis and CN. Optimization is examined by quantifying changes in penile morphology, with decreased apoptosis and preserved smooth muscle indicative of more normal penile morphology and preserved function.
Methods
Animals:
Ninety-seven Sprague-Dawley rats were obtained from Charles River. The rat age was between postnatal day 115-120 (P115-P120). The study was performed according to the recommendations outlined in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and the Office of Animal Care, and Institutional Biosafety at the University of Illinois at Chicago approved the protocol.
Surgical procedures for CN crush (bilateral) and sham surgeries:
After exposing the PG/CN, it was crushed for 30 seconds (mild crush) using microforceps (0.02 X 0.06mm). This CN crush methodology is routinely used in the literature27-28 and the reproducibility and extent of injury were previously defined in our laboratory15. Control (sham) surgical procedures were performed by exposing and identifying the CN without performing crush injury. Rats were sacrificed 4 and 9 days post injury. Severe crush injury (3 consecutive 30 second crushes, n=4) and CN resection (removal of a 5mm portion of the CN >5mm from the pelvic ganglia, n=4) were also performed to examine the effect of injury severity on the apoptotic index, and rats were sacrificed 4 days after damage.
Peptide amphiphile (PA) delivery of SHH protein to the penis for 4 days after CN injury:
CN damage was performed as cited in the previous section. V3A3E3-COOH PA hydrogel was used to deliver either SHH (n=5) or mouse serum albumin (MSA, control, n=4) proteins to the penis. Briefly V3A3E3-COOH PA (50 μl of a 20-mM solution), SHH or MSA protein (5 μl of a 1.25 μg/μl solution, R&D Systems, Minneapolis, MN, USA) and CaCl2 (50 μl of a 40 mM solution) were injected directly into the corpora cavernosa of the penis as described previously13. The penis was exposed, a silk tourniquet was fixed at the proximal portion of the penis, and PA was injected with a 26-gauge needle (1-5μl volume) directly into the distal portion of the corpora cavernosa. The PA gelled within 30 seconds to 1 minute, forming a thin layer coating the sinusoidal spaces. Final PA concentration was 10 mM, CaCl2 was 20 mM, and SHH protein was 6.25 μg per rat (1X) or 2X (n=5). Additional rats underwent sham (n=6) and CN crush (n=6) surgeries. Penises were removed by sharp dissection from euthanized males 4 days after SHH protein/PA/CaCl2 injection and were snap frozen in liquid nitrogen.
CN injury with increased duration of SHH PA treatment of the penis for 9 days:
We performed CN crush injury and injected rats immediately with SHH or MSA/bovine serum albumin (BSA, control) PA as described above. At day 5, when SHH protein was largely depleted from the PA as it lost its structural integrity13, a second SHH (n=8) or MSA/BSA (n=7) PA injection was performed and rats were sacrificed after an additional 4 days (9 days total since CN crush surgery). An additional group that had been given one SHH PA injection at the time of CN crush was sacrificed after 9 days without further intervention (n=4). Sham (n=5) and CN crush (n=5) were also performed for comparison.
CN crush injury with simultaneous delivery of SHH PA to the penis and CN for 4 days:
CN crush injury (bilateral) was performed and rats were immediately injected with SHH (n=7) or MSA (control, n=4, 1 μg/μl) PA into the corpora cavernosa as described above. Following injection into the penis, SHH or MSA was delivered bilaterally to the CN using a second type of PA15. The highly-aligned “noodle” PAs used for CN protein delivery were made as described previously15 by heating a 100mM solution of V2A2E2-NH2 PA to 80°C (30 minutes), and slowly cooling to room temperature. SHH protein (2.27 μg in 1.5 μl volume) was added to 8.5 μl PA, immediately prior to use in the animal. 500 μl 20mM CaCl2 was placed on a slide, and cooled PA/SHH mixture was slowly expressed through a pipet tip, into the CaCl2 solution, forming a noodle-like hydrogel that could be laid on the exposed CN.
Apoptotic index:
Apoptosis was quantified using the Apoptag kit (Millipore) on penis tissue that was frozen, cut 11 μM in thickness, and the tissue was post fixed in acetone for 15 minutes at 4°C as described previously15. DAPI (0.005 μg/ml) was used to stain all cells for comparison. Fluorescence was observed using a Leica DM2500 microscope. Photography was performed using a Qicam 1394 digital camera. The apoptotic index (apoptotic cells/all cells) was determined by counting the number of apoptotic cells and all cells in five fields from each section and five sections per penis and was reported ± standard error of the mean.
Trichrome stain:
Trichrome stain of penis tissue was performed and quantified by Image J (version 1.45 s, down load date 5/22/2012)29. The area of smooth muscle (red) and collagen (blue) were quantified individually in trichrome photos after background subtraction. Smooth muscle and collagen were quantified in 25 photos (200X) from each penis tissue that were selected randomly (5 photos per section and 5 sections per penis tissue).
PA delivery of SHH or BSA (control) protein to the penis:
Sprague Dawley rats (P120) were randomly assigned to two groups: CN resection (bilateral) with either SHH PA injection into the penis (n=12), or with BSA (control) (n=11). The methodology for CN injury and SHH and BSA distribution by PA were previously documented13. Penises were removed at 2, 4, and 7 days after injury, by sharp dissection.
Quantification of proliferation:
The proliferative index was quantified in penis tissue treated with SHH (n=9) or BSA (n=9) protein by PA, by counting Ki67 stained cells and all cells which were stained with DAPI (0.005μg/ml). Stained cells were observed with a fluorescent microscope (Leitz) and were photographed with a digital camera (Nikon). The number of proliferating cells/all cells was reported from five fields in each section and five sections per penis and was reported ± the standard error of the mean.
Immunohistochemical analysis (IHC):
Dual staining using fluorescent IHC for Ki67/CD31 (endothelial marker) and Ki67/α-ACTIN (smooth muscle marker) were performed as described previously11 assaying for 1/50 goat Ki67 (Santa Cruz, SC-7846), 1/100 mouse α-ACTIN (Sigma, A-5691) and 1/100 mouse CD31 (Millipore, MAB1393). Alexa Fluor 488 chicken anti-goat (1/150), and Alexa Fluor 594 donkey anti-mouse (1/350, Molecular Probes) were used as secondary antibodies.
Statistical Analysis:
Statistics were performed by ANOVA with a Scheffe’s posthoc test using the SSPS statistical program or using a Student’s t-test. Differences were significant when p≤0.05.
Results
PA delivery of SHH protein to the penis for 4 days after CN injury:
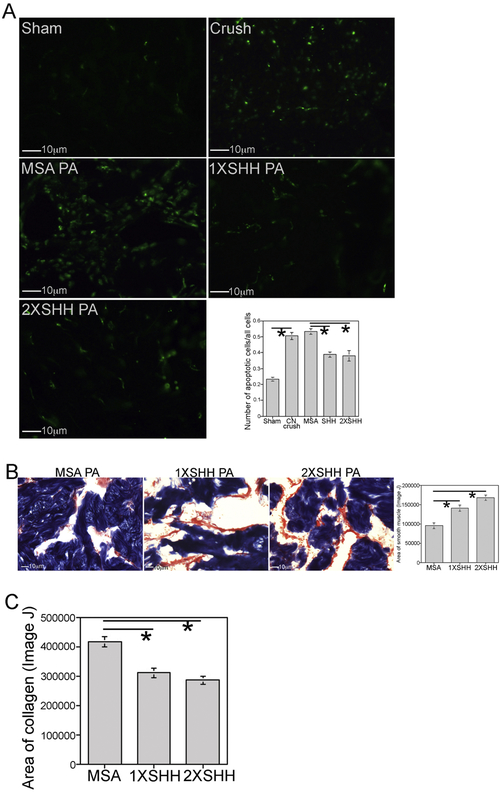
We previously used self-assembling PA hydrogels for extended release of SHH protein to the penis of a CN resection ED model, in order to prevent apoptosis in the more severe, but less common (10% of surgical injury), prostatectomy model13, 30. The PA (V3A3E3-COOH) with SHH protein undergo targeted injection into the corpora cavernosa of the penis with subsequent gelation of the PA in vivo in the corpora cavernosa. It forms as a soft gel layer along the surface of the corpora cavernosal sinuses. SHH protein is enmeshed in the hydrogel as it forms during cation-based assembly and crosslinking, and is released as the PA breaks down. With the advent of nerve sparing techniques, the majority of radical prostatectomy induced injury derives from CN crush and manipulation of the PG/CN. The PAs were applied here in a CN crush ED model to examine if SHH protein released by PA in vivo in the penis, suppresses the apoptotic index (ratio of apoptotic cells/all cells stained with DAPI) and collagen induction, in a similar manner to effects in the more severe resection model. The SHH concentration delivered for maximal apoptosis suppression was also optimized, with two concentrations being examined. The apoptotic index is elevated for tissue injury/death, and decreases with effective therapeutic delivery.
The apoptotic index increased 117% 4 days after CN injury (n=6) relative to sham controls (n=6, p=0.0001, Figure 1A). SHH PA (n=5) suppressed apoptosis 27% in comparison to MSA PA (n=4) treated controls (p=0.005, Figure 1A). Doubling the SHH protein concentration given to the penis (2X, n=5) decreased the apoptotic index 29% (p=0.003), which was not significantly different apoptosis suppression than the 1X SHH treatment group (p=0.999, Figure 1A).
Figure 1:
(A) Apoptotic index was quantified at 4 days after injury in corpora cavernosal tissue of Sprague Dawley rats that underwent sham surgery or CN crush and1X SHH, 2X SHH or MSA (control) treatment by PA injected into the corpora cavernosa. Apoptosis increased 117% 4 days after CN injury (p=0.0001). SHH PA suppressed apoptosis 27% (p=0.005). Doubling the concentration of SHH protein delivered to the penis decreased the apoptotic index 29% (p=0.003), which was not significantly different from the 1X SHH treated group (p=0.999). (B) Trichrome stain quantification of smooth muscle showed 48% more smooth muscle in the 1X SHH treated group (p=0.005). Doubling the concentration of SHH resulted in 76% more smooth muscle (p=0.0001). There was no significant difference in smooth muscle between the 1X and 2X SHH treated groups (p=0.066). (C) Trichrome stain quantification of collagen showed 26% less collagen in the 1X SHH treated group (p=0.002), and 32% less collagen with 2X SHH treatment (p=0.0001). There was no difference in collagen abundance between the 1X and 2X SHH treated groups (p=0.522).
Trichrome stain quantification of smooth muscle showed 48% more smooth muscle in the 1X SHH treated group (n=5) compared to MSA treated controls (n=4, p=0.005, Figure 1B). Doubling the concentration of SHH (2X, n=5) resulted in 76% more smooth muscle than in MSA treated controls (p=0.0001, Figure 1B). No significant difference in smooth muscle was observed between the 1X and 2X SHH treated groups (p=0.066, Figure 1B), likely resulting from differences in distribution or break down of the PA in the penis, which would effect the SHH release rate. Since sheer stress within the sinusoidal lining where the PA forms may vary from penis to penis depending on the erectile state of the animal over several days during delivery, this can induce variability in apoptotic suppression and smooth muscle preservation which make reliable quantification of penile smooth muscle by western analysis challenging.
Trichrome stain quantification of collagen showed 26% less collagen in the 1X SHH treated group (n=5) compared to MSA treated controls (n=4, p=0.002, Figure 1C). Doubling the concentration of SHH (2X, n=5) resulted in 32% less collagen than in MSA treated controls (p=0.0001, Figure 1C). A significant difference in collagen abundance was not observed between the 1X and 2X SHH treated groups (p=0.522, Figure 1C).
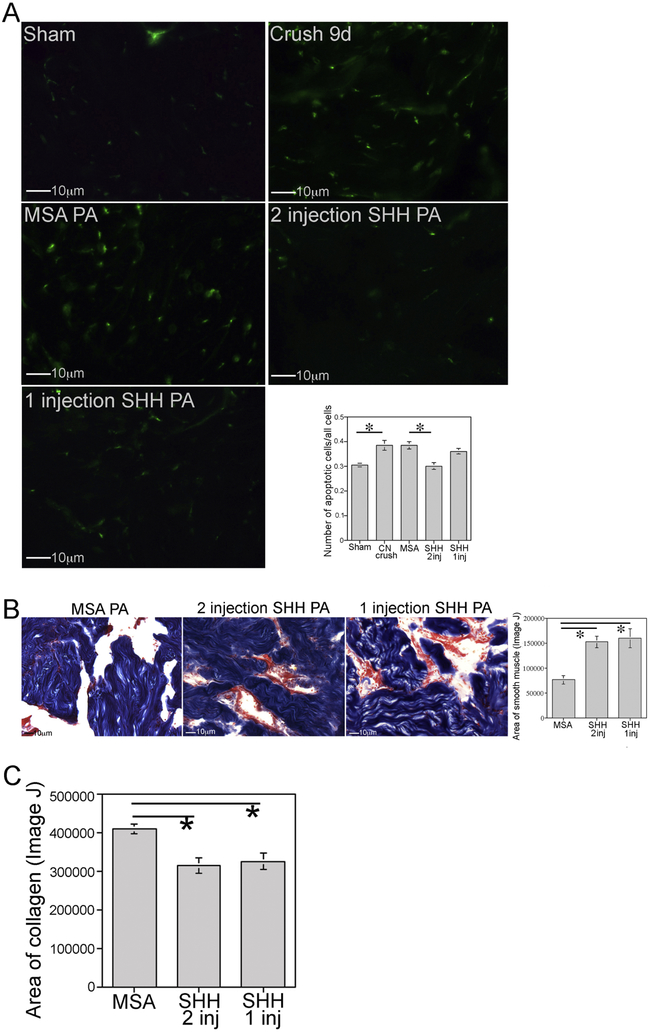
CN injury with increased duration of SHH PA treatment of the penis for 9 days:
In order to examine how long SHH PA injection can suppress apoptosis, if apoptosis is suppressed or delayed with SHH treatment, and to examine if multiple SHH PA injections would be advantageous to suppress apoptosis for a longer time after CN injury, CN crush was performed with SHH or MSA/BSA PA injection at time of surgery. A second SHH and MSA/BSA PA injection was performed at five days after injury, and rats were sacrificed nine days post CN crush. The apoptotic index was increased 26% at 9 days after CN injury (n=5) in comparison to sham (n=5, p=0.014, Figure 2A). Two SHH protein PA injections (n=4) caused a 22% reduction in apoptosis at 9 days after CN crush in comparison to two MSA/BSA PA injections (n=4, p=0.021, Figure 2A). Rats that had been given only one SHH PA injection (n=4) and were sacrificed at 9 days after CN crush, had apoptotic levels that were not significantly different than untreated CN crushed rats at 9 days post injury (p=0.830, Figure 2A), indicating that once SHH protein is depleted from the PA, the corpora cavernosal tissue reverts to the apoptotic level that occurs at that time post injury, thus avoiding the initial bolus of apoptosis that occurs in the first few days (2-4 days) after injury.
Figure 2:
(A) Apoptotic index was quantified 9 days after CN injury in corpora cavernosal tissue of Sprague Dawley rats that underwent sham surgery or CN crush with one or two SHH PA injections into the corpora cavernosa. Apoptosis index increased 26% at 9 days after CN injury (p=0.014). Two SHH PA injections decreased apoptosis 22% (p=0.021). One SHH PA injection (protein depleted by ~6 days), had apoptotic levels that were not different from untreated 9 day CN crushed rats (p=0.830). (B) Trichrome stain quantification of smooth muscle showed 100% more muscle in the two SHH injection group (p=0.001). With one SHH injection, 110% more smooth muscle was identified compared to MSA controls (p=0.001). There was no difference in smooth muscle preservation between the one and two SHH PA injected penis (p=0.921). (C) Trichrome stain quantification of collagen showed 24% less collagen in the two SHH injection group (p=0.003), and 21% less in the one SHH PA injection group (p=0.022). The one and two SHH injection groups were not different from each other with regard to collagen preservation (p=0.919).
Trichrome stain quantification of smooth muscle showed 100% more smooth muscle in the two SHH injection group (n=7) compared to MSA controls (n=7, p=0.001, Figure 2B). With one SHH injection (n=4), 110% more smooth muscle was present in comparison to MSA treated controls (p=0.001, Figure 2B). The one and two SHH injection groups were not significantly different from each other with regard to smooth muscle preservation (p=0.921, Figure 2B).
Trichrome stain quantification of collagen showed 24% less collagen in the two SHH injection group (n=7) compared to MSA treated controls (n=7, p=0.003, Figure 2C). With one SHH injection (n=4), 21% less collagen was present in comparison to MSA treated controls (p=0.022, Figure 2C). The one and two SHH injection groups were not significantly different from each other with regard to collagen preservation (p=0.919, Figure 2C).
CN crush injury with simultaneous delivery of SHH PA to the penis and CN for 4 days:
Potential additive effects on suppression of morphology changes in the penis with simultaneous SHH distribution to the penis and CN, were examined. When SHH (n=7) or MSA (control, n=4) protein were delivered by PA into the penis and CN concurrent with CN injury, the apoptotic index decreased 27% 4 days after injury with SHH treatment in comparison to MSA controls (p=0.0001, Figure 3A).
Figure 3:
(A) Apoptotic index was quantified 4 days after CN crush in Sprague Dawley rats that underwent CN crush and SHH or MSA (control) protein was delivered by PA to the penis and PG/CN. Apoptosis decreased 27% with SHH treatment (p=0.0001). (B) Trichrome stain quantification of penile smooth muscle showed 127% more smooth muscle in SHH treated rats (p=0.0004). (C) Trichrome stain quantification of collagen showed 30% less collagen in the rats treated with SHH in the penis and PG/CN (n=7) (p=0.0003).
Trichrome stain quantification of penile smooth muscle showed 127% more smooth muscle in the rats treated with SHH in the penis and CN (n=7) versus MSA treated controls (n=4, p=0.0004, Figure 3B). This was the highest smooth muscle preservation observed in all groups examined.
Trichrome stain quantification of penile collagen showed 30% less collagen in the rats treated with SHH in the penis and CN (n=7) versus MSA treated controls (n=4, p=0.0003, Figure 3C).
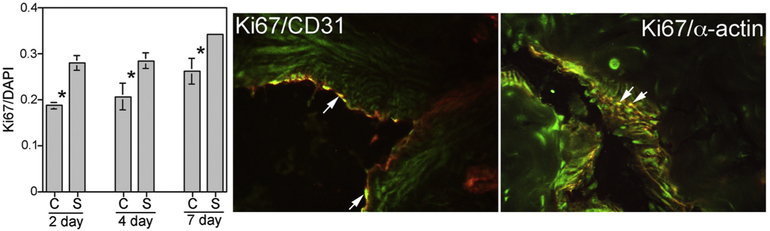
Quantification of proliferation in CN injured penis at 2, 4 and 7 days post injury:
The proliferative index Ki67 (proliferation marker)/DAPI (stains all cells) was quantified in corpora cavernosal tissue from Sprague Dawley rats that had bilateral CN injury and either SHH (n=9) or BSA (control, n=9) PA injection into the corpora cavernosa at the time of injury. Tissues were examined at 2, 4 and 7 days after injury. SHH PA treatment increased the proliferative index 50% (p=0,003) at two days after injury, 38% (p=0.042) at 4 days after injury, and 31% (p=0.022) at 7 days after injury (Figure 4). Dual IHC analysis for Ki67/CD31 and Ki67/α-ACTIN show that proliferation is taking place in both smooth muscle and endothelium (Figure 4).
Figure 4:
Proliferative index (Ki67/DAPI) quantified in SHH and BSA (control) PA treated penis, shows increased proliferation at 2, 4 and 7 days of SHH treatment (p=0.003, p=0.042, and p=0.022). Co-localization of Ki67(green)/CD31 (red) and Ki67(green)/α-ACTIN (red), indicate proliferation in smooth muscle and endothelium.
Effect of severity of CN injury on penile apoptotic response:
The apoptotic index was quantified by TUNEL assay on penis tissue from rats that underwent, sham, mild CN crush, severe CN crush or CN resection injury, and tissues were assayed 4 days after CN damage. The apoptotic index increased 117%, 119% and 125% for mild crush, severe crush, and CN resection, relative to sham controls (p=0.0001, Figure 5). There was no difference in the apoptotic index between the mild and severe CN crush and CN resection groups, indicating an all or nothing response of the penis to loss of innervation (mild to severe p=0.996, mild to resection p=0.894, severe to resection p=0.979, Figure 5).
Figure 5:
Apoptotic index was quantified on penis tissue from rats that underwent sham, mild or severe crush, or CN resection (4 days). Apoptosis increased 117%, 119% and 125% for mild and severe crush, and CN resection, relative to sham controls (p=0.0001). There was no difference in apoptosis between the injury groups.
Discussion
This manuscript examines development of a nanoparticle based vehicle for sonic hedgehog protein delivery to the penis to preserve penile morphology and regenerate erectile function after cavernous nerve (CN) crush injury (radical prostatectomy model). We show that optimization of sonic hedgehog delivery conditions by peptide amphiphile (PA) nanofiber hydrogel increases smooth muscle preservation 80%, with implications towards preservation of erectile function. CN crush is the most common injury that occurs during radical prostatectomy, however CN neuropathy also occurs in diabetic and aging patients, with parallel changes observed in penile morphology and sonic hedgehog signaling, suggesting potential SHH PA application in these difficult to treat ED populations. Optimization of sonic hedgehog delivery is indispensable for clinical translation to radical prostatectomy patients to impede erectile dysfunction and the PA nanofiber distribution mechanism, may be broadly applicable as an in vivo delivery tool.
Our work shows the importance of SHH signaling in preserving the smooth muscle morphology of the penis after CN injury, which is critical for preservation of erectile function. Suppression of smooth muscle apoptosis and accelerated CN regeneration, are key components required for development of ED therapies. SHH delivery by PA, both within the corpora cavernosa as a thin interior lining adjacent to the smooth muscle and endothelial tissue, and adjacent to the injured CN via a PA “noodle”, offers a dual approach to addressing two key mechanisms of ED progression. PAs are particularly attractive for eventual clinical application as they provide extended release of protein, are biodegradable, and are readily produced by synthetic means and versatile enough that both modalities (injection as an aqueous hydrogel precursor, or physical deposition along the CN) are feasible clinical steps, post-prostatectomy.
Clinical advances toward nerve-sparing procedures have shifted CN injuries from full resections to partial physical damage (“crush”). In this study we examined if SHH delivered by PA to the penis at the time of CN crush (mimicking radical prostatectomy induced injury) suppresses the morphological changes that occur in the corpora cavernosa of the penis in response to loss of innervation. These include induction of apoptosis, primarily in penile smooth muscle, followed by increased collagen deposition. The corpora cavernosal remodeling is significant and may result in loss of physiological function/erectile function. We show that apoptosis is suppressed 27% in response to SHH PA treatment at four days after CN injury, a time when apoptosis induction is most abundant in response to loss of CN innervation5,3. In our previous studies of the more severe CN resection prostatectomy model at the same time post injury (4 days), apoptosis was reduced 25%, indicating that SHH PA delivery suppresses penile apoptosis in a similar manner in the crush and resection models. SHH PA treatment has the added benefit of suppressing collagen induction (26%), which leads to penile fibrosis and a less compliant penis. Doubling the concentration of SHH protein did not further suppress the apoptotic index. This can occur because we are in the target range for optimal SHH suppression of apoptosis, there is a threshold above which further addition of SHH does not further improve penile morphology, or may result due to technical challenges in vivo with the PA such as variation in PA break down due to differences in sheer stress between rats, and limitations of targeted PA injection into the corpora cavernosa of the rat. In patients, the penis tissue is much larger and targeted injection into the corpora cavernosa is not a difficulty. Dampening the intensity of the early apoptotic response is critical to preserving smooth muscle and erectile function.
The time course of apoptosis that occurs in the rat penis in response to CN resection3 and CN crush5, have been well documented. For the more severe case of CN resection, apoptosis peaks at 2 days after CN injury, begins decreasing at 7 days after injury, and remains barely detectable above baseline from day 14-60 post injury. For CN crush, apoptosis induction is slower with a peak at 4 days after injury, and decreases significantly by 7 days, and returns to baseline by 14 days post crush injury5. In this study we show 117% increase in apoptosis at 4 days after bilateral CN crush. The apoptotic index was reduced to 26% by 9 days after CN injury. Indicating that the majority of apoptosis occurs in the first few days after injury and if this could be prevented, than the majority of penile remodeling would be avoided, and thus erectile function preserved. When one SHH PA injection was given at the time of CN injury, apoptosis was suppressed until SHH was depleted from the PA. Addition of a second SHH PA injection, maintains apoptosis suppression longer, however smooth muscle preservation was not statistically different with one and two SHH PA injections. This likely occurs because apoptosis in the rat after CN crush injury is most abundant 2-4 days after injury and then remains only slightly elevated above baseline between 7 and 14 days. One SHH PA injection may thus be sufficient to suppress most of the apoptotic response and preserve smooth muscle. A similar trend was shown with suppression of collagen with one and two SHH PA injections.
The highest smooth muscle preservation was observed with simultaneous SHH PA treatment of the penis and CN at the time of crush injury. With SHH PA injection into the penis alone, smooth muscle was preserved 48%, versus simultaneous delivery to the CN and penis resulted in 127% more smooth muscle retention at 4 days after injury. This 80% improvement in smooth muscle retention likely results due to direct effects of SHH protein in both locations. In previous studies, we’ve shown that SHH is critical to establish and maintain the sinusoidal architecture of the corpora cavernosa of the penis, and inhibition of SHH pathway in the penis increases smooth muscle apoptosis10-11. We’ve also shown that SHH is essential for normal CN/peripheral nerve function and that SHH supplementation can regenerate the CN more quickly, re-establishing normal nerve morphology and regenerating erectile function15. Thus it is not surprising that simultaneous SHH protein supplementation in both tissues is the most effective means of preserving penile smooth muscle. The amount of smooth muscle preservation may be high relative to the apoptotic index suppression observed. This may result since we are only quantifying the apoptotic response at small windows of time post injury. More likely is that SHH PA treatment not only affects the apoptotic response, it also effects proliferation of smooth muscle (Figure 4). This is a concept that has not previous been explored after CN injury. It is assumed that smooth muscle decreases without the tissue attempting to reestablish itself. In this study we demonstrate that proliferation occurs in the corpora cavernosa after CN injury, and this response is increased in smooth muscle and endothelium in the presence of SHH PA in the penis. The proliferation that is observed appears to be normal patterned penile architecture, suggesting up-regulation of normal developmental mechanisms in response to SHH. Thus SHH protein treatment of the penis by PA both suppresses smooth muscle apoptosis and increases proliferation.
Collagen deposition was 20-30% lower in all SHH treated groups after CN crush in comparison to controls. It is unclear how this suppression of collagen induction occurs. Our previous studies suggest that the morphological remodeling that results in the penis in response to loss of innervation is an orchestrated process, with smooth muscle apoptosis occurring prior to increased collagen induction. For CN crush, the majority of apoptosis occurs 2-4 days after injury and decreases by 7 days5. Collagen is not measurably increased until 7-14 days after CN injury6. There is minimal study in this area however cells undergoing apoptosis release factors into the microenvironment that may affect proliferation/morphology in target cells31-33. Further study beyond the scope of this work will be required to understand this remodeling process.
These studies show that SHH PA treatment of the penis and CN reduces apoptosis, preserves smooth muscle, and suppresses collagen induction that occurs in response to CN crush injury. Optimizing the method and duration of PA delivery improved smooth muscle preservation 80%, with implications towards preservation of erectile function. An all or nothing apoptotic response of the penis was observed with loss of innervation, irrespective of the severity of CN damage. CN crush is the most common injury that occurs during radical prostatectomy, however CN neuropathy also occurs in diabetic patients and with aging, with parallel changes observed in penile morphology and SHH signaling, suggesting potential SHH PA application in these difficult to treat ED populations. Optimization of SHH delivery is essential for clinical translation to radical prostatectomy patients to prevent ED and the PA nanofiber vehicle may be widely applicable as an in vivo delivery tool.
Statement of Significance: We use self-assembling peptide amphiphiles (PA) as biological delivery vehicles to prevent cavernous nerve (CN) injury-induced changes in penile morphology that cause erectile dysfunction (ED). These versatile hydrogels were molecularly pre-programmed for Sonic hedgehog (SHH) protein delivery, either from an injectable solution with fast, in situ assembly into a soft hydrogel, or by highly aligned monodomain nanofiber bundles. SHH PA treatment of the penis reduces apoptosis, preserves smooth muscle, and suppresses collagen induction that occurs in response to CN crush injury. Optimizing SHH delivery conditions improved smooth muscle preservation 80%, with implications towards preservation of erectile function. Optimization of SHH PA delivery is essential for clinical translation to prostatectomy patients to prevent ED and the PA nanofiber vehicle may be widely applicable as an in vivo delivery tool.
Acknowledgments
This project was supported by NIH/NIDDK Award number R01DK101536. Peptide synthesis, purification, and characterization were performed by staff in the Peptide Synthesis Core Facility of the Simpson Querrey Institute at Northwestern University. The U.S. Army Research Office, the U.S. Army Medical Research and Material Command, and Northwestern University provided funding to develop this facility.
Grant Sponsor: National Institutes of Health DK101536
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994; 151: 54–61. [DOI] [PubMed] [Google Scholar]
- 2.Heruti R, Shochat T, Tekes-Manova D, Ashkenazi I, Justo D (2004) Prevalence of erectile dysfunction among young adults: results of a large-scale survey. J Sex Med 2004; 1: 284–291. [DOI] [PubMed] [Google Scholar]
- 3.User HM, Hairston JH, Zelner DJ, McKenna KE, McVary KT. Penile weight and cell subtype specific changes in a post-radical prostatectomy model of erectile dysfunction. J Urol 2003; 169: 1175–1179. [DOI] [PubMed] [Google Scholar]
- 4.Angeloni NL, Bond CW, McVary KT, Podlasek CA. Sonic hedgehog protein is decreased and penile morphology is altered in prostatectomy and diabetic patients. PLOS ONE 2013; 8: e70985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angeloni N, Bond CW, Harrington D, Stupp S, Podlasek CA. Sonic hedgehog is neuroprotective in the cavernous nerve with crush injury. J Sex Med 2013; 10: 1240–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choe S, Veliceasa D, Bond CW, Harrington DA, Stupp SI, McVary KT, Podlasek CA. Sonic hedgehog delivery from self-assembled nanofiber hydrogels reduces the fibrotic response in models of erectile dysfunction. Acta Biomateralia 2016; 32: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pace G, Del Rosso A, Vicentini C. Penile rehabilitation therapy following radical prostatectomy. Disabil Rehabil 2010; 32: 1204–1208. [DOI] [PubMed] [Google Scholar]
- 8.Perimenis P, Markou S, Gyftopoulos K, Athanasopoulos A, Giannitsas K, Barbalias G. Switching from long-term treatment with self-injections to oral sildenafil in diabetic patients with severe erectile dysfunction. European Urology 2002; 41: 387–391. [DOI] [PubMed] [Google Scholar]
- 9.Kendirci M, Hellstrom WJ (2004) Current concepts in the management of erectile dysfunction in men with prostate cancer. Clin Prostate Cancer 3: 87–92. [DOI] [PubMed] [Google Scholar]
- 10.Podlasek CA, Zelner DJ, Jiang HB, Tang Y, Houston J, McKenna KE, McVary KT. Sonic hedgehog cascade is required for penile postnatal morphogenesis, differentiation and adult homeostasis. Biology of Reproduction 2003; 68: 423–438. [DOI] [PubMed] [Google Scholar]
- 11.Podlasek CA, Meroz CL, Tang Y, McKenna KE, McVary KT. Regulation of cavernous nerve injury-induced apoptosis by sonic hedgehog. Biol Reprod 2007; 76: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bond C, Tang Y, Podlasek CA. Neural influences on sonic hedgehog and apoptosis in the penis. Biology of Reproduction 2008; 78: 947–956. PMC282718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bond CW, Angeloni NL, Harrington DA, Stupp SI, McKenna KE, Podlasek CA. Peptide amphiphile nanofiber delivery of sonic hedgehog protein to reduce smooth muscle apoptosis in the penis after cavernous nerve resection. J Sex Med 2011; 8: 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Podlasek CA, Zelner DJ, Harris JD, Meroz CL, Tang Y, McKenna KE, McVary KT. Altered sonic hedgehog signaling is associated with morphological abnormalities in the penis of the BB/WOR diabetic rat. Biology of Reproduction 2003; 69: 816–827. [DOI] [PubMed] [Google Scholar]
- 15.Angeloni NL, Bond CW, Tang Y, Harrington DA, Zhang S, Stupp SI, McKenna KE, Podlasek CA. Regeneration of the cavernous nerve by Sonic hedgehog using aligned peptide amphiphile nanofibers. Biomaterials 2011; 32: 1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001; 294:1684. [DOI] [PubMed] [Google Scholar]
- 17.Hartgerink JD, Beniash E, Stupp SI. Peptide-amphiphile nanofibers: A versatile scaffold for the preparation of self-assembling materials. Proc Natl Acad Sci USA 2002; 99: 5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S, Greenfield MA, Mata A, Palmer LC, Bitton R, Mantei JR, Aparicio C, Olvera de la Cruz M, Stupp SI.A self-assembly pathway to aligned monodomain gels. Nature Materials 2010; 9: 594–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webber MJ, Tongers J, Renault M-A, Roncalli JG, Losordo DW, Stipp SI. Development of bioactive peptide amphiphiles for therapeutic cell delivery. Acta Biomaterialia 2015; 23: S42–S51. [DOI] [PubMed] [Google Scholar]
- 20.Mata A, Geng Y, Henrikson KJ, Aparicio C, Stock SR, Satcher RL, Stupp SI. Bone regeneration mediated by biomimetic mineralization of a nanofiber matrix. Biomaterials 2010; 31: 6004–6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tysseling VM, Sahni V, Pashuck ET, Birch D, Hebert A, Czeisler C, Stupp SI, Kessler JA. Self-assembling peptide amphiphile promotes plasticity of serotonergic fibers following spinal cord injury. J Neurosci Res 2010; 88: 3161–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SS, Hsu EL, Mendoza M, Ghodasra J, Nickoli MS, Ashtekar A, Polavarapu M, Babu J, Riaz RM, Nicolas JD, Nelson D, Hashmi SZ, Kaltz SR, Earhart JS, Merk BR, McKee JS, Bairstow SF, Shah RN, Hsu WK, Stupp SI. Gel scaffolds of BMP-2-binding peptide amphiphile nanofibers for spinal arthrodesis. Adv Healthc Mater 2015; 4: 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berns EJ, Sur S, Pan L, Goldberger JE, Suresh S, Zhang S, Kessler JA, Stupp SI. Aligned neurite outgrowth and directed ell migration in self-assembled monodomain gels. Biomaterials 2014; 35: 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moyer TJ, Kassam HA, Bahnson ESM, Morgan CE, Tantakitti F, Chew TL, Kibbe MR, Stupp SI. Shape dependent targeting of injured blood vessels by peptide amphiphile supramolecular nanostructures. Small 2015; 11: 2750–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choe S, Bond CW, Harrington DA, Stupp SI, McVary KT, Podlasek CA. Peptide amphiphile nanofiber hydrogel delivery of sonic hedgehog protein to the cavernous nerve to promote regeneration and prevent erectile dysfunction. Nanomedicine: Nanotechnology, Biology, and Medicine 2017; 13: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobbs R, Choe S, Kalmanek E Harrington DA, Stupp SI, McVary KT, Podlasek CA. Peptide amphiphile delivery of sonic hedgehog protein promotes neurite formation in penile projection neurons. Nanomedicine 2018; 7: 2087–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mullerad M, Donohue JF, Li PS, Scardino PT, Mulhall JP. Functional Sequelae of Cavernous Nerve Injury in the Rat: is There Model Dependency. J Sex Med 2006; 3: 77–83. [DOI] [PubMed] [Google Scholar]
- 28.Nangle MR, Keast JR. Reduced Efficacy of Nitrergic Neurotransmission Exacerbates Erectile Dysfunction After Penile Nerve Injury Despite Axonal Regeneration. Exp Neurol 2007; 207: 30–41. [DOI] [PubMed] [Google Scholar]
- 29.Sheehan DC, Hrapchak BB (1980) Theory and practice of histotechnology, 2nd ed. St. Louis: C.V. Mosby Company: 141–154. [Google Scholar]
- 30.Krishnan R, Katz D, Nelson CJ, Mulhall JP. Erectile dysfunction recovery in patients after non-nerve sparing radical prostatectomy. Andrology 2014; 2: 951–954. [DOI] [PubMed] [Google Scholar]
- 31.Soleti R, Benameur T, Porro C, Panaro MA, Andriantsitohjaina R, Martínez MC. Microparticles harboring Sonic hedgehog promote angiogenesis through the uprgulation of adhesion proteins and proangiogenic factors. Carcinogenesis 2009; 30: 580–588. [DOI] [PubMed] [Google Scholar]
- 32.Agouni A, Mostefai A, Porro C, Carusia N, Favre J, Richard V, Henrion D, Martínez MC, Andriantsitohaina R. Sonic hedgehog carried by microparticles corrects endothelial injury through nitric oxide release. FASEB J 2007; 21: 2735–2741. [DOI] [PubMed] [Google Scholar]
- 33.Mezentsev A, Merks RMH, O’Riordan E, Chen J, Mendelev N, Goligorsky MS, Brodsky SV. Endothelial microparticles affect angiogenesis in vitro: role of oxidative stress. Am J Physiol Heart Circ Physiol 2005; 289: H1106–H1114. [DOI] [PubMed] [Google Scholar]