Abstract
Yellow head virus (YHV) is known as a major pathogen in the black tiger shrimp, Penaeus (Penaeus) monodon. It can also cause serious mortality in farmed whiteleg shrimp, Penaeus (Litopenaeus) vannamei. However, there is no published information on the economic and/or production impact of the disease in P. vannamei. Shrimp with gross signs of YHV disease (faded body colour and 60–70% mortality) were observed in 20 study farms rearing P. vannamei in the central part of Thailand from the end of 2007 through early 2008. The estimated economic loss for these farms according to the Thai Animal Aquaculture Association was approximately US$3 million. Detailed sequence analysis of RT‐PCR amplicons from shrimp in all the study ponds revealed the presence of YHV Type 1b (YHV‐1b) alone (characterized by a 162‐bp deletion in the ORF3 region encoding the structural gene for gp116) and the absence of YHV Type 1a (YHV‐1a), the original YHV type reported from Thailand. Despite the large 162‐bp deletion (= 54 deduced amino acids) in the gp116 structural gene, histopathology of YHV‐1b infections was identical to that of YHV‐1a infections, and electron microscopy revealed that YHV‐1b virions were morphologically indistinguishable from those previously reported for YHV‐1a. In addition, an existing commercial RT‐PCR detection kit and an immunochromatographic test strip for the detection of YHV were proven to have been valid tests for both YHV‐1b and YHV‐1a. The source of the virus for these outbreaks was unlikely to have been the post‐larvae used to stock the ponds, as they were derived from domesticated specific pathogen‐free stocks free of YHV. Thus, it is possible that they originated from an unknown, natural reservoir.
Keywords: detection, Penaeus vannamei, shrimp, Thailand, virus, yellow head virus
Introduction
Domesticated and genetically selected, specific pathogen‐free (SPF) Penaeus (Litopenaeus) vannamei (Boone) has replaced Penaeus (Penaeus) monodon as the dominant cultivated shrimp species in Thailand since 2002–2003 (Wyban 2007a, 2007b). The change in cultivated species has been accompanied by a change in the focus on shrimp pathogens, because those with significant negative impact on P. vannamei are not always the same as on P. monodon and vice versa (Flegel 2006). By the same token, some are serious pathogens for both species, and among these, the RNA virus yellow head virus (YHV) is one of the most important. Since the first report of YHV in Thailand in 1990 (Limsuwan 1991), six geographical types have been described (YHV‐1 to YHV‐6) (Wijegoonawardane 2008; Wijegoonawardane, Cowley & Walker 2008a; Wijegoonawardane, Cowley, Phan, Hodgson, Nielsen, Kiatpathomchai & Walker 2008b). These types differ in virulence and in overall genome sequences by up to 20% (Cowley, Dimmock, Wongteerasupaya, Boonsaeng, Panyim & Walker 1999; Sittidilokratna, Hodgson, Cowley, Jitrapakdee, Boonsaeng, Panyim & Walker 2002; Wijegoonawardane 2008). The presence of different YHV genotypes in various countries in Indo‐Pacific areas and phylogenetic comparison of specific regions of their genome sequences have also been reported (Wijegoonawardane 2008; Wijegoonawardane et al. 2008b).
The complete genome sequences of YHV‐1 and YHV‐2 (the latter also called GAV or gill‐associated virus) have been published (Cowley, Dimmock, Spann & Walker 2000; Sittidilokratna et al. 2002; Sittidilokratna, Dangtip, Cowley & Walker 2008). They are approximately 26 kb in length and composed of five open reading frames (ORF1a, ORF1b, ORF2, ORF3 and ORF4). However, a functional ORF4 may not exist in YHV‐1 (Sittidilokratna et al. 2008). A methodology for designation of 6 YHV genotypes based on regions in ORF1b and ORF3 has been recently presented (Wijegoonawardane 2008; 2008a, 2008b). Additionally, genotype YHV‐1 was further subdivided into YHV‐1a and YHV‐1b based on a 54‐amino acid deletion in the portion of ORF3 encoding structural protein gp116 in YHV‐1b when compared to YHV‐1a (Sittidilokratna, Chotwiwatthanakun, Wijegoonawardane, Unajak, Boonnad, Wangnai, Cowley & Walker 2009). Recombinant YHV genotypes have also been reported (Wijegoonawardane 2008; Gangnonngiw, Anantasomboon, Sang‐oum, Sriurairatana, Sritunyalucksana & Flegel 2009).
Since 1994, it has been known that other crustacean species including the whiteleg shrimp, P. vannamei, are susceptible to disease caused by YHV (Lu, Tapay, Bock & Loh 1994; Castro‐Longoria, Quintero‐Arredondo, Grijalva‐Chon & Ramos‐Paredes 2008), but according to Wijegoonawardane (2008) and Wijegoonawardane et al. (2008b), the most virulent types YHV‐1a and YHV‐1b have been found so far only in Thailand. Since 2006, sporadic YHV outbreaks have occurred in P. vannamei‐rearing ponds in areas of low salinity cultivation in Thailand, and one of these outbreaks was attributed to YHV‐1b (Sittidilokratna et al. 2009). However, there have been no published reports of estimated loss from YHV outbreaks in P. vannamei anywhere or any assessment of the prevalence of YHV types that may be the cause of the continuing outbreaks in Thailand since 2006. There are also open questions regarding the correlation between the gp116 deletion in YHV‐1b and its consequences for virion structure and virulence. For example, one study (Gangnonngiw et al. 2009) described a YHV5‐1b type recombinant that was non‐virulent for P. monodon and showed the lack of complete virions in infected shrimp tissue. Another study reported that YHV‐1b was somewhat less virulent than YHV‐1a in laboratory studies of one isolate from a disease outbreak pond in Ratchaburi province, Thailand, but no histological analysis or ultrastructure work was carried out to show whether this was related to changes in histopathology or virion structure (Gangnonngiw et al. 2009; Sittidilokratna et al. 2009). Nor was there any report on the scale of losses to YHV‐1b or whether the farm outbreak from which the isolate was derived resulted from single infections of YHV‐1b or mixed infections with YHV‐1a and YHV‐1b. Here, economic losses estimated by a farmer association for recent YHV disease outbreaks in farmed P. vannamei in two provinces in Thailand from late 2007 through early 2008 are presented together with a detailed analysis of the YHV type associated with these outbreaks.
Materials and methods
Shrimp samples
Juvenile Penaeus vannamei were collected from YHV‐outbreak ponds reported in the central part of Thailand. These included samples of living shrimp from four ponds in Ratchaburi province on 11 December 2007, from three ponds in Nakhon Pathom province on 5 March 2008 and from one pond in Nakhon Pathom province on 1 April 2008. The four live samples from Ratchaburi province and three from Nakhon Pathom province were used for detailed molecular analysis. A reference isolate of YHV‐1a genotype RNA was also extracted from frozen shrimp collected during a YHV outbreak in P. monodon from Thailand in 2002.
Gill tissues for RNA extraction and histopathological examination
Gill tissues from individual shrimp were homogenized in Trizol reagent (Invitrogen), and RNA was extracted following the manufacturer’s instructions. RNA concentration and quality were measured by spectrophotometric analysis at 260 and 280 nm. Parts of the remaining gills were fixed in Davidson’s fixative solution and processed for paraffin sectioning as previously described (Bell & Lightner 1988). Haematoxylin and eosin (H&E) staining was carried out to screen for histopathological features, characteristic of YHV (Flegel 2006).
YHV typing and nested PCR
Based on a recent genotype assignment (Wijegoonawardane 2008; Wijegoonawardane et al. 2008b), YHV typing regions were located in ORF1b and ORF3 of the viral genome. Primers for the ORF1b region were designed according to Wijegoonawardane et al. (2008b) and were YH30‐F2 (CTA CCA YTC AAA CAT CAT CAA YAA YCA) and YH30‐R2 (GAG ATG ATY TGR TKC TTG AAT TTC TG) with an expected amplicon size of ∼1 kb. Primers for the ORF3 typing region yielding a product of ∼1.2–1.4 kb were designed based on Gangnonngiw et al. (2009) and were gp116‐1F (CTA TCA ACG TGC AAC AAC ACA A) and gp116‐1R (GTG TGG ATG CAT AAA TTT CAT). RT‐PCR was performed according to the references with slight modification. To determine whether the samples were mixed‐type infections between Type‐1a and Type‐1b, a second PCR step (i.e. nested PCR) was carried out targeting the primary amplicon from the ORF3 typing region. Primers (HRM‐ORF3‐F; CCT ATC GCT ARA TCY TTC AT and HRM‐ORF3‐R; GTA TTT RAT RGC KTG TGT, where Y = C/T; R = A/G; K = G/T) were designed corresponding to nucleotides 21462–22016 of the YHV‐1a genome (GenBank accession no. EU487200) in such a manner as to bracket the region of deletion in YHV‐1b. Using these degenerate primers would yield expected nested PCR products of 555 bp for YHV‐1a, 393 bp for YHV‐1b and 480 bp for YHV‐2. The purpose was to determine whether even a small amount of YHV‐1a was mixed with YHV‐1b in the outbreak infections. This second, nested amplification was carried out in 20‐μL reaction solution containing 1 μL of 1:100 diluted solution from the first RT‐PCR reaction vial, 250 nm of each forward and reverse primer, 1 unit of Taq polymerase (Invitrogen), 0.2 mm dNTPs and 1× reaction buffer. The reaction protocol comprised denaturation at 94 °C for 5 min followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 30 s.
YHV detection using commercial tests
To determine whether commercial tests designed for YHV‐1a were still valid for detection of YHV‐1b, two common kits were used. One was a lateral‐flow chromatographic immunodiagnostic strip designed for the simultaneous detection of white spot syndrome virus (WSSV) and YHV (generously provided by Prof. P. Sithigorngul, Srinakharinwirot University, Thailand) (Sithigorngul, Rukpratanporn, Pecharaburanin, Longyant, Chaivisuthangkura & Sithigorngul 2006; Sithigorngul, Rukpratanporn, Sittidilokratna, Pecharaburanin, Longyant, Chaivisuthangkura & Sithigorngul 2007). For these tests, gill tissues were homogenized in the application buffer and loaded into the sample chamber. Photographs were taken after intensity of the control line had stabilized. The other test consisted of an IQ2000 YHV/GAV RT‐PCR detection kit (Farming IntelliGene Biotechnology Corporation). RT‐PCR was performed according to the manufacturer’s protocol, and amplified products were analysed on 2% agarose gels.
DNA cloning and sequence analysis
Amplicons were purified using a NucleoSpin Extract II kit (Macherey‐Nagel) and cloned into pDrive cloning vector (QIAGEN). Recombinant plasmids were verified by colony PCR (data not shown) prior to DNA sequencing by 1st BASE, Malaysia. DNA and protein analysis were carried out using the ExPASy Web server (http://au.expasy.org/). Alignments of nucleotide and deduced amino acid sequences were performed by ClustalW (http://www.ebi.ac.uk/clustalw/).
Transmission electron microscopy (TEM)
Lymphoid organs (a major target for YHV) were removed and divided into pieces of approximately 1 mm3. They were immediately fixed in 4.5% glutaraldehyde in 0.1 m phosphate buffer, pH 7.4 at 4 °C for 2 h. The fixing solution was removed, and the tissues were post‐fixed in 1% (w/v) osmium tetroxide in 0.1 m phosphate buffer at pH 7.4 for 2 h, washed twice with distilled water, dehydrated in a graded series of ethanol solutions from 50 to 100% and twice in 100% propylene oxide. Tissues were embedded in Epon‐812 resin (EMS) by successive 1‐h infiltration of 1:1 and 2:1 resin: propylene oxide, and 100% resin. The tissue blocks were then polymerized by incubating at 70 °C for 48 h in fresh 100% epoxy resin. Thin sections were stained in 2% (w/v) uranyl acetate and 0.3% (w/v) lead citrate solutions and viewed in a Hitachi H7100 electron microscope at 100 kV.
Results and discussion
Occurrence of YHV outbreaks in Penaeus vannamei
Outbreaks in Penaeus vannamei were reported in the central part of Thailand in the provinces of Ratchaburi and Nakhon Pathom, especially in areas practising low salinity cultivation (i.e. approximately 3–5 ppt). These outbreaks are still occurring. The period of our study spanned the end of 2007 through early 2008 and included 20 farms with altogether 500 culture ponds that passed approximately five crop cycles, although not every pond was operated in every cycle. Our molecular data (below) came from samples taken in 2007–2008. Affected shrimp had faded overall body colour, and mortality started after around 45–60 days of culture, with a cumulative mortality of 60–70%. Histological examination of YHV‐1b‐infected tissues (especially the lymphoid organ and gills) revealed typical characteristics of severe YHV infections (Flegel 2006), with abundant pyknotic and karyorrhectic nuclei (data not shown). The crop loss for the 20 farms included in this study was estimated by the Thai Animal Aquaculture Association to be approximately US$ 3 million. Because this loss did not include outbreaks in other freshwater cultivation areas, the national total must have been higher but is unlikely to have approached the US$ 30 million estimated loss to YHV for the whole of Thailand in 1992 (Flegel, Sriurairatana, Wongterrasupaya, Boonsaeng, Panyim & Withyachumnarnkul 1995), when P. monodon was the main cultured species, and all cultivation areas (not just fresh water) were affected. Despite the decrease in overall negative impact on national production, localized losses for individual farmers can be severe, and a continued effort is needed to control outbreaks because they can spread rapidly to nearby ponds.
Because the ponds included in the study were all stocked with post‐larvae derived from domesticated SPF stocks of P. vannamei certified free of YHV, they were unlikely to be the source of YHV for these outbreaks. This raises the possibility that the virus may have originated from an unknown, natural reservoir. For example, it is known that Metapenaeus brevicornis and some palaemonid shrimp species can be infected with YHV (Longyant, Sithigorngul, Chaivisuthangkura, Rukpratanporn, Sithigorngul & Menasveta 2005; Longyant, Sattaman, Chaivisuthangkura, Rukpratanporn, Sithigorngul & Sithigorngul 2006). However, these potential carriers can be eliminated from the culture system by proper pond preparation and by subsequent filtration of exchange water. Curiously, these practices to avoid natural crustacean carriers have allowed shrimp farmers in the study area to successfully avoid WSSV disease outbreaks (many potential crustacean carriers) but not YHV outbreaks. In response, many farmers have resorted to less profitable cultivation of fish or the freshwater prawn, Macrobrachium rosenbergii, singly or in combination.
After successive losses to YHV, even after a 2‐year intervening period of fish cultivation, one farm from an adjacent province (Samut Songkhram) has very recently carried out tests (unpublished) on 24 cultivation ponds arranged in rows of four ponds each. Alternate rows were completely covered with mosquito netting (i.e. 3 rows = 12 ponds) while the remaining rows (3 = 12 ponds) were left uncovered. Stocking, feeding and water management were identical for each of the three paired rows. At 60 days of cultivation, one of the uncovered ponds experienced a YHV disease outbreak, and all of the remaining uncovered ponds followed within 1 month. By contrast, none of the covered ponds were affected, and all reached successful harvests approximately 2 months after the initial YHV disease outbreak. The farm manager has concluded that YHV is spread by an airborne carrier and now plans to cover all the ponds in the farm. The possibility of an airborne carrier(s) should be pursued in a detailed study. For example, an appropriate study has shown that Macrobrachium rosenbergii nodavirus and its partner extra small virus (XSV) can be carried by aquatic insects that are capable of initiating white tail disease in cultivated freshwater prawns (Sudhakaran, Parameswaran & Sahul Hameed 2007; Sudhakaran, Haribabu, Kumar, Sarathi, Ahmed, Babu, Venkatesan & Sahul Hameed 2008).
YHV typing revealed YHV‐1b
As several geographical types of YHV have been reported (Wijegoonawardane 2008; 2008a, 2008b), it was of epidemiological interest to know which type of YHV was responsible for the disease outbreaks. Gill RNA extracts from P. vannamei samples from four ponds from Ratchaburi province (11 December 2007) and three ponds from Nakhon Pathom province (5 March 2008) were used for YHV typing. RT‐PCR of the ORF1b and ORF3 typing regions yielded expected amplicon sizes of 1002 bp and 1252 bp, respectively (data not shown). Some sequence variation was found between samples from Ratchaburi and Nakhon Pathom provinces, i.e. four and six nucleotide differences in ORF1b and ORF3 typing regions, respectively. Sequence comparison for the ORF1b typing region of YHV‐1a (GenBank accession no. EU487200) revealed five nucleotide differences but 100% amino acid identity with the Ratchaburi sequence and six nucleotide differences but 99.7% amino acid identity with the Nakhon Pathom sequence. Sequence comparison for the ORF3 typing region of YHV‐1b (GenBank accession no. FJ194949) revealed 98.9% nucleic acid identity and 98.1% amino acid identity with the Ratchaburi sequence and 98.9% nucleic acid identity and 98.3% amino acid identity with the Nakhon Pathom sequence (Fig. 1a). In addition, the identical 162‐bp deletion was present in ORF3 sequences from both samples (Fig. 1a). The sequencing results clearly indicated that these YHV outbreaks in P. vannamei from the two provinces in Thailand were caused by YHV‐1b. Sequences from the Ratchaburi sample were combined into a single sequence with gaps to separate each section and deposited at GenBank under accession no. FJ627274.
Figure 1.
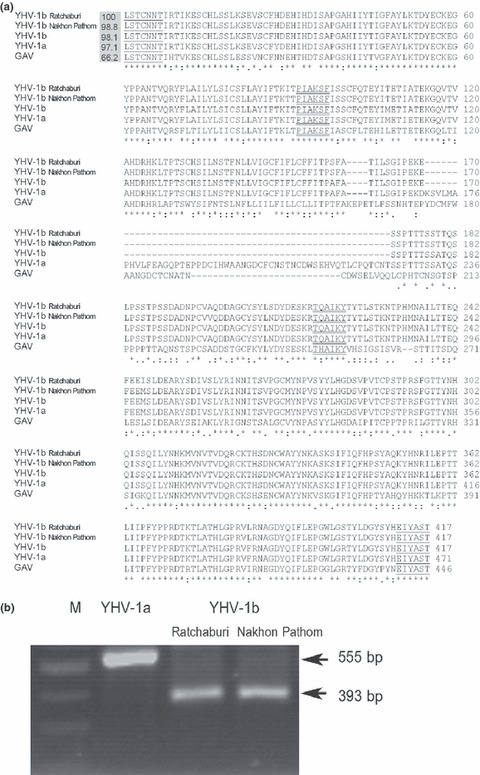
ORF3 typing regions of yellow head virus (YHV). (a) Sequence alignment of deduced amino acid sequences of ORF3 typing regions. Sequences were YHV‐1b from Ratchaburi and Nakhon Pathom provinces (FJ627274), previously identified as YHV‐1b (FJ194949), YHV‐1a (EU487200) and GAV (NC_010306). The percentage identity of Ratchaburi YHV‐1b to other sequences is indicated in the grey box. Positions of primers used to investigate mixed‐type YHV infections are underlined and double underlined for the first and nested PCR, respectively. (b) Example agarose gel showing nested PCR amplicons of ORF3 typing region obtained by using 1st‐step RT‐PCR products of YHV‐1a (reference) and YHV‐1b (diseased shrimp) as templates. M = DNA marker (2‐Log DNA Ladder; New England Biolabs).
So far, there have been four reports on four YHV isolates with a deletion sequence in ORF3 (Wijegoonawardane 2008; 2008a, 2008b; Gangnonngiw et al. 2009; Sittidilokratna et al. 2009). Two virulent YHV isolates from Thailand were used to define Type‐1b (GenBank accession no. FJ194949) (Wijegoonawardane 2008; 2008a, 2008b). A third was a virulent type from Ratchaburi province, Thailand, also defined as Type‐1b (Sittidilokratna et al. 2009). A fourth was a non‐virulent type from Thailand defined as a recombinant between Type‐5 and Type‐1b (Gangnonngiw et al. 2009) (GenBank accession no. EU123854) based on the typing system of Wijegoonawardane et al. (Wijegoonawardane 2008; 2008a, 2008b). Our Ratchaburi and adjacent Nakhon Pathom isolates matched the originally defined Type‐1b (GenBank accession no. FJ194949) by high sequence identity at both typing loci and by reported high mortality in P. vannamei.
Infected samples did not have mixed YHV‐type infections
Because some questions have been raised about the ability of the YHV‐1b deletion form of gp116 to participate in the formation of normal YHV virions (Gangnonngiw et al. 2009), we tested our outbreak pond samples for the possibility of mixed infections with YHV‐1a using a nested RT‐PCR that would reveal the presence of YHV‐1a even if present together with YHV‐1b at very low levels. A diagram of the primers and their target regions is shown in Fig. 1a. The nested‐PCR results (samples in Fig. 1b) revealed the expected amplicon of 555 bp from the YHV‐1a reference isolate but only a single 393 bp amplicon from samples of whiteleg shrimp from the outbreak ponds in Ratchaburi and Nakhon Pathom provinces. The lack of two or more amplicon bands in any of these tests indicated that neither YHV‐1a nor YHV‐2 previously reported from Thailand was mixed with YHV‐1b in these infections, at least within the limits of the detection method used. Our test could not exclude the possible presence of other defined types of YHV or previously unreported variants.
Analysis of proteins from purified virions from a previous YHV‐1b isolate from Ratchaburi Province in 2006 (Sittidilokratna et al. 2009) showed absence of full‐length gp116 together with the deletion version, suggesting that it was also a single infection of YHV‐1b. In addition, our sample from Nakhon Pathom province in April 2008 was also for YHV‐1b alone. The earlier report by Wijegoonawardane et al. (2008b) listed results for 31 shrimp samples from Thailand. Out of these, 13 samples of P. monodon from 2000, 2003 and 2004 were infected with non‐virulent YHV Type‐2 (n = 6) and Type‐3 (n = 7), while the remaining 18 samples were infected with virulent Type‐1. From these 18 Type‐1 isolates, five from 2000, four from 2001, one from 2002 and four from 2003 (total 14) were Type‐1a. In addition, the four from 2001 plus three from 2003 (total 7) were obtained from one of the provinces (Nakhon Pathom) included in our study. Only three isolates, one from Ratchaburi province in 2003 and two from Chachoengsao province in 2003 were Type‐1b. Thus, the first records for Type‐1b were in 2003, both in P. vannamei from fresh water cultivation areas after P. vannamei had become the dominant cultured species in Thailand. We hypothesize that YHV‐1b may have originated as a mutant of YHV‐1a that might be more suitable for the infection of P. vannamei than Type‐1a.
TEM revealed mature YHV virions
TEM was used to further examine the question regarding capability of the deletion form of gp116 from YHV‐1b to participate in formation of normal YHV virions. A representative TEM micrograph of lymphoid organ tissue (Fig. 2) of shrimp from such ponds revealed typical mature and immature viral structures previously reported for acute infections of YHV‐1 and YHV‐2 (Chantanachookin, Boonyaratanapalin, Kasornchandra, Direkbusarakom, Ekpanithanpong, Supamataya, Sriurairatana & Flegel 1993; Spann, Cowley, Walker & Lester 1997), indicating that the 162‐bp deletion in ORF3 did not interfere with morphologically normal YHV particle assembly. This is despite the fact that previous work (Gangnonngiw et al. 2009; Sittidilokratna et al. 2009) revealed that the ORF3 locus of YHV‐1b produced a small, conformationally altered version of gp116 that reacted poorly with polyclonal antibodies and not at all with a monoclonal antibody raised against normal gp116.
Figure 2.
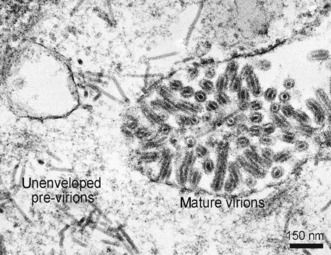
Transmission electron micrograph of lymphoid organ tissue of YHV‐1b‐infected Penaeus vannamei. Both yellow head virus (YHV) previrions and mature virions are indicated.
TEM from previous work on a non‐virulent recombinant between YHV‐5 and YHV‐1b (YHV‐5/1b) revealed only immature unenveloped, previrions in the lymphoid organ (a prime target for YHV), suggesting that the conformationally altered gp116 and normal gp64 proteins produced could not be assembled into mature virions by the recombinant isolate (Gangnonngiw et al. 2009). Our TEM work with YHV‐1b confirming that the altered gp116 could participate in assembly of morphologically normal virions supported an earlier proposal (based on structural protein analysis) that YHV‐1b produced infectious virions, despite somewhat lower virulence than YHV‐1a in laboratory challenge tests (Sittidilokratna et al. 2009). In summary, the recombinant’s inability to form mature viral particles could not be attributed to conformationally altered gp116, and this supported an earlier suggestion that the inability of the recombinant to form normal virions was more likely associated with its ORF1b gene region.
At the same time, the region of ORF3 upstream of gp116 and gp64 comprises a putative gene of currently uncertain function (Sittidilokratna et al. 2008) that is transcribed and translated together with gp116 and gp64 to yield a single polyprotein. In the YHV‐5/1b recombinant, this gene region contained many stop codons, suggesting that it would not produce a functional protein. In contrast, the sequence of YHV‐1b from our study and those previously reported lacked these stop codons and translated into an uninterrupted, deduced protein, identical to those reported for both YHV‐1 types (Fig. 1a). Such YHV‐1b isolates would be able to produce an intact protein while the YHV‐5/1b recombinant would not, opening an alternative possibility that the relevant gene product may play a role in YHV viral assembly. In some coronaviruses and arteriviruses with genome organization similar to that of YHV, the comparable ORF3 region is called the M protein region (Snijder & Meulenberg 1998; Sawicki, Sawicki & Siddell 2007) that is responsible for production of the virion matrix protein. Although no matrix protein has ever been reported for YHV, we cannot yet exclude the possibility that the related (M‐like) YHV transcript also plays some role in the virion assembly process. It would be interesting to specifically knock down expression of this M‐like transcript in YHV‐1a or YHV‐1b to see whether TEM examination of lymphoid organ tissue in challenged shrimp would reveal reduced ability to form normal, mature virions.
Commercial kits were valid for YHV‐1b diagnosis
Despite any differences in virulence from YHV‐1a in laboratory studies (Sittidilokratna et al. 2009), YHV‐1b was shown to cause disease outbreaks and crop losses in P. vannamei that were equally destructive to those previously reported for the original type (Flegel et al. 1995). Because no effective control solution has been found other than prevention, effective YHV detection methods for shrimp breeding stocks and post‐larvae are essential. We confirmed that two kinds of commercial test kits designed for detection of YHV‐1a are also applicable for YHV‐1b diagnosis. The kits were an immunological‐based dual WSSV/YHV test strip kit and an RT‐PCR–based IQ2000 YHV/GAV detection kit. Gill homogenates of our shrimp samples from Ratchaburi and Nakhon Pathom provinces gave positive results for YHV only, using the dual WSSV/YHV test strip kit. Representative immunoassay test strip results (Fig. 3a) and RT‐PCR assay results using the IQ2000 YHV/GAV detection kit (Fig. 3b) are shown. Because amplicons for both the outer (777 bp) and the inner (277 bp) primer sets were observed, the infections were classified as severe, according to the kit manual, and this was consistent with the histopathological picture of severe infections. Thus, the tested commercial kits and conventional histopathology could be routinely used for YHV‐1b diagnosis. These results were expected as the commercial kits targeted features of high similarity between YHV‐1a and YHV‐1b. That is, the immunodiagnostic strip was specific for YHV p20 nucleocapsid protein (ORF2 of the viral genome), and the RT‐PCR detection kit targeted a region upstream of the ORF1b typing sequence (Cowley, Cadogan, Wongteerasupaya, Hodgson, Boonsaeng & Walker 2004) where YHV‐1a (GenBank accession no. EU487200) and our YHV‐1b (GenBank accession no. FJ627274) displayed 97–98% nucleic acid sequence identity.
Figure 3.
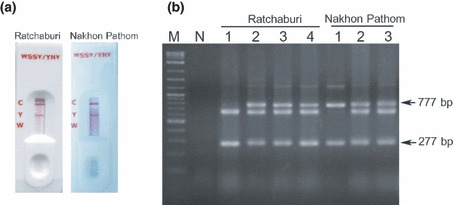
Diagnostic results of YHV‐1b‐infected samples from two provinces in central Thailand using commercial kits. (a) Representative examples of results obtained for samples from each province using gill homogenates with dual WSSV/YHV immunochromatographic test strips. C = control line; Y = YHV; W = WSSV. (b) Results using a commercial RT‐PCR detection kit. Bands at 277 and/or 777 bp indicate a YHV infection according to the kit instructions (arrows). The band just below 777 bp is the kit internal control amplicon. M = DNA marker (2‐Log DNA Ladder; New England Biolabs); N = no template control. YHV, yellow head virus; WSSV, white spot syndrome virus.
Conclusions
We have shown that recent outbreaks of acute yellow head disease in P. vannamei reared in central Thailand have been caused predominantly by YHV Type‐1b in single infections and not mixed infections with two other YHV types known to occur in Thailand. The first recorded occurrence of YHV‐1b in 2003 and its current dominance in disease outbreaks in Thailand coincided with a shift in the major cultivated species from P. monodon to P. vannamei beginning in 2002–2003. Severity of the outbreaks and economic losses for affected ponds was similar to those caused by YHV‐1a, despite laboratory tests suggesting that YHV‐1b is somewhat less virulent than YHV‐1a. TEM of lymphoid organ tissue from the diseased shrimp revealed that the large deletion in the YHV‐1b structural protein gp116 did not interfere with the formation of viral particles morphologically identical to those previously described for acute infections caused by YHV‐1a. Because YHV‐1b from the diseased shrimp had a normal M‐like gene, while a non‐virulent YHV‐5/1b recombinant previously reported from Thailand did not, we speculate that abnormal virion assembly seen for the recombinant may have resulted from the lack of a functional M‐like gene. Despite the differences between YHV‐1a and YHV‐1b, commonly used detection kits designed based on YHV‐1a and YHV‐2 were shown to be effective for the detection of YHV‐1b.
Acknowledgements
This work was supported by a Mahidol University grant and by a Thailand Research Fund grant to S. Senapin (grant no. TRG5280005). The authors would like to thank Prof. P. Sithigorngul, Srinakharinwirot University, Thailand, for providing the WSSV/YHV test strips and K. Phewsaiya, Centex Shrimp, for skilled technical assistance.
The GenBank accession number for the sequence reported in this paper is FJ627274.
References
- Bell T. & Lightner D. (1988) Handbook of Normal Penaeid Shrimp Histology. The World Aquaculture Society, LA, USA. [Google Scholar]
- Castro‐Longoria R., Quintero‐Arredondo N., Grijalva‐Chon J.M. & Ramos‐Paredes J. (2008) Detection of the yellow‐head virus (YHV) in wild blue shrimp, Penaeus stylirostris, from the Gulf of California and its experimental transmission to the Pacific white shrimp, Penaeus vannamei . Journal of Fish Diseases 31, 953–956. [DOI] [PubMed] [Google Scholar]
- Chantanachookin C., Boonyaratanapalin S., Kasornchandra J., Direkbusarakom S., Ekpanithanpong U., Supamataya K., Sriurairatana S. & Flegel T.W. (1993) Histology and ultrastructure reveal a new granulosis‐like virus in Penaeus monodon affected by “yellow‐head” disease. Diseases of Aquatic Organisms 17, 145–157. [Google Scholar]
- Cowley J.A., Dimmock C.M., Wongteerasupaya C., Boonsaeng V., Panyim S. & Walker P.J. (1999) Yellow head virus from Thailand and gill‐associated virus from Australia are closely related but distinct prawn viruses. Diseases of Aquatic Organisms 36, 153–157. [DOI] [PubMed] [Google Scholar]
- Cowley J.A., Dimmock C.M., Spann K.M. & Walker P.J. (2000) Gill‐associated virus of Penaeus monodon prawns: an invertebrate virus with ORF1a and ORF1b genes related to arteri‐ and coronaviruses. Journal of General Virology 81, 1473–1484. [DOI] [PubMed] [Google Scholar]
- Cowley J.A., Cadogan L.C., Wongteerasupaya C., Hodgson R.A., Boonsaeng V. & Walker P.J. (2004) Multiplex RT‐nested PCR differentiation of gill‐associated virus (Australia) from yellow head virus (Thailand) of Penaeus monodon . Journal of Virological Methods 117, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegel T.W. (2006) Detection of major penaeid shrimp viruses in Asia, a historical perspective with emphasis on Thailand. Aquaculture 258, 1–33. [Google Scholar]
- Flegel T.W., Sriurairatana S., Wongterrasupaya C., Boonsaeng V., Panyim S. & Withyachumnarnkul B. (1995) Progress in characterization and control of yellow‐head virus of Penaeus monodon In: Swimming Through Troubled Water, Proceedings of the Special Session on Shrimp Farming (ed. by Browdy C.L. & Hopkins J.S.), pp. 76–83. World Aquaculture Society, Baton Rouge, LA. [Google Scholar]
- Gangnonngiw W., Anantasomboon G., Sang‐oum W., Sriurairatana S., Sritunyalucksana K. & Flegel T.W. (2009) Non‐virulence of a recombinant shrimp nidovirus is associated with its non structural gene sequence and not a large structural gene deletion. Virology 385, 161–168. [DOI] [PubMed] [Google Scholar]
- Limsuwan C. (1991) Handbook for Cultivation of Black Tiger Prawns. Tamsetakit Co. Ltd., Bangkok (in Thai). [Google Scholar]
- Longyant S., Sithigorngul P., Chaivisuthangkura P., Rukpratanporn S., Sithigorngul W. & Menasveta P. (2005) Differences in susceptibility of palaemonid shrimp species to yellow head virus (YHV) infection. Diseases of Aquatic Organisms 64, 5–12. [DOI] [PubMed] [Google Scholar]
- Longyant S., Sattaman S., Chaivisuthangkura P., Rukpratanporn S., Sithigorngul W. & Sithigorngul P. (2006) Experimental infection of some penaeid shrimps and crabs by yellow head virus (YHV). Aquaculture 257, 83–91. [Google Scholar]
- Lu Y., Tapay L.M., Bock J.A. & Loh P.C. (1994) Infection of the yellow head baculo‐like virus (YBV) in two species of penaeid shrimp, Penaeus stylirostris (Stimpson) and Penaeus vannamei (Boone). Journal of Fish Diseases 17, 649–656. [Google Scholar]
- Sawicki S.G., Sawicki D.L. & Siddell S.G. (2007) A contemporary view of coronavirus transcription. Journal of Virology 81, 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sithigorngul W., Rukpratanporn S., Pecharaburanin N., Longyant S., Chaivisuthangkura P. & Sithigorngul P. (2006) A simple and rapid immunochromatographic test strip for detection of white spot syndrome virus (WSSV) of shrimp. Diseases of Aquatic Organisms 72, 101–106. [DOI] [PubMed] [Google Scholar]
- Sithigorngul W., Rukpratanporn S., Sittidilokratna N., Pecharaburanin N., Longyant S., Chaivisuthangkura P. & Sithigorngul P. (2007) A convenient immunochromatographic test strip for rapid diagnosis of yellow head virus infection in shrimp. Journal of Virological Methods 149, 193–199. [DOI] [PubMed] [Google Scholar]
- Sittidilokratna N., Hodgson R.A., Cowley J.A., Jitrapakdee S., Boonsaeng V., Panyim S. & Walker P.J. (2002) Complete ORF1b‐gene sequence indicates yellow head virus is an invertebrate nidovirus. Diseases of Aquatic Organisms 50, 87–93. [DOI] [PubMed] [Google Scholar]
- Sittidilokratna N., Dangtip S., Cowley J.A. & Walker P.J. (2008) RNA transcription analysis and completion of the genome sequence of yellow head nidovirus. Virus Research 136, 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittidilokratna N., Chotwiwatthanakun C., Wijegoonawardane P.K.M., Unajak S., Boonnad A., Wangnai W., Cowley J.A. & Walker P.J. (2009) A virulent isolate of yellow head nidovirus contains a deformed envelope glycoprotein gp116. Virology 384, 192–200. [DOI] [PubMed] [Google Scholar]
- Snijder E.J. & Meulenberg J.J.M. (1998) The molecular biology of arteriviruses. Journal of General Virology 79, 961–979. [DOI] [PubMed] [Google Scholar]
- Spann K.M., Cowley J.A., Walker P.J. & Lester R.J.G. (1997) A yellow‐head‐like virus from Penaeus monodon cultured in Australia. Diseases of Aquatic Organisms 31, 169–179. [Google Scholar]
- Sudhakaran R., Parameswaran V. & Sahul Hameed A. (2007) In vitro replication of Macrobrachium rosenbergii nodavirus and extra small virus in C6/36 mosquito cell line. Journal of Virological Methods 146, 112–118. [DOI] [PubMed] [Google Scholar]
- Sudhakaran R., Haribabu P., Kumar S., Sarathi M., Ahmed V., Babu V., Venkatesan C. & Sahul Hameed A. (2008) Natural aquatic insect carriers of Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus (XSV). Diseases of Aquatic Organisms 79, 141–145. [DOI] [PubMed] [Google Scholar]
- Wijegoonawardane P.K. (2008) Molecular epidemiology of yellow head‐complex viruses of cultured prawns in the Asian region. University of Queensland, Brisbane, Australia. [Google Scholar]
- Wijegoonawardane P.K., Cowley J.A. & Walker P.J. (2008a) Consensus RT‐nested PCR detection of yellow head complex genotypes in penaeid shrimp. Journal of Virological Methods 153, 168–175. [DOI] [PubMed] [Google Scholar]
- Wijegoonawardane P.K., Cowley J.A., Phan T., Hodgson R.A., Nielsen L., Kiatpathomchai W. & Walker P.J. (2008b) Genetic diversity in the yellow head nidovirus complex. Virology 380, 213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyban J. (2007a) Domestication of Pacific white shrimp revolutionizes aquaculture. Global Aquaculture Advocate July/August, 42–44. [Google Scholar]
- Wyban J. (2007b) Thailand’s white shrimp revolution. Global Aquaculture Advocate May/June, 56–58. [Google Scholar]


