Abstract
Chronic pain is long-lasting nociceptive state, impairing the patient's quality of life. Existing analgesics are generally not effective in the treatment of chronic pain, some of which such as opioids have the risk of tolerance/dependence and overdose death with higher daily opioid doses for increasing analgesic effect. Opioid use disorders have already reached an epidemic level in the United States; therefore, nonopioid analgesic approach and/or use of nonpharmacologic interventions will be employed with increasing frequency. Viral vector–mediated gene therapy is promising in clinical trials in the nervous system diseases. Glutamic acid decarboxylase (GAD) enzyme, a key enzyme in biosynthesis of γ-aminobutyric acid (GABA), plays an important role in analgesic mechanism. In the literature review, we used PubMed and bioRxiv to search the studies, and the eligible criteria include (1) article written in English, (2) use of viral vectors expressing GAD67 or GAD65, and (3) preclinical pain models. We identified 13 eligible original research articles, in which the pain models include nerve injury, HIV-related pain, painful diabetic neuropathy, and formalin test. GAD expressed by the viral vectors from all the reports produced antinociceptive effects. Restoring GABA systems is a promising therapeutic strategy for chronic pain, which provides evidence for the clinical trial of gene therapy for pain in the near future.
Keywords: glutamic acid decarboxylase, chronic pain, gene therapy, viral vectors
Introduction
Acute pain acts as a necessary protective response to alert the body to the presence of a noxious stimulation.1 However, chronic pain is long-lasting nociceptive state greatly impairing the patient's quality of life, even if after tissue injury has healed. Therefore, it is necessary to proactively manage chronic pain. Existing analgesics are generally not effective in the treatment of chronic pain, some of which such as opioids have the risk of tolerance/dependence and/or overdose death with higher daily opioid doses for increasing analgesic effect.2 Opioid use disorders have already reached an epidemic level in the United States.3 Therefore, nonopioid analgesic approach and/or use of nonpharmacologic interventions will be promising to be employed with increasing frequency to fight opioid crisis.
Therapeutic gene transfer may locally produce neurotransmitters/neuropeptides while avoiding unwanted side effects that would cause activation of the same receptors in other locations/pathways by a systemically administered drug.4 Using viral vectors to express target gene products could represent an alternative to standard pharmacological approaches.4,5 Gene therapy is a promising choice for the treatment of many central nervous system (CNS) disorders in clinical trials including chronic pain.5–8 It is known that γ-aminobutyric acid (GABA) is the main inhibitory neurotransmitter reducing neural excitability in the spinal cord. GABA systems modulate a facilitated state of nociceptive processing.9,10 Glutamic acid decarboxylase (GAD) enzyme is a key enzyme in biosynthesis of GABA. GAD has two major isoforms GAD67 and GAD65, encoded by gad1 and gad2 genes, respectively. Spinal GAD is lowered in the nerve injury states (Fig. 1).11–13 In this literature review, we synthesize the current available literature of viral vector–mediated GAD gene therapy for pain.
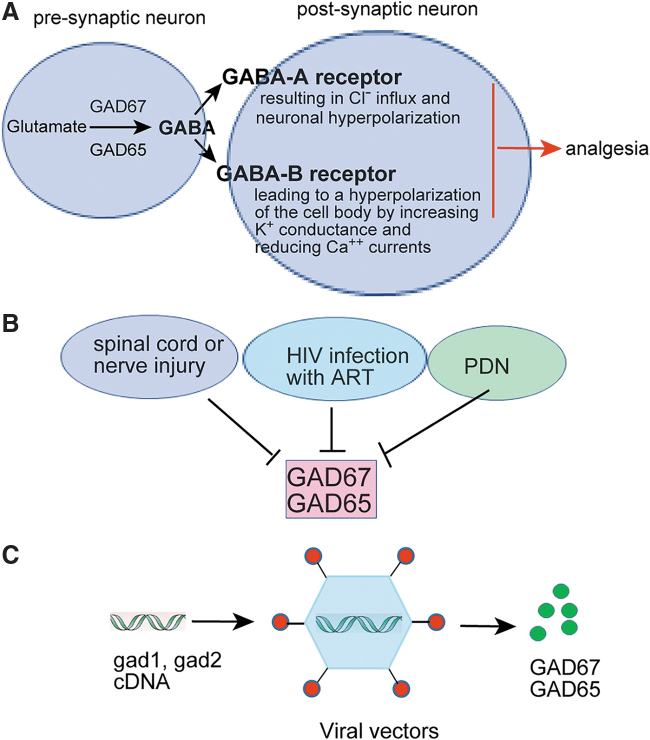
Figure 1.
The role of GABAergic system in the modulation of nociception in the spinal cord dorsal horn. (A) Two glutamic acid decarboxylases GAD67 and GAD65 induce cytoplasmic GABA synthesis from glutamate in the spinal cord dorsal horn. Within the dorsal horn, GABAergic neurons regulate nociceptive signals by GABA-A/B receptors. GABA-A receptor results in Cl− influx and neuronal hyperpolarization, which are mainly located postsynaptically.9 Metabotropic GABA-B receptors is linked by a Gi protein to both K+ channels and calcium channels, leading to a hyperpolarization of the cell body by increasing K+ conductance and reducing Ca++ currents,82 finally inducing analgesia. (B) Spinal cord or peripheral nerve injury, HIV infection with ART, and PDN lower the GAD67/GAD65 expression, finally decreasing the synthesis of GABA and inducing functional disinhibition.11–13,56,59,79,105 (C) Viral vectors encoding gad1 and/or gad2 gene can increase GAD67/GAD65 synthesis for chronic pain treatment. ART, antiretroviral therapy; GABA, γ-aminobutyric acid; PDN, painful diabetic neuropathy.
Review of the Literature for Gene Therapy Approach by Viral Vector–Mediated GAD for Chronic Pain in Animals Models
Literature searches from PubMed, BioRxiv, and Cochrane Database of Systematic Review (CDSR) were performed in December 2019. In the PubMed searching, the studies range covers from 1946 to 2019. We searched “glutamic acid decarboxylase” and found that there were 11,386 publications in PubMed. In the BioRxiv searching, there were 356 publications for term “glutamic acid decarboxylase” from 2010 to 2019. In the CDSR searching, there are two articles for term “glutamic acid decarboxylase.” The eligible criteria include (1) article written in English, (2) use of viral vectors expressing GAD enzyme, and (3) pain models. Studies not meeting the inclusion criteria were excluded. Based on our criteria, we identified 13 eligible original research articles, in which the pain models include spinal cord/nerve injury, HIV-related pain, painful diabetic neuropathy (PDN), and formalin test. Brief summaries from these literatures were given for gene therapy approach by herpes simplex virus (HSV) vector-mediated GAD (Table 1) and by non-HSV vector–mediated GAD (Table 2).
Table 1.
Summary of gene therapy approach by herpes simplex virus–mediated glutamic acid decarboxylase for chronic pain in animals models
| Animal Models | Genes | Routes of HSV Delivery | Results | References |
|---|---|---|---|---|
| Rat SCI | GAD67 | Hind paw injection | Reduced mechanical allodynia and thermal hyperalgesia at 1–6 weeks postinfection; bicuculline reverses the antinociceptive effect | 23 |
| Rat SCI | GAD67 | Bladder wall injection | Decreased number and amplitude of nonvoiding contractions, which was reversed by bicuculline | 26 |
| Rat SNL | GAD67 | Hind paw injection | Reduced mechanical allodynia and thermal hyperalgesia and suppressed spinal c-fos and pERK1/2 | 27 |
| Rat ligation of lumbar roots | GAD67 | Hind paw injection | Reduction in mechanical allodynia | 28 |
| Rat sciatic gp120 | GAD67 | Hind paw injection | Reduced mechanical allodynia at 3–28 days postinfection; bicuculline/CGP54626 reversed the anti-allodynic effect; decrease spinal ROS and Wnt5a | 51 |
| Rat sciatic gp120+ddC | GAD67 | Hind paw injection | Reduced mechanical allodynia at 3–28 days postinfection; decrease spinal ROS, pCREB, pC/EBPβ | 56 |
| Rat PDN | GAD67 | Hind paw injection | Reduced mechanical hyperalgesia, thermal hyperalgesia, and cold allodynia and lowered the voltage-gated sodium channel isoform 1.7 | 61 |
| Mice and rat PDN | GAD65, GAD67 | Hind paw injection | Reduced mechanical allodynia and thermal hyperalgesia | 62 |
GAD, glutamic acid decarboxylase; HSV, herpes simplex virus; PDN, painful diabetic neuropathy; pERK1/2, phosphorylated extracellular signal-regulated kinase 1/2; ROS, reactive oxygen species; SCI, spinal cord injury; SNL, spinal nerve ligation; Wnt5a, Wingless-Type Mammary Tumor Virus Integration-Site Family Member 5a.
Table 2.
Other viral vectors mediating glutamic acid decarboxylase expression for analgesia
| Vector Type | Gene | Animal Model | Routes of Vector Delivery | Results | References |
|---|---|---|---|---|---|
| AV | GAD67 | Mice SNT | L4 spinal nerve | Reduced mechanical allodynia; preventing SNT-induced neuropathic pain, suppressed the elevation of Cav3.2 mRNA in the DRG | 63 |
| AV | GAD65 | Rat orofacial formalin test | Trigeminal ganglion | Decrease pain behavior, which was blocked by bicuculline | 71 |
| AAV | GAD65 | Rat tibial and sural nerve ligation | Sciatic nerve injection | Reduced mechanical hyperalgesia and allodynia, and c-fos activation | 66 |
| AAV | GAD65 | Rat tibial and sural nerve transected | L4/5 DRG | Reduced mechanical hyperalgesia and allodynia | 67 |
| HFV | GAD67 | Rat SCI | Hind paws | Reversed mechanical allodynia and thermal hyperalgesia | 68 |
AAV, adeno-associated virus; AV, adenoviral vector; HFV, human foamy virus; SCI, spinal cord injury; SNT, spinal nerve transection.
HSV-mediated GAD gene transfer for chronic pain
In a culture of cerebellar granule cell (CGC), treatment with HSV vectors containing GAD cDNA increases expression of GAD65 and GAD67 and stimulation-evoked GABA release.14 Treatment of CGCs with kainic acid, which destroys most of the GABAergic neurons, did not prevent vector-derived expression of GAD nor synthesis of GABA, suggesting that defective HSV vector–derived GAD expression can be used to increase GABA synthesis and release in CNS tissue, even in the relative absence of GABAergic neurons.14 HSV is suitable for altering neurotransmitter and/or neuropeptides release after subcutaneous inoculation for preclinical pain treatment.15–18 After injection of nonreplicating HSV vectors into the skin, the vectors are picked up by the peripheral terminals of the dorsal root ganglion (DRG) neurons and travel to the cell bodies of primary afferents by retrograde axonal transport to produce target molecules (Fig. 2). In in vivo studies, we and others used HSV vector containing the sequence of the human gad1 gene encoding GAD67 in preclinical pain models (Table 1).
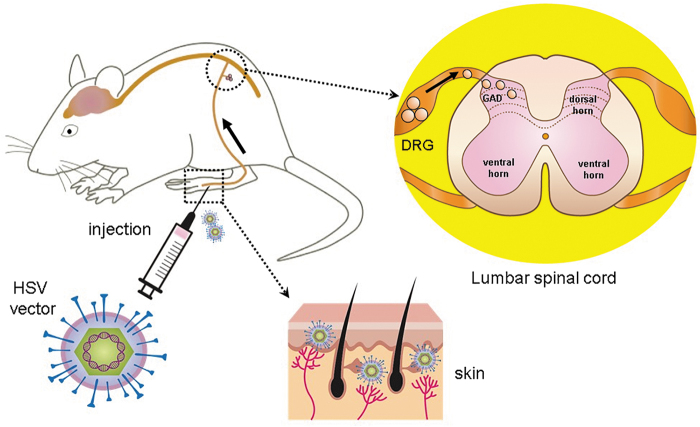
Figure 2.
Gene therapy for pain using HSV vectors expressing GAD. After injection of nonreplicating HSV vectors into the skin, the vectors are picked up by the peripheral terminals of the DRG neurons and travel to the cell bodies of primary afferents by retrograde axonal transport. The vectors can establish life-long quiescent state (latency) in the DRG neurons as an intranuclear episomal element. The transgene product GAD from the HSV vectors can be released from nerve terminals in the spinal cord dorsal horn. DRG, dorsal root ganglion; HSV, herpes simplex virus.
HSV vector for nerve injury–induced neuropathic pain
Neuropathic pain following spinal cord injury (SCI) is a critical issue that impedes effective rehabilitation.19 Lowered GAD expression in the superficial laminae of the spinal cord may contribute to neuropathic pain.11–13 Thoracic 13 segment spinal cord hemisected rats show central neuropathic pain manifested by mechanical allodynia and thermal hyperalgesia. Administration of spinal GABA-A/GABA-B receptor agonists, muscimol or baclofen, reduces SCI-induced mechanical allodynia in both rat hind paws.20 Intrathecal GABA-B receptor agonist baclofen suppresses spontaneous and evoked dysesthetic pain (mechanical allodynia), suggesting that a dysfunctional spinal GABA-B receptor system is associated with the phenomenon of central pain among patients with spinal lesions.21 Recent studies show that there is a GABA autocrine feedback mechanism at nociceptive nerve terminals and GABA serves as a modulator of nociceptor sensitization in the periphery.22 Subcutaneous inoculation of replication-defective HSV vector expressing GAD67 into the hind paw reduced manifestations of SCI pain; the antinociceptive effect was partially blocked by the GABA-A antagonist bicuculline or by the GABA-B antagonist phaclofen,23 suggesting that the antinociceptive effect mediated by the HSV-expressing GAD67 is through GABA system activity. SCI rostral to the lumbosacral level impairs voluntary and supraspinal control of voiding and disrupts the coordination of the urinary bladder and external urethral sphincter. SCI initially induces a detrusor overactivity (DO) mediated by spinal reflex mechanisms.24 GABA-B receptor agonist is approved for treatment of DO in SCI patients.25 Spinal dysfunction of inhibitory GABAergic neuronal activity plays a role in the genesis of DO after SCI induced by Surgifoam sponge compression to lumbar spine cord.26 HSV vectors expressing GAD67 injected into the bladder wall significantly decreased the number and amplitude of nonvoiding contractions (a biomarker of urinary bladder pathologies) along with an increase in voiding efficiency, and intrathecal bicuculline (a GABA-A receptor antagonist) reversed the decreased number and amplitude of nonvoiding contractions, suggesting that HSV-based GAD gene transfer represents a novel approach for treatment of neurogenic DO.26 In rats with spinal nerve ligation, inoculation with the HSV vector expressing GAD67 into the hind paw reduced mechanical allodynia and thermal hyperalgesia, and the antiallodynic effect lasted 6 weeks and was reestablished by reinoculation; the vector expressing GAD67 also suppressed induction of c-fos (a biomarker of neuron activity) and phosphorylated extracellular signal-regulated kinase 1/2 in the spinal cord.27 In a model of lumbar radiculopathy by ligation of the dorsal and ventral lumbar roots proximal to the DRG, HSV-based vectors coding for GAD67 subcutaneously into the foot resulted in a substantial and significant reduction of mechanical allodynia.28 The vector-mediated reduction in pain-related behavior was higher in magnitude and longer in duration after inoculation of the HSV vector expressing GAD67.28
HSV vector for HIV neuropathic pain
Human immunodeficiency virus–associated sensory neuropathy (HIV-SN) is one of three commonly studied diseases of peripheral neuropathy with huge public health impact; other two are chemotherapy-induced peripheral neuropathy and diabetic peripheral neuropathy.29 Approximately 10% of HIV individuals suffer from painful sensory neuropathy.30 Traditional HIV-SN mainly contains HIV infection–related distal sensory polyneuropathy and antiretroviral toxic neuropathies. At the early stage, HIV-infected microglia to release neurotoxins including proinflammatory factors, activating astrocytes.31 Astrocytes may contribute to the production or maintenance of excitotoxins.31 Astrocytes release proinflammatory factors, such as tumor necrosis factor (TNF)-α in the spinal cord dorsal horn (SCDH) of HIV patients.32 HIV infects astrocytes in the brain as viral reservoir.33 HIV replication releases viral protein HIV-1 envelope protein gp120, Tat, and so on, generating reactive oxygen species, which in turn initiate immune activation/inflammation and neurotoxicity.34–40 The recombinant gp120 application to the sciatic nerve resulted in neuropathic pain.41–43 Spinal gp120 protein induces an acute painful behavior and proinflammatory cytokine release in the spinal cord.44 Repeated intrathecal gp120 induces chronic pain in mice.45 HIV gp120–induced oxidative damage is involved in this neuropathogenic process through disrupting mitochondrial function and biogenesis.46–49 Improvement in oxidative changes is used as a marker of HIV treatment.50 We used HSV vectors expressing GAD67 in peripheral HIV gp120–induced neuropathic pain in rats.51 HSV vector–mediated GAD67 attenuated mechanical allodynia; the anti-allodynic effect of GAD67 was reduced by GABA-A and GABA-B receptor antagonists; and the HSV vectors expressing GAD67 suppressed the upregulated mitochondrial superoxide and Wnt5a (a mechanism related to HIV pain52) in the spinal dorsal horn. To determine if peripheral GABA receptors were involved in the antinociceptive effect of GAD67 expressed by HSV, we intraplantarly injected two antagonists that poorly penetrates the blood–brain barrier, bicuculline methiodide (GABA-A receptor antagonist) and CGP54626 (GABA-B antagonist) in peripheral HIV gp120–induced neuropathic pain state.51 Our data showed that intraplantar administration of bicuculline methiodide significantly lowered mechanical threshold compared with the vehicle group. Intraplantar CGP54626 treatment decreased mechanical threshold compared with vehicle.
Antiretroviral therapy (ART) usually contains three or more different drugs, such as two nucleoside reverse transcriptase inhibitors (NRTIs) and a protease inhibitor, two NRTIs and a non-NRTI, or other such combinations. While ART has been effective in HIV-infected individuals since 1996, ART causes painful peripheral neuropathy due to its neurotoxicity. NRTI reduces plasma viral load and improves immune function.53 However, some NRTIs (e.g., zalcitabine/2′,3′-dideoxycytidine/ddC, didanosine/2′,3′-dideoxyinosine/ddI, and stavudine/didehydro-deoxythymidine/d4T) are neurotoxic and cause dose-dependent peripheral neuropathy.54 NRTIs can alter mitochondrial DNA (mtDNA) content by inhibiting polymerase gamma, an enzyme involved in mtDNA replication.55 mtDNA is required for many oxidative phosphorylation complex I proteins. mtDNA depletion causes complex I depletion and complex II overuse, resulting in elevated superoxide levels.55 Although old neurotoxic NRTIs have been discontinued in clinic in the United States, HIV-SN induced by neurotoxic NRTIs still exist in HIV patients who used them before. In a neuropathic pain model of combination of HIV gp120 and ddC, we also demonstrated that subcutaneous inoculation of HSV vectors expressing GAD67 attenuated mechanical allodynia for more than 28 days.56 The HSV vectors decreased signals of mitochondrial superoxide in the spinal dorsal horn and lowered the expression of phosphorylation of cAMP response element binding protein (pCREB) and phosphorylated CCAAT/enhancer binding protein β in the spinal dorsal horn in rats.
HSV vector for diabetic neuropathy-induced neuropathic pain
It is known that diabetes causes peripheral nerve damage, and PDN is very common. The most common type of diabetic peripheral neuropathy is bilateral and symmetric damage to the distal nerves of the feet, known as stocking–glove neuropathy distribution.57 In the early stages, PDN patients complain of pain, tingling, stinging sensations (sensory abnormalities), and numbness. Then, impaired sensory processing causes allodynia and hyperalgesia, which has been reported in animal models of streptozotocin (STZ)-diabetic rats.58
Expression of GAD67 was downregulated by STZ in the spinal cord, suggesting decreased GABA availability in diabetic rats.59 Neuropathic pain induced by STZ-induced diabetic peripheral neuropathy increases the voltage-gated sodium channel α subunit isoform 1.7 (NaV1.7) in DRG.60 Subcutaneous inoculation of HSV vector expressing GAD67 in rats with PDN reduced mechanical hyperalgesia, thermal hyperalgesia, and cold allodynia and prevented the increase in the voltage-gated sodium channel isoform 1.7 (NaV1.7) protein.61 In STZ-induced diabetic peripheral neuropathic pain model, Wang et al. transduced replication-defective HSV-based vectors to the DRG to produce two isoforms of GAD65 or GAD67, alone and in combination.62 They found that vectors expressing GAD65 or GAD67 decreased diabetes-induced mechanical allodynia to a degree that was greater than daily injections of gabapentin in rats. The results showed that either GAD65 or GAD67 vectors are very effective in the treatment of diabetic pain. Combinations of HSV expressing GAD67 with endomorphin or enkephalins produced a significant antinociceptive effect. These findings above provide further evidence for the clinical development of antinociceptive gene therapies for diabetic peripheral neuropathic pain.62
Other viral vectors mediating GAD expression for analgesia
An adenovirus strategy to target therapeutic genes to DRG neurons was developed. Ogawa et al. reported that in a mice neuropathic pain model induced by spinal nerve transection (SNT), they engineered a helper-dependent adenoviral vector expressing GAD67 with a modified fiber containing the DRG homing peptide sequence (HDAd-DRG-GAD67).63 They administrated the vectors into the L4 spinal nerve immediately after SNT and found that HDAd-DRG-GAD67 significantly reduced allodynia in SNT mice. In addition, HDAd-DRG-GAD67 had a much greater transduction efficiency and expressed the therapeutic gene for a much longer time than wild-type HDAd. HDAd-DRG-GAD67 suppressed the upregulated Cav3.2 mRNA in the DRG.63 Furthermore, the anti-allodynic effect of HDAd-DRG-GAD67 occurred even when the therapeutic vector was given day 7 after SNT.
Recombinant adeno-associated virus (AAV) is also an optimal gene transfer vehicle for the nervous system with stable and safe gene expression. Recent CNS clinical trials show that AAV vectors have been used for single gene genetic disorders, such as spinal muscular atrophy and giant axon neuropathy.64,65 An AAV vector was constructed using the AAV helper-free system to express GAD65.66 In a neuropathic pain model induced by the tibial and sural nerves ligation, the rAAV2-GAD65 vectors applied to sciatic nerve induced GABA overexpression in the spinal cord and reduced pain symptoms. The direct administration of rAAV-GAD65 to DRG induced GAD65 expression and reduced allodynic and hyperalgesic behavior.67 The sciatic nerve is a highly promising route for delivering rAAV2 to the DRG, clinically viable gene therapy option.
A replication-defective human foamy virus (HFV) vector was developed.68 In the study of a thoracic T13 left hemisection model of SCI in rats, mechanical allodynia was observed at 1 week after hemisection. Subcutaneous inoculation of replication-defective HFV vector expressing GAD (rdvGAD67) into the plantar surface of the hind paws significantly increased hind paw withdrawal threshold at 1 week after inoculation (2 weeks after injury). The maximal anti-allodynic effect occurred 2 weeks after the inoculation. The antiallodynic effect persisted for 5 weeks. Subcutaneous inoculation of rdvGAD67 resulted in enhanced production of GAD and GABA release from transduced DRG neurons, suggesting that HFV-mediated gene transfer to DRG treats central neuropathic pain after incomplete SCI.
In the DRG, a layer of satellite glial cells (SGCs) surrounds primary sensory neurons.69–71 Vit et al. developed an adenovirus encoding the human GAD under the transcriptional control of chicken actin ubiquitous promoter.71 Control adenovirus express either green fluorescent protein or β-galactosidase under the control of cytomegalovirus promoter. Rats were injected with the vectors into the left trigeminal ganglion, leading to sustained expression of the GAD65 over the 4 weeks observation period. The adenovirus-mediated GAD expression and GABA synthesis were mainly in SGCs; GABA-A receptors decorated the neuronal surface. The adenovirus-mediated GAD into the trigeminal ganglion significantly decreased pain behavior in the orofacial formalin-induced inflammatory pain, which was blocked by bicuculline, a selective GABA-A receptor antagonist.
Discussion
GABA is synthesized from glutamate by GAD, and two isoforms of this enzyme, GAD65 and GAD67, exist. GAD plays an important role in analgesic mechanism. In the literature review, we searched 13 original research articles using the viral vectors including HSV, adenovirus-based vector (AV), AAV, and HFV to express GAD for chronic pain treatment. These articles showed that GAD expressed by the viral vectors produced antinociceptive effects in preclinical pain models.
Primary input high-threshold C- and Aδ-fibers transmitting pain signal project to the laminae I–II in the SCDH; low-threshold C-/Aδ-fibers carrying touch signal project to lamina II inner and III.72,73 A-fibers transmit touch information through the dorsal columns to the brainstem as well as send collaterals into the deeper dorsal horn laminae IIi–IV.72,73 Inhibitory interneurons in the SCDH control the relay of nociceptive signals from the periphery to a higher region of the CNS.74,75 A growing body of literature indicates that inhibitory GABAergic and glycinergic neurons play an important role in pain modulation.11,76,77 The interneurons of the SCDH contribute to inhibitory control of “pain” transmission and to the transmission of pain messages by projection neurons to the brain.72 GABA-mediated inhibition works in both presynaptic neuronal and postsynaptic synapses.78 Pharmacologic inhibition of intrinsic GABAergic tone in normal rats in the spinal cord causes mechanical hypersensitivity similar to neuropathic pain.10 Furthermore, inhibition of GABA receptor increases polysynaptic excitatory transmission to the superficial dorsal horn,79 indicating inhibitory interneurons control nociceptive input in dorsal horn neurons.
Neuropathic pain induces dysregulation of inhibitory networks. Spared nerve injury induces the cumulative loss of GABAergic inhibitory interneurons in the SCDH and a marked decrease in inhibitory postsynaptic currents of lamina II neurons,80 leading to decreased GABA synthesis and release. Early report showed that chronic constriction injury (CCI) decreased the number of GABA- and GAD-immunoreactive cells in the lumbar spinal cord.81 GABA may interact with both GABA-A and GABA-B receptors. GABA-A receptor is Cl− ionophores increasing Cl− conductance to induce hyperpolarize the cell body.9 GABA-B receptor is linked by a Gi protein to both K+ channels and calcium channels, leading to a hyperpolarization of the cell body by increasing K+ conductance and reducing Ca2+ currents.82 Administration of spinal GABA-A and GABA-B agonist resulted in a dose-dependent antagonism of the allodynia induced by spinal nerve injury.9
Viral vector development has a complex history to treat nervous system disorders during the last two decades.83,84 Effective gene delivery is key to the success of gene therapy, which is dependent on the level and duration of transgene expression, cellular specificity, and safety issues.5 We summarized the characteristics of vectors used for GAD expression (Table 3). HSV is a large double-stranded DNA virus with 152 kb genome DNA. In HSV, there are four immediate early (IE) genes, and deletion of one or more essential IE genes makes recombinant HSV-based vectors available to retain the neuronal targeting properties of the wild-type virus; recombinant HSV-based vectors can be propagated to high titers on complementing cells without replicating in animals in vivo.85 Because its large genome size, it is possible to insert a large foreign gene.85 Therefore, HSV as a vector can be engineered by incorporating a foreign gene into the viral genome. Like nature infection of HSV on the mouth and lips, including cold sores and fever blisters, HSV vector delivery to the peripheral nervous system (PNS) is achieved by simple subcutaneous inoculation of the skin nerve terminals and travels retrogradely to the DRG nucleus, where the target gene is expressed in the absence of viral lytic functions (Fig. 2). HSV is used as a vector for delivery of genes to the nervous system due to its natural tropism to the nervous system. The dorsal root and trigeminal ganglia are important primary neurons for pain signal transduction. HSV vector establishes life-long quiescent state (latency) in neurons as an intranuclear episomal element.5,85–87 In the review, we found eight articles using HSV vectors expressing GAD67 and/or GAD65 (Table 1) to reduce pain in rats with SCI and peripheral injury,23,26–28 HIV-related pain models in rats,51,56 and PDN.61,62 HSV vectors expressing the opioid peptide preproenkephalin substantially reduced acute, inflammatory, and neuropathic pain in preclinical studies17,84,88,89 In addition, HSV vectors that expressed soluble TNF receptor I and anti-inflammatory peptides (interleukin-4 and -10) reduced pain in models of CNS and PNS neuropathic pain.90–93 To assess the safety of this approach, the Phase I clinical trial of HSV expressing human preproenkephalin in subjects with intractable focal pain caused by terminal cancer was reported.8
Table 3.
Summary of characteristics of vectors used for glutamic acid decarboxylase expression in pain treatment (modulated from the report5)
| HSV | AV | AAV vector | FV vector | |
|---|---|---|---|---|
| Wild type | DS-DNA | DS-DNA | SS-DNA | a |
| Genome size (kb) | 152 | 36 | ∼4.7 | 13 |
| Capacity of gene inserted (kb) | ∼40 | ∼7.5 | ∼4.5 | 9.2 |
| Persistence of gene expression | Days–weeks | Days–weeks | Months–years | Days–weeks |
| Safety issues | Immune and inflammatory response | Immune and inflammatory response | Insertional mutagenesis | Apathogenicb |
The adenovirus genome is composed of linear double-stranded DNA of ∼36 kb.
Deletion of the region coding for early gene of the adenoviral genome makes it a replication impaired vector for gene therapy.94 AV is used to achieve an in vivo gene transfer paradigm for treating chronic pain in animal.95 A recombinant helper-dependent AV vector expressing GAD67 reduced allodynia and Cav3.2 mRNA in the DRG in SNT mice.63 Administration of adenoviral vectors encoding GAD65 gene into the trigeminal ganglion leads to sustained expression of the GAD65 isoform over the 4 weeks observation period and decrease inflammatory pain behavior in rats.71
AAV is a single-strand nonenveloped DNA virus with a 4.7-kb genome, and AAV is nonpathogenic and affords long-term gene expression; however, it has limited transgene capacity compared with HSV, AV, or HFV (Table 3) and are readily eliminated by humoral immune responses in patients previously exposed to the virus.96 Recombinant AAV vectors are suitable for in vitro and in vivo gene transfer. GAD65 mediated by AAV vectors reduced mechanical hyperalgesia and allodynia induced by tibial and sural nerves ligation or transection.66,67 AAV2-human aromatic l-amino acid decarboxylase (AADC) might improve motor development in children with AADC deficiency.6,97 Gene therapy for Parkinson's disease was reported using AAV2 encoding the complementary DNA for AADC in a phase 1 clinical trial.7
Foamy viruses (FVs) are unique retroviruses without pathology, have the largest genome size of all retroviruses, and can infect cells from many vertebrate species and diverse tissue types; however, the ability of FV vectors to efficiently deliver any specific transgene cassette must be determined experimentally.98 A high multiplicity of infection of FV in vitro induced a marked cytopathic effect.99 The best-studied FV species is the prototype FV, which for many years was known as HFV. Liu et al. reported that replication-defective HFV vector mediating human GAD67 reduces neuropathic pain following SCI.68
Translating gene therapy to the human trials poses specific challenges with careful considerations, such as the route and mode of delivery of the vector, the efficacy and safety of the vector, and the choice of the patient population.100,101 A previous clinical trial with single intratumoral injection of a replication-defective AV vector bearing thymidine kinase gene into patients with advanced recurrent malignant brain tumors was reported.102 In a phase II trial, bilateral infusion of AAV2 vector expressing GAD into the subthalamic nucleus improved the unified Parkinson disease rating scale.103 A clinical study with HSV vector in patients with relapsed malignant glioma demonstrates the feasibility of HSV in human therapy.104 HSV for pain Phase I trial demonstrated that the HSV-based vector expressing human preproenkephalin in subjects with intractable focal pain caused by cancer was well tolerated with no study agent-related serious adverse events and had pain relief, warranting further clinical investigation.8 With continued commitment from researchers in this field, the current advances in preclinical gene therapy with GAD expression support the growing and promising clinical trials for pain treatment in the near future.
Summary
Inhibitory GABA system plays an important role in analgesic mechanism. Chronic pain induces the loss of GAD. Expression of GAD by viral vectors may complement the decrease in GABAergic systems for chronic pain treatment. The literature review summarizes original research articles using the viral vectors including HSV, AV, AAV, and HFV to express GAD67 or GAD65 for chronic pain treatment. These studies above provide a promising support for the clinical trial using viral vector–mediated GAD for therapeutic options of chronic pain.
Author Disclosure
No competing financial interests exist.
Funding Information
This work was supported by grants from the U.S. National Institutes of Health R01NS066792 (S.H.), R01DA34749 (S.H.), R01DA047089 (S.R. and S.H.), R01DA047157 (S.H.), and by JSPS KAKENHI Grant JP19K09316 and JP18K16469.
References
- 1. Yekkirala AS, Roberson DP, Bean BP, et al. Breaking barriers to novel analgesic drug development. Nat Rev Drug Discov 2017;16:545–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harned M, Sloan P. Safety concerns with long-term opioid use. Expert Opin Drug Saf 2016:1–8 [DOI] [PubMed] [Google Scholar]
- 3. White House: True Cost of Opioid Epidemic Tops $500 Billion. Washington, DC: CNBC, 2017 [Google Scholar]
- 4. Glorioso JC, Mata M, Fink DJ. Therapeutic gene transfer to the nervous system using viral vectors. J Neurovirol 2003;9:165–172 [DOI] [PubMed] [Google Scholar]
- 5. Simonato M, Bennett J, Boulis NM, et al. Progress in gene therapy for neurological disorders. Nat Rev Neurol 2013;9:277–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tseng CH, Chien YH, Lee NC, et al. Gene therapy improves brain white matter in aromatic l-amino acid decarboxylase deficiency. Ann Neurol 2019;85:644–652 [DOI] [PubMed] [Google Scholar]
- 7. Christine CW, Bankiewicz KS, Van Laar AD, et al. Magnetic resonance imaging-guided phase 1 trial of putaminal AADC gene therapy for Parkinson's disease. Ann Neurol 2019;85:704–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fink DJ, Wechuck J, Mata M, et al. Gene therapy for pain: results of a phase I clinical trial. Ann Neurol 2011;70:207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hwang JH, Yaksh TL. The effect of spinal GABA receptor agonists on tactile allodynia in a surgically-induced neuropathic pain model in the rat. Pain 1997;70:15–22 [DOI] [PubMed] [Google Scholar]
- 10. Malan TP, Mata HP, Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology 2002;96:1161–1167 [DOI] [PubMed] [Google Scholar]
- 11. Moore KA, Kohno T, Karchewski LA, et al. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci 2002;22:6724–6731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lorenzo LE, Magnussen C, Bailey AL, et al. Spatial and temporal pattern of changes in the number of GAD65-immunoreactive inhibitory terminals in the rat superficial dorsal horn following peripheral nerve injury. Mol Pain 2014;10:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meisner JG, Marsh AD, Marsh DR. Loss of GABAergic interneurons in laminae I-III of the spinal cord dorsal horn contributes to reduced GABAergic tone and neuropathic pain after spinal cord injury. J Neurotrauma 2010;27:729–737 [DOI] [PubMed] [Google Scholar]
- 14. New KC, Gale K, Martuza RL, et al. Novel synthesis and release of GABA in cerebellar granule cell cultures after infection with defective herpes simplex virus vectors expressing glutamic acid decarboxylase. Brain Res Mol Brain Res 1998;61:121–135 [DOI] [PubMed] [Google Scholar]
- 15. Glorioso JC, Fink DJ. Herpes vector-mediated gene transfer in treatment of diseases of the nervous system. Annu Rev Microbiol 2004;58:253–271 [DOI] [PubMed] [Google Scholar]
- 16. Hao S, Mata M, Wolfe D, et al. HSV-mediated gene transfer of the glial cell-derived neurotrophic factor provides an antiallodynic effect on neuropathic pain. Mol Ther 2003;8:367–375 [DOI] [PubMed] [Google Scholar]
- 17. Hao S, Mata M, Goins W, et al. Transgene-mediated enkephalin release enhances the effect of morphine and evades tolerance to produce a sustained antiallodynic effect in neuropathic pain. Pain 2003;102:135–142 [DOI] [PubMed] [Google Scholar]
- 18. Wolfe D, Hao S, Hu J, et al. Engineering an endomorphin-2 gene for use in neuropathic pain therapy. Pain 2007;133:29–38 [DOI] [PubMed] [Google Scholar]
- 19. Siddall PJ, Loeser JD. Pain following spinal cord injury. Spinal Cord 2001;39:63–73 [DOI] [PubMed] [Google Scholar]
- 20. Gwak YS, Tan HY, Nam TS, et al. Activation of spinal GABA receptors attenuates chronic central neuropathic pain after spinal cord injury. J Neurotrauma 2006;23:1111–1124 [DOI] [PubMed] [Google Scholar]
- 21. Herman RM, D'Luzansky SC, Ippolito R. Intrathecal baclofen suppresses central pain in patients with spinal lesions. A pilot study. Clin J Pain 1992;8:338–345 [PubMed] [Google Scholar]
- 22. Hanack C, Moroni M, Lima WC, et al. GABA blocks pathological but not acute TRPV1 pain signals. Cell 2015;160:759–770 [DOI] [PubMed] [Google Scholar]
- 23. Liu J, Wolfe D, Hao S, et al. Peripherally delivered glutamic acid decarboxylase gene therapy for spinal cord injury pain. Mol Ther 2004;10:57–66 [DOI] [PubMed] [Google Scholar]
- 24. de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Progr Brain Res 2006;152:59–84 [DOI] [PubMed] [Google Scholar]
- 25. Steers WD, Meythaler JM, Haworth C, et al. Effects of acute bolus and chronic continuous intrathecal baclofen on genitourinary dysfunction due to spinal cord pathology. J Urol 1992;148:1849–1855 [DOI] [PubMed] [Google Scholar]
- 26. Miyazato M, Sugaya K, Goins WF, et al. Herpes simplex virus vector-mediated gene delivery of glutamic acid decarboxylase reduces detrusor overactivity in spinal cord-injured rats. Gene Ther 2009;16:660–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hao S, Mata M, Wolfe D, et al. Gene transfer of glutamic acid decarboxylase reduces neuropathic pain. Ann Neurol 2005;57:914–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee JY, Fink DJ, Mata M. Vector-mediated gene transfer to express inhibitory neurotransmitters in dorsal root ganglion reduces pain in a rodent model of lumbar radiculopathy. Spine 2006;31:1555–1558 [DOI] [PubMed] [Google Scholar]
- 29. Hoke A. Animal models of peripheral neuropathies. Neurotherapeutics 2012;9:262–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schifitto G, McDermott MP, McArthur JC, et al. Incidence of and risk factors for HIV-associated distal sensory polyneuropathy. Neurology 2002;58:1764–1768 [DOI] [PubMed] [Google Scholar]
- 31. Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 2001;410:988–994 [DOI] [PubMed] [Google Scholar]
- 32. Shi Y, Gelman BB, Lisinicchia JG, et al. Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients. J Neurosci 2012;32:10833–10840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li GH, Henderson L, Nath A. Astrocytes as an HIV reservoir: mechanism of HIV infection. Curr HIV Res 2016;14:373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ivanov AV, Valuev-Elliston VT, Ivanova ON, et al. Oxidative stress during HIV infection: mechanisms and consequences. Oxid Med Cell Longev 2016;2016:8910396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Porter KM, Sutliff RL. HIV-1, reactive oxygen species, and vascular complications. Free Radic Biol Med 2012;53:143–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu Z, Zang Y, Qiao L, et al. ASPP2 involvement in p53-mediated HIV-1 envelope glycoprotein gp120 neurotoxicity in mice cerebrocortical neurons. Sci Rep 2016;6:33378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stevens PR, Gawryluk JW, Hui L, et al. Creatine protects against mitochondrial dysfunction associated with HIV-1 tat-induced neuronal injury. Curr HIV Res 2015;12:378–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haughey NJ, Zhu X, Bandaru VV. A biological perspective of CSF lipids as surrogate markers for cognitive status in HIV. J Neuroimmune Pharmacol 2013;8:1136–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Campbell LA, Avdoshina V, Day C, et al. Pharmacological induction of CCL5 in vivo prevents gp120-mediated neuronal injury. Neuropharmacology 2015;92:98–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carey AN, Sypek EI, Singh HD, et al. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res 2012;229:48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Herzberg U, Sagen J. Peripheral nerve exposure to HIV viral envelope protein gp120 induces neuropathic pain and spinal gliosis. J Neuroimmunol 2001;116:29–39 [DOI] [PubMed] [Google Scholar]
- 42. Zheng W, Ouyang H, Zheng X, et al. Glial TNFalpha in the spinal cord regulates neuropathic pain induced by HIV gp120 application in rats. Mol Pain 2011;7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wallace VC, Blackbeard J, Segerdahl AR, et al. Characterization of rodent models of HIV-gp120 and anti-retroviral-associated neuropathic pain. Brain 2007;130:2688–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Milligan ED, O'Connor KA, Nguyen KT, et al. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J Neurosci 2001;21:2808–2819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yuan SB, Shi Y, Chen J, et al. Gp120 in the pathogenesis of human immunodeficiency virus-associated pain. Ann Neurol 2014;75:837–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hu S, Sheng WS, Lokensgard JR, et al. Preferential sensitivity of human dopaminergic neurons to gp120-induced oxidative damage. J Neurovirol 2009;15:401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hu S, Sheng WS, Schachtele SJ, et al. Reactive oxygen species drive herpes simplex virus (HSV)-1-induced proinflammatory cytokine production by murine microglia. J Neuroinflammation 2011;8:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fields JA, Overk C, Adame A, et al. Neuroprotective effects of the immunomodulatory drug FK506 in a model of HIV1-gp120 neurotoxicity. J Neuroinflammation 2016;13:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shah A, Kumar A. HIV-1 gp120-mediated mitochondrial dysfunction and HIV-associated neurological disorders. Neurotox Res 2016;30:135–137 [DOI] [PubMed] [Google Scholar]
- 50. Gerschenson M, Kim C, Berzins B, et al. Mitochondrial function, morphology and metabolic parameters improve after switching from stavudine to a tenofovir-containing regimen. J Antimicrob Chemother 2009;63:1244–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kanda H, Kanao M, Liu S, et al. HSV vector-mediated GAD67 suppresses neuropathic pain induced by perineural HIV gp120 in rats through inhibition of ROS and Wnt5a. Gene Ther 2016;23:340–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yuan SB, Ji G, Li B, et al. A Wnt5a signaling pathway in the pathogenesis of HIV-1 gp120-induced pain. Pain 2015;156:1311–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Haas DW, Geraghty DE, Andersen J, et al. Immunogenetics of CD4 lymphocyte count recovery during antiretroviral therapy: an AIDS Clinical Trials Group study. J Infect Dis 2006;194:1098–1107 [DOI] [PubMed] [Google Scholar]
- 54. Dalakas MC. Peripheral neuropathy and antiretroviral drugs. J Peripher Nerv Syst 2001;6:14–20 [DOI] [PubMed] [Google Scholar]
- 55. Hao S. The molecular and pharmacological mechanisms of HIV-related neuropathic pain. Curr Neuropharmacol 2013;11:499–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kanao M, Kanda H, Huang W, et al. Gene transfer of glutamic acid decarboxylase 67 by herpes simplex virus vectors suppresses neuropathic pain induced by human immunodeficiency virus gp120 combined with ddC in rats. Anesth Analg 2015;120:1394–1404 [DOI] [PubMed] [Google Scholar]
- 57. Feldman EL, Nave KA, Jensen TS, et al. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron 2017;93:1296–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Suzuki Y, Sato J, Kawanishi M, et al. Tissue glucose level modulates the mechanical responses of cutaneous nociceptors in streptozotocin-diabetic rats but not normal rats in vitro. Pain 2002;99:475–484 [DOI] [PubMed] [Google Scholar]
- 59. Manni L, Florenzano F, Aloe L. Electroacupuncture counteracts the development of thermal hyperalgesia and the alteration of nerve growth factor and sensory neuromodulators induced by streptozotocin in adult rats. Diabetologia 2011;54:1900–1908 [DOI] [PubMed] [Google Scholar]
- 60. Hong S, Wiley JW. Altered expression and function of sodium channels in large DRG neurons and myelinated A-fibers in early diabetic neuropathy in the rat. Biochem Biophys Res Commun 2006;339:652–660 [DOI] [PubMed] [Google Scholar]
- 61. Chattopadhyay M, Mata M, Fink DJ. Vector-mediated release of GABA attenuates pain-related behaviors and reduces Na(V)1.7 in DRG neurons. Eur J Pain 2011;15:913–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang Y, Nowicki MO, Wang X, et al. Comparative effectiveness of antinociceptive gene therapies in animal models of diabetic neuropathic pain. Gene Ther 2013;20:742–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ogawa N, Terashima T, Oka K, et al. Gene therapy for neuropathic pain using dorsal root ganglion-targeted helper-dependent adenoviral vectors with GAD67 expression. Pain Rep 2018;3:e695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mendell JR, Al-Zaidy S, Shell R, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med 2017;377:1713–1722 [DOI] [PubMed] [Google Scholar]
- 65. Bailey RM, Armao D, Nagabhushan Kalburgi S, et al. Development of intrathecal AAV9 gene therapy for giant axonal neuropathy. Mol Ther Methods Clin Dev 2018;9:160–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kim J, Kim SJ, Lee H, et al. Effective neuropathic pain relief through sciatic nerve administration of GAD65-expressing rAAV2. Biochem Biophys Res Commun 2009;388:73–78 [DOI] [PubMed] [Google Scholar]
- 67. Lee B, Kim J, Kim SJ, et al. Constitutive GABA expression via a recombinant adeno-associated virus consistently attenuates neuropathic pain. Biochem Biophys Res Commun 2007;357:971–976 [DOI] [PubMed] [Google Scholar]
- 68. Liu W, Liu Z, Liu L, et al. A novel human foamy virus mediated gene transfer of GAD67 reduces neuropathic pain following spinal cord injury. Neurosci Lett 2008;432:13–18 [DOI] [PubMed] [Google Scholar]
- 69. Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev 2005;48:457–476 [DOI] [PubMed] [Google Scholar]
- 70. Huang TY, Cherkas PS, Rosenthal DW, et al. Dye coupling among satellite glial cells in mammalian dorsal root ganglia. Brain Res 2005;1036:42–49 [DOI] [PubMed] [Google Scholar]
- 71. Vit JP, Ohara PT, Sundberg C, et al. Adenovector GAD65 gene delivery into the rat trigeminal ganglion produces orofacial analgesia. Mol Pain 2009;5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Braz J, Solorzano C, Wang X, et al. Transmitting pain and itch messages: a contemporary view of the spinal cord circuits that generate gate control. Neuron 2014;82:522–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Moehring F, Halder P, Seal RP, et al. Uncovering the cells and circuits of touch in normal and pathological settings. Neuron 2018;100:349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150:971–979 [DOI] [PubMed] [Google Scholar]
- 75. Knabl J, Witschi R, Hosl K, et al. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature 2008;451:330–334 [DOI] [PubMed] [Google Scholar]
- 76. Harvey RJ, Depner UB, Wassle H, et al. GlyR alpha3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 2004;304:884–887 [DOI] [PubMed] [Google Scholar]
- 77. Basbaum AI, Bautista DM, Scherrer G, et al. Cellular and molecular mechanisms of pain. Cell 2009;139:267–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Malcangio M, Bowery NG. GABA and its receptors in the spinal cord. Trends Pharmacol Sci 1996;17:457–462 [DOI] [PubMed] [Google Scholar]
- 79. Baba H, Ji RR, Kohno T, et al. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci 2003;24:818–830 [DOI] [PubMed] [Google Scholar]
- 80. Scholz J, Broom DC, Youn DH, et al. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci 2005;25:7317–7323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Eaton MJ, Plunkett JA, Karmally S, et al. Changes in GAD- and GABA-immunoreactivity in the spinal dorsal horn after peripheral nerve injury and promotion of recovery by lumbar transplant of immortalized serotonergic precursors. J Chem Neuroanat 1998;16:57–72 [DOI] [PubMed] [Google Scholar]
- 82. Filip M, Frankowska M. GABA(B) receptors in drug addiction. Pharmacol Rep 2008;60:755–770 [PubMed] [Google Scholar]
- 83. Antunes Bras JM, Epstein AL, Bourgoin S, et al. Herpes simplex virus 1-mediated transfer of preproenkephalin A in rat dorsal root ganglia. J Neurochem 1998;70:1299–1303 [DOI] [PubMed] [Google Scholar]
- 84. Wilson SP, Yeomans DC, Bender MA, et al. Antihyperalgesic effects of infection with a preproenkephalin-encoding herpes virus. Proc Natl Acad Sci U S A 1999;96:3211–3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fink DJ, DeLuca NA, Goins WF, et al. Gene transfer to neurons using herpes simplex virus-based vectors. Annu Rev Neurosci 1996;19:265–287 [DOI] [PubMed] [Google Scholar]
- 86. Mata M, Hao S, Fink DJ. Applications of gene therapy to the treatment of chronic pain. Curr Gene Ther 2008;8:42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Glorioso JC, Fink DJ. Herpes vector-mediated gene transfer in the treatment of chronic pain. Mol Ther 2009;17:13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Goss JR, Mata M, Goins WF, et al. Antinociceptive effect of a genomic herpes simplex virus-based vector expressing human proenkephalin in rat dorsal root ganglion. Gene Ther 2001;8:551–556 [DOI] [PubMed] [Google Scholar]
- 89. Goss JR, Harley CF, Mata M, et al. Herpes vector-mediated expression of proenkephalin reduces bone cancer pain. Ann Neurol 2002;52:662–665 [DOI] [PubMed] [Google Scholar]
- 90. Peng XM, Zhou ZG, Glorioso JC, et al. Tumor necrosis factor-alpha contributes to below-level neuropathic pain after spinal cord injury. Ann Neurol 2006;59:843–851 [DOI] [PubMed] [Google Scholar]
- 91. Hao S, Mata M, Glorioso JC, et al. Gene transfer to interfere with TNFalpha signaling in neuropathic pain. Gene Ther 2007;14:1010–1016 [DOI] [PubMed] [Google Scholar]
- 92. Hao S, Mata M, Glorioso JC, et al. HSV-mediated expression of interleukin-4 in dorsal root ganglion neurons reduces neuropathic pain. Mol Pain 2006;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhou Z, Peng X, Hao S, et al. HSV-mediated transfer of interleukin-10 reduces inflammatory pain through modulation of membrane tumor necrosis factor alpha in spinal cord microglia. Gene Ther 2008;15:183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hao S, Mata M, Fink DJ. Viral vector-based gene transfer for treatment of chronic pain. Int Anesthesiol Clin 2007;45:59–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Finegold AA, Mannes AJ, Iadarola MJ. A paracrine paradigm for in vivo gene therapy in the central nervous system: treatment of chronic pain. Hum Gene Ther 1999;10:1251–1257 [DOI] [PubMed] [Google Scholar]
- 96. Goncalves MA. Adeno-associated virus: from defective virus to effective vector. Virol J 2005;2:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chien YH, Lee NC, Tseng SH, et al. Efficacy and safety of AAV2 gene therapy in children with aromatic L-amino acid decarboxylase deficiency: an open-label, phase 1/2 trial. Lancet Child Adolesc Health 2017;1:265–273 [DOI] [PubMed] [Google Scholar]
- 98. Trobridge GD. Foamy virus vectors for gene transfer. Expert Opin Biol Ther 2009;9:1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sweeney NP, Regan C, Liu J, et al. Rapid and efficient stable gene transfer to mesenchymal stromal cells using a modified foamy virus vector. Mol Ther 2016;24:1227–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hoyng SA, de Winter F, Tannemaat MR, et al. Gene therapy and peripheral nerve repair: a perspective. Front Mol Neurosci 2015;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pleticha J, Heilmann LF, Evans CH, et al. Preclinical toxicity evaluation of AAV for pain: evidence from human AAV studies and from the pharmacology of analgesic drugs. Mol Pain 2014;10:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Trask TW, Trask RP, Aguilar-Cordova E, et al. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol Ther 2000;1:195–203 [DOI] [PubMed] [Google Scholar]
- 103. LeWitt PA, Rezai AR, Leehey MA, et al. AAV2-GAD gene therapy for advanced Parkinson's disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol 2011;10:309–319 [DOI] [PubMed] [Google Scholar]
- 104. Rampling R, Cruickshank G, Papanastassiou V, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther 2000;7:859–866 [DOI] [PubMed] [Google Scholar]
- 105. Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. J Neurophysiol 1994;72:169–179 [DOI] [PubMed] [Google Scholar]