ABSTRACT
Cellular dynamics of KORRIGAN 1 (KOR1) is closely linked with cellulose biosynthesis and plant osmotic stress tolerance. Cycling of KOR1 between the plasma membrane (PM) and trans-Golgi Network (TGN) is maintained by sequence motifs and protein structures that are recognized by cellular transport and quality control mechanisms. Several mutations in KOR1, as well as in the host genetic background, promote the mistargeting of KOR1 and induce KOR1 accumulation in the tonoplast (TP). Yet, little is known about how retention and sorting of KOR1 are regulated in the PM-TGN cycle. Forward genetic screening for GFP-KOR1 mislocalizing phenotype resulted in several mutant lines with different localization patterns or signal intensity of GFP-KOR1. One of the identified mutants were disrupted at UDP-glucose:glycoprotein glucosyltransferase (UGGT) locus, which is essential for the protein quality control in the ER. Our finding suggests the mis/unfolded structure of KOR1 triggers the TP targeting.
KEYWORDS: Arabidopsis thaliana, KORRIGAN1, UDP-glucose:glycoprotein glucosyltransferase, tonoplast, membrane protein, osmotic stress
KORRIGAN 1 (KOR1) is a type II membrane protein essential for cellulose biosynthesis and osmotic stress tolerance in plants.1-3 Under the normal growth condition, newly produced KOR1 proteins travel through the secretory pathway and cycles between the plasma membrane (PM) and trans-Golgi Network (TGN), and during the cell division, KOR1 accumulates in the cell plate.4-6 Multiple sequence motifs in the KOR1 peptide govern the KOR1 distribution. Indeed, all regions of the protein, namely, the N-terminal cytoplasmic domain, transmembrane domain, and the C-terminal extracellular catalytic domain contain one or more sequences or structures that regulate transport behavior of the KOR1 in the secretory pathway. Disruptions of these sequence motifs often cause destabilization and mistargeting of KOR1, typically to the tonoplasts (TP). For example, G429 R mutation (known as rsw2-1) decrease KOR1 at the PM and increases it at the TP.7 rsw2-1 causes root growth defects with the characteristic radial swelling at high tempatures.7,8 Therefore, the proper targeting of KOR1 is necessary for normal plant growth. Similar to rsw2-1 mutation, modifications at the carboxyl terminal proline-rich motif (P-motif) as well as elimination of N-glycosylations also direct KOR1 to the TP.7 In addition to the mutations in KOR1 peptide itself, the host genotype affects the intracellular targeting of KOR1. Mutations in a catalytic subunit of oligosaccharyl transferase (OST) complex, which catalyzes the attachment of asparagine (N)-linked glycans to nascent proteins, changes N-glycosylation pattern of KOR1 and causes KOR1 accumulation in the TP.6 In each case, TP targeting of KOR1 is associated with a reduction of overall KOR1 protein levels and with growth reduction. Therefore, the TP targeting of KOR1 is likely a part of the degradation process that governs the essential cellular KOR1 homeostasis for the growth and stress tolerance of the host plants.
To identify the gene networks that control the subcellular distribution of KOR1 protein, we have conducted a forward genetic screen and identified Arabidopsis mutants based on the mislocalization of the GFP-KOR1 reporter gene. (Figure 1a). Two mutant lines, 2C11 and 6E13, showed the accumulation of GFP-KOR1 at the TP like GFP-KOR1 variants described above. The line 6E13 showed a more distinctive GFP signal at the TP than 2C11 did. The line 5A4 has an approximately two-fold higher GFP signal than the parental line, but the overall GFP-KOR1 pattern remained the same (Figure 1a,b). The line 15A2 exhibited abnormal TGN pattern like aggregation. This pattern of TGN is similar to that in the gnom mutant, which is an ARF-guanine nucleotide exchange factor (ARF-GEF) in membrane trafficking regulators.9
Figure 1.
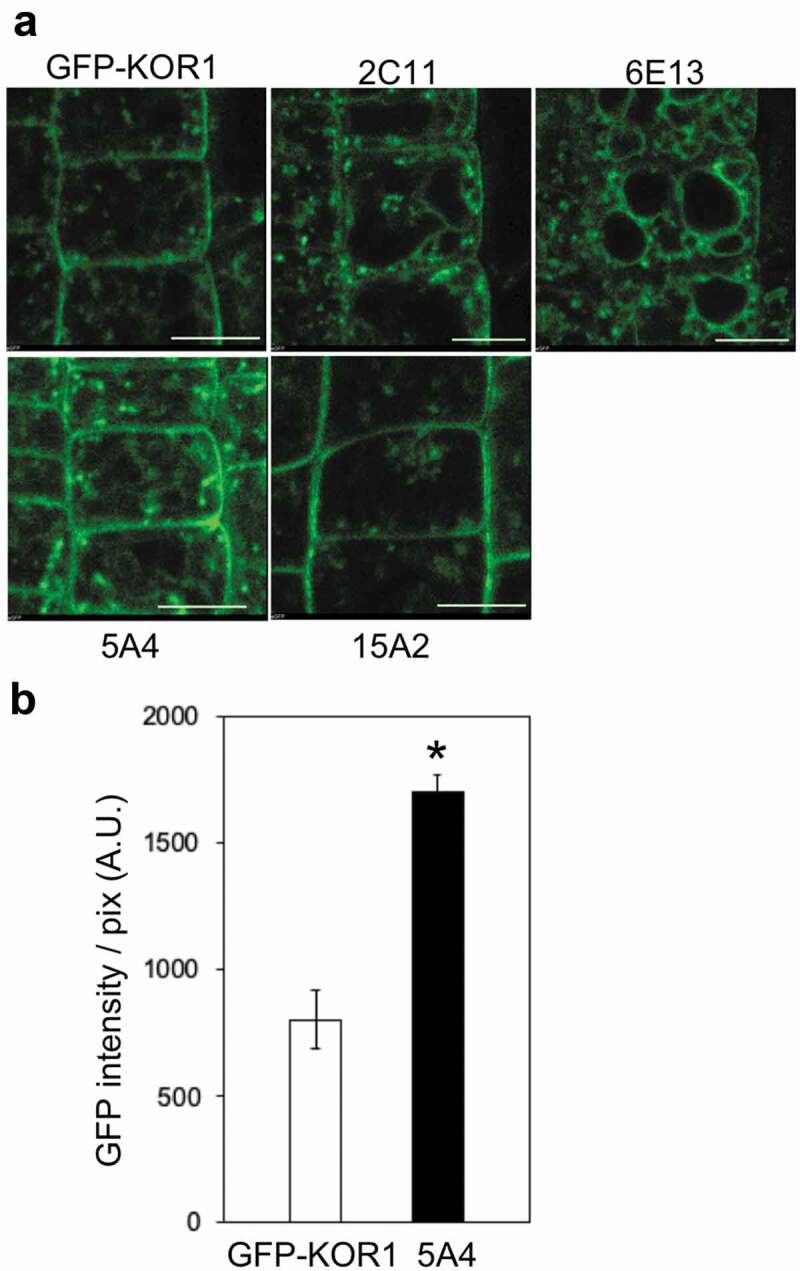
GFP-KOR1 in the mutant lines isolated from the screening. (a) Confocal microscopy images of GFP-KOR1 in the mutant lines. Four-day-old plants grown on 1 x MS, 3% sucrose vertical plate medium were used for observation. The microscope settings were kept constant for the series of pictures. Scale bars indicate 10 µm. (b) Comparison of GFP signal intensity in root tip area in GFP-KOR1 and 5A4 mutant. GFP signal intensity was divided by area (pix). A. U. = arbitrary unit. Error bars represent the SE of the mean (n = 5). The asterisk indicates a significant difference between genotypes (p < .01, Welch’s t-test). Fluorescent images were acquired using a C1si confocal microscope and measured by NIS Elements (Nikon).
Two TP-targeting mutations have been analyzed further. The mutation in the line 6E13 was identified allelic to stt3a-2 based on the genetic cross and phenotype analysis of the F1 plants. All 20 6E13/stt3a-2 F1 plants showed TP-localized GFP-KOR1 profile (Figure 2). For the mutation in line 2C11, on the other hand, we have conducted map-based cloning using Next Generation Sequencing.10 We identified the mutation as a nonsense mutation at the 81st Serine of AT1G71220, which encodes UDP-glucose:glycoprotein glucosyltransferase (UGGT). To confirm uggt mutation was responsible for the KOR1 localization phenotype, we obtained two T-DNA insertion lines of UGGT, uggt-11 and uggt-1211 and crossed the T-DNA lines and 2C11 for further analysis (Figure 3a). Both uggt insertion lines had shorter roots than wild-type even under the normal growth condition as already reported.12 A similar short-root phenotype was also observed in 2C11 and the uggt x 2C11 F1 plants (Figure 3b), establishing uggt mutation is responsible for the short-root phenotype of 2C11. Microscopic observation exhibited TP accumulation of GFP-KOR1 in uggt-11 x 2C11 and uggt-12 x 2C11 F1 plants, as well as uggt-11 plants with introgressed GFP-KOR1 (Figure 3c). These data indicate that the uggt mutation promotes TP targeting of GFP-KOR1 in the secretory pathway. UGGT is a part of the ER quality control (ERQC) system, which is a mechanism to check the folding of glycoproteins and eliminate mis/unfolded proteins.13 Indeed, UGGT is important in quality control of membrane proteins like BRI1 and EFR in Arabidopsis.14,15 Therefore, it assumes that a lack of UGGT increases the chance that mis/unfolded KOR1 proteins escape from the ER. However, another post-ER mechanism likely recognizes and sorts the mis/unfolded KOR1 to the TP for degradation. This model is consistent with the observations that the impaired GFP-KOR1 variants accumulate in the TP. The understanding of the sorting mechanisms of KOR1 in the secretory pathway is still in its infancy, but at least two routes exist to target KOR1 to the TP.13 Further dissection of KOR1 transport mutants could help us understand the regulatory mechanism of the KOR1 sorting and the factors governing the localization of KOR1.
Figure 2.
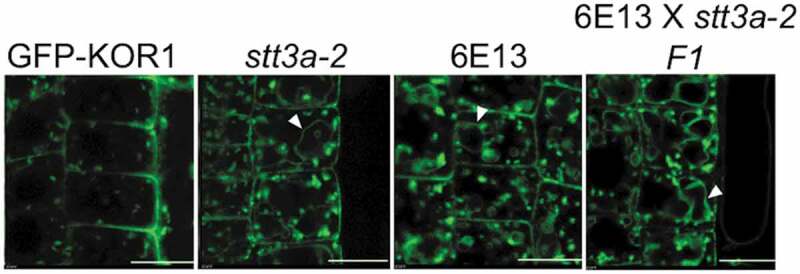
Genetic analysis revealed 6E13 is a new mutant allele of STT3a. Confocal microscopy images of GFP-KOR1 in 4-day-old 6E13 plants. F1 lines were grown on 1 x MS, 3% sucrose medium. The white arrowheads indicate GFP signals at the TP. The scale bars indicate 10 µm.
Figure 3.
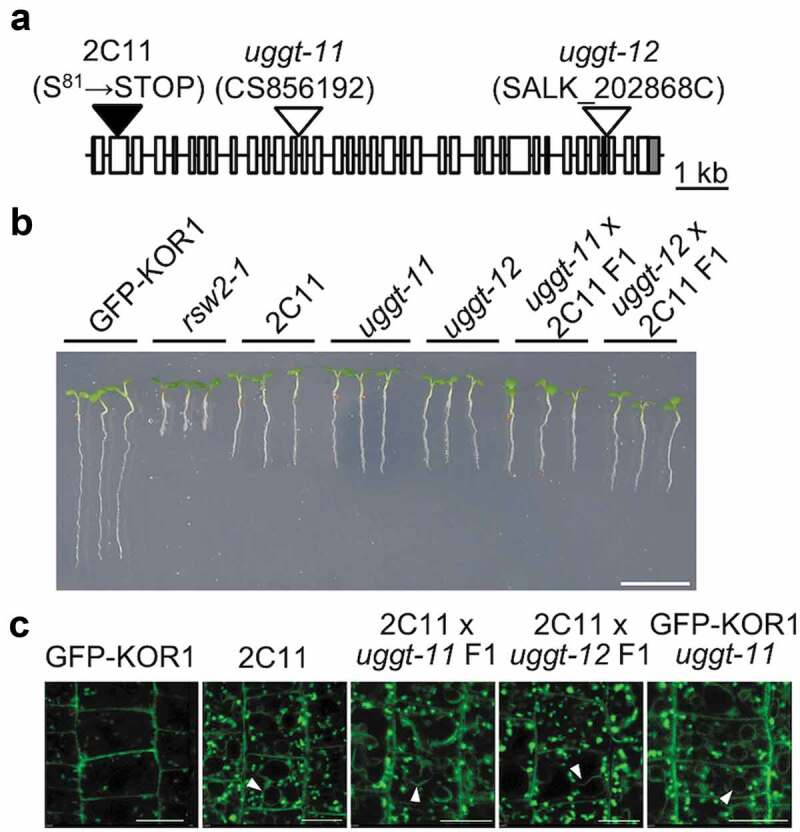
Mutation in ER-resident UDP-glucose: glycoprotein glucosyltransferase (UGGT) induces the mislocalization of GFP-KOR1. (a) Schematic diagram of the UGGT locus (open boxes represent exons). Positions for mutations are indicated. (b) Subcellular localization of GFP-KOR1 in the 2C11 mutant and F1 lines obtained from genetic crosses between 2C11 and indicated uggt T-DNA insertion lines (uggt-11 and uggt-12). The scale bar indicates 10 mm. (c) Confocal microscopy images of GFP-KOR1 in 7-day-old 2C11 plants, uggt-11, uggt-12, and F1 lines grown on 1 x MS, 3% sucrose medium. The white arrowheads indicate GFP signals at the TP. The scale bars indicate 10 µm.
Funding Statement
This work was supported by the National Science Foundation (IOS1547551 to H.K.) and by the German Research Foundation (SCHA541 to A.v.S.).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Nicol F, His I, Jauneau A, Vernhettes S, Canut H, Höfte H.. A plasma membrane-bound putative endo-1,4-beta-D-glucanase is required for normal wall assembly and cell elongation in Arabidopsis. Embo J. 1998;17:1–3. doi: 10.1093/emboj/17.19.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peng L, Hocart CH, Redmond JW, Williamson RE.. Fractionation of carbohydrates in Arabidopsis root cell walls shows that three radial swelling loci are specifically involved in cellulose production. Planta. 2000;211:406–414. doi: 10.1007/s004250000301. [DOI] [PubMed] [Google Scholar]
- 3.Kang JS, Frank J, Kang CH, Kajiura H, Vikram M, Ueda A, Kim S, Bahk JD, Triplett B, Fujiyama K, et al. Salt tolerance of Arabidopsis thaliana requires maturation of N-glycosylated proteins in the Golgi apparatus. Proc Natl Acad Sci U S A. 2008;105(15):5933–5938. doi: 10.1073/pnas.0800237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuo J, Niu QW, Nishizawa N, Wu Y, Kost B, Chua NH. KORRIGAN, an Arabidopsis endo-1,4-beta-glucanase, localizes to the cell plate by polarized targeting and is essential for cytokinesis. Plant Cell. 2000;12:1137–1152. doi: 10.1105/tpc.12.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert S, Bichet A, Grandjean O, Kierzkowski D, Satiat-Jeunemaître B, Pelletier S, Hauser M-T, Höfte H, Vernhettes S. An Arabidopsis endo-1,4-beta-D-glucanase involved in cellulose synthesis undergoes regulated intracellular cycling. Plant Cell. 2005;17:3378–3389. doi: 10.1105/tpc.105.036228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rips S, Bentley N, Jeong IS, Welch JL, von Schaewen A, Koiwa H. Multiple N-glycans cooperate in the subcellular targeting and functioning of Arabidopsis KORRIGAN1. Plant Cell. 2014;26:3792–3808. doi: 10.1105/tpc.114.129718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Z, Liu X, Qian X, Zhang X, von Schaewen AKoiwa H. Multiple quality control mechanisms in the er and tgn determine subcellular dynamics and salt-stress tolerance function of korrigan 1. Plant Cell. 2020;32:470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lane DR, Wiedemeier A, Peng L, Höfte H, Vernhettes S, Desprez T, Hocart CH, Birch RJ, Baskin TI, Burn JE, et al. Temperature-sensitive alleles of RSW2 link the KORRIGAN endo-1,4-beta-glucanase to cellulose synthesis and cytokinesis in Arabidopsis. Plant Physiol. 2001;126:278–288. doi: 10.1104/pp.126.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naramoto S, Otegui MS, Kutsuna N, de Rycke R, Dainobu T, Karampelias M, Fujimoto M, Feraru E, Miki D, Fukuda H, et al. Insights into the localization and function of the membrane trafficking regulator GNOM ARF-GEF at the Golgi apparatus in Arabidopsis. Plant Cell. 2014;26:3062–3076. doi: 10.1105/tpc.114.125880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Austin RS, Vidaurre D, Stamatiou G, Breit R, Provart NJ, Bonetta D, Zhang J, Fung P, Gong Y, Wang PW, et al. Next-generation mapping of Arabidopsis genes. Plant J. 2011;67:715–725. doi: 10.1111/tpj.2011.67.issue-4. [DOI] [PubMed] [Google Scholar]
- 11.Berardini TZ, Reiser L, Li D, Mezheritsky Y, Muller R, Strait E, Huala E. The Arabidopsis information resource: making and mining the “gold standard” annotated reference plant genome. Genesis. 2015;53:474–485. doi: 10.1002/dvg.22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanco-Herrera F, Moreno AA, Tapia R, Reyes F, Araya M, D’Alessio C, Parodi A, Orellana A. The UDP-glucose: glycoprotein glucosyltransferase (UGGT), a key enzyme in ER quality control, plays a significant role in plant growth as well as biotic and abiotic stress in Arabidopsis thaliana. BMC Plant Biol. 2015;15(1):127. doi: 10.1186/s12870-015-0525-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagashima Y, von Schaewen A, Koiwa H. Function of N-glycosylation in plants. Plant Sci. 2018;274:70–79. doi: 10.1016/j.plantsci.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Zhao-Hui C, Batoux M, Nekrasov V, Roux M, Chinchilla D, Zipfel C, Jones JDG. Specific ER quality control components required for biogenesis of the plant innate immune receptor EFR. Proc Natl Acad Sci U S A. 2009;106(37):15973–15978. doi: 10.1073/pnas.0905532106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin H, Yan Z, Nam KH, Li J. Allele-specific suppression of a defective brassinosteroid receptor reveals a physiological role of UGGT in ER quality control. Mol Cell. 2007;26:821–830. doi: 10.1016/j.molcel.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


