ABSTRACT
Nitrogen in soil directly influences the production and quality of tea. However, high nitrogen application in tea plantation leads to soil acidification and environmental pollution. Studies in model plants showed that plasma membrane localized amino acid transporter can regulate the distribution of amino acids to enhance nitrogen use efficiency. Our recent study identified six CsAAPs as transporters for theanine, a unique and most abundant non-proteinaceous amino acid in tea plant. In this work, we found these theanine transporters can also transport Glutamine, Glutamate, aspartate, alanine and γ-aminobutyric acid. Tissue-specific expression analyses showed that CsAAP1, CsAAP5 and CsAAP6 mainly expressed in leaves, CsAAP8 in root, CsAAP4 and CsAAP2 in stem. Furthermore, the expression of these CsAAPs was induced by nitrogen deficiency in a tissue-specific manner. Subcellular localization analyses showed that CsAAP1, CsAAP2 and CsAAP6 location were in the plasma membrane and endoplasmic reticulum. Taken together, these results suggested theanine transporters are involved in nitrogen deficiency response probably by mediating amino acid transport from roots to new shoots and from source to sink tissues in tea plants.
KEYWORDS: Tea, amino acid transporter, CsAAPs, nitrogen, Camellia sinensis L
Tea plant, Camellia sinensis L., is an important economic crop in the world. It requires a lot of nitrogen fertilizer for normally growth, due to mainly growing on mountain area with little nutrients and picking tea leaves many times in one year.1,2 Nitrogen fertilizer is also closely related to tea quality, by improving the accumulation of free amino acids, caffeine and aroma compounds in tea leaves.1-3 However, high N application in a tea plantation results to soil acidification and environmental pollution.4 So, it is very important to reduce nitrogen application by increasing nitrogen utilization.
Nitrogen transport in tea plant is mainly in the form of amino acids.5 When ammonium and nitrate forms of nitrogen are applied to tea plants, they are mainly assimilated into amino acids in the roots, and high content of glutamine (Gln), glutamate (Glu), aspartate (Asp), alanine (Ala) and γ-aminobutyric acid (GABA) are present in the xylem sap and transported to the shoots.5 Studies in model plants have shown that the amino acids transport between different organs is mediated by plasm membrane-localized amino acid transporters.6 Impressively, pea (Pisum sativum) plants overexpressing AAP1 allocated more nitrogen via the vasculature to the shoot and seeds and produced more biomass and higher seed yields than wild-type plants, and improved nitrogen uptake and utilization efficiency.7,8 So, it is intriguing to alter amino acid transporter encoding gene expression to improve nitrogen utilization efficiency in tea plants.
Theanine is the most abundant free amino acid in tea, and is responsible for the “Umami” taste and anti-anxiety effects of tea infusion. Theanine is mainly synthesized in roots and subsequent transport to shoots by theanine transporters.9,10 Our recent study showed that six CsAAPs can transport theanine with moderate theanine affinities, in a H+-dependent manner.11 These CsAAPs express tissue-specifically, and CsAAP1 play an critical role in theanine transport from root to leaf buds.
To examine whether the six CsAAPs can transport other amino acids, we transformed pYES2-CsAAPs to the yeast 22Δ10α line, an amino acid transporter mutant cannot grow in medium with amino acid as the sole nitrogen source. 23344c, the wild-type background of 22Δ10α, was used as a positive control. 22Δ10α and 22Δ10α tranformed pYES2 empty vector as a negative control. The results showed that yeast mutant 22Δ10α expressed CsAAP1, CsAAP2, CsAAP4, CsAAP5, CsAAP6 and CsAAP8 was able to grow on medium with Gln, Glu, Asp, or Ala as the sole nitrogen source (Figure 1), indicating that these theanine transporters can also transport these proteinaceous amino acids present in xylem sap of tea plants. However, only yeast mutant 22Δ10α expressed CsAAP4, CsAAP5 and CsAAP8 was able to grow on medium with GABA as the sole nitrogen source (Figure 1). These results indicate that CsAAP1, CsAAP2, CsAAP4, CsAAP5, CsAAP6 and CsAAP8 have the capacity to transport a wide spectrum of amino acids with different substrate specificity.
Figure 1.
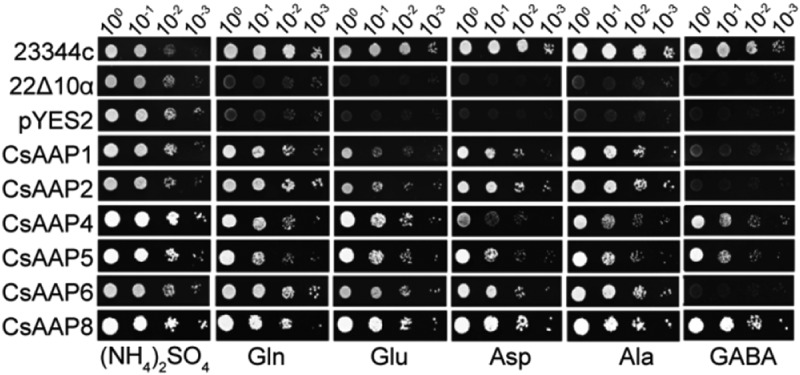
Yeast 22Δ10α growth complementation assay with amino acid as the sole nitrogen source. Yeast mutant line 22Δ10α was used to analyze growth of yeast cells expressing CsAAPs on medium with Gln, Glu, Asp, Ala, or GABA with the sole nitrogen source, respectively. For control, yeast cells grow with the supply of 2-mM ammonium sulfate. The yeast strains were transformed with pYES2-CsAAPs. 4 yeast colonies in each row represent different dilutions.
To explore the role of these theanine transporters in nitrogen deficiency response, we treated the tea plants under nitrogen deficiency (without nitrogen) for ten days, with normal nitrogen supply as a control condition. Then, we harvested the first and second leaves, the third and fourth leaves, tender stem and root for RNA extraction to examine gene expression. qRT-PCR results showed that N deficiency did not change the tissue-specific expression pattern of CsAAPs (Figure 2). CsAAP1, CsAAP5 and CsAAP6 mainly expressed in leaves; CsAAP4 and CsAAP2 mainly expressed in stem; CsAAP8 mainly expressed in root and stem. Interestingly, the expression of these CsAAPs was significantly induced by nitrogen deficiency in their mainly expressed tissues. The tissue-specific expression is often connected with its physiological function.12 This tissue-specific response of CsAAPs expression to nitrogen deficiency suggested they may play tissue-specific roles in amino acids transport in tea plants. We speculate that, under nitrogen deficiency, the CsAAP6 and CsAAP8 may retrieve amino acids from the apoplast of root, and mediate amino acid loading into the xylem; CsAAP2 and CsAAP4 may mediate amino acid exchange between xylem and phloem in vascular bundle; CsAAP1 and CsAAP5 may enhance amino acids targeting to developing leaves. Thus, the nitrogen in tea plant will probably be transported and used more efficiently under the coordinated act of these CsAAPs. This speculation is supported by the previous report that over-expression of PsAAP1 in pea increased nitrogen use efficiency in nitrogen-deficient soil.7
Figure 2.
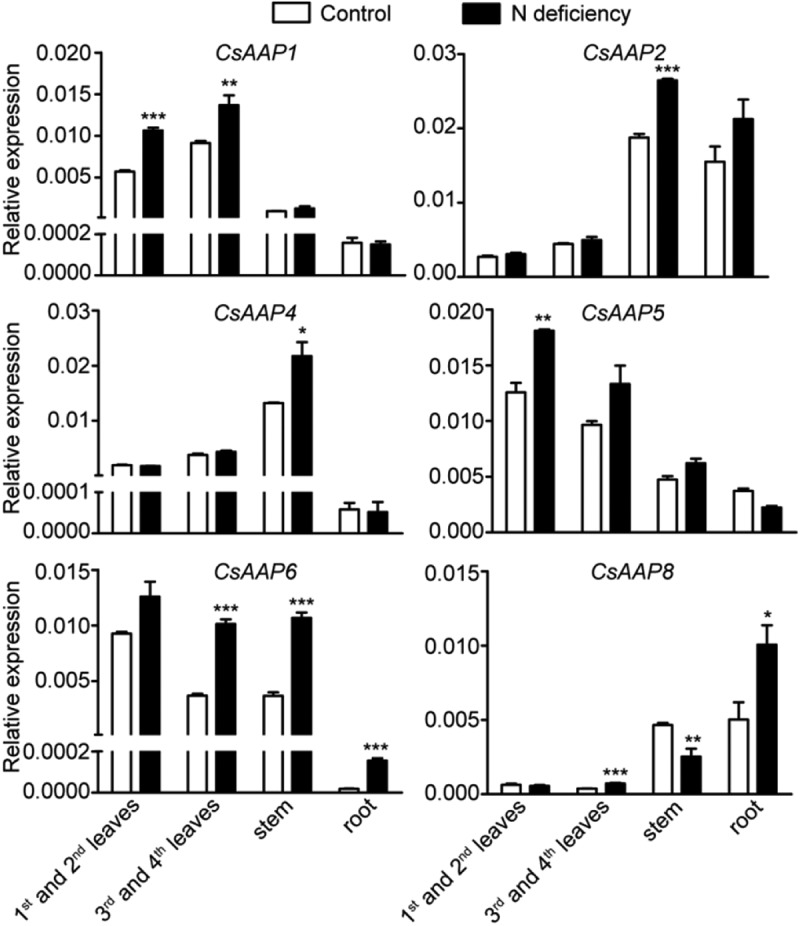
CsAAP expression in various tissues in response to nitrogen (n) deficiency. Results of RT-qPCR of CsAAP1, CsAAP2, CsAAP4, CsAAP5, CsAAP6 and CsAAP8 expression in first and second leaves, third and fourth leaves, tender stem and root of the tea plants under normal N supply (Control) and N deficiency conditions. Mean of three independent biological replicates of 4 plants with three technical replicates. Error bars are standard error of the mean. Asterisks represent statistical significance determined by Student’s t-test (*p = 0.05, **p = 0.01, ***p = 0.01).
Partitioning of amino acids among different organelles, cells, tissues, and organs is mediated by various membrane-localized amino acid transporters.6,8 Plasma membrane-localized amino acid transporters import amino acids into or export amino acids out of cells, whereas the transporters located in endometrial system mainly contribute to the distribution of amino acids in different organelles. To analyze the subcellular localization of these CsAAPs, GFP-CsAAP1, GFP-CsAAP2 and GFP-CsAAP6 under control of the CMV 35S promoter were introduced into protoplasts of Arabidopsis. Laser confocal imaging results showed that green fluorescence of GFP-CsAAP1, GFP-CsAAP2 or GFP-CsAAP6 were in plasma membrane and endometrial system (Figure 3(a)). To further confirm the endometrial location of these proteins, we co-transformed GFP-CsAAP1 with an endoplasmic reticulum (ER) marker AtWAK2-mCherry13 to the tobacco epidermis cells (Figure 3(b)). We observed that AtWAK2-mCherry signal and GFP-CsAAP1 signal overlapped completely in the cells. Previous reports in rice and Arabidopsis also showed that AAPs are plasma membrane proteins and that fluorescence observed in the endoplasmic reticulum was probably due to the biosynthesis or trafficking.14-16
Figure 3.
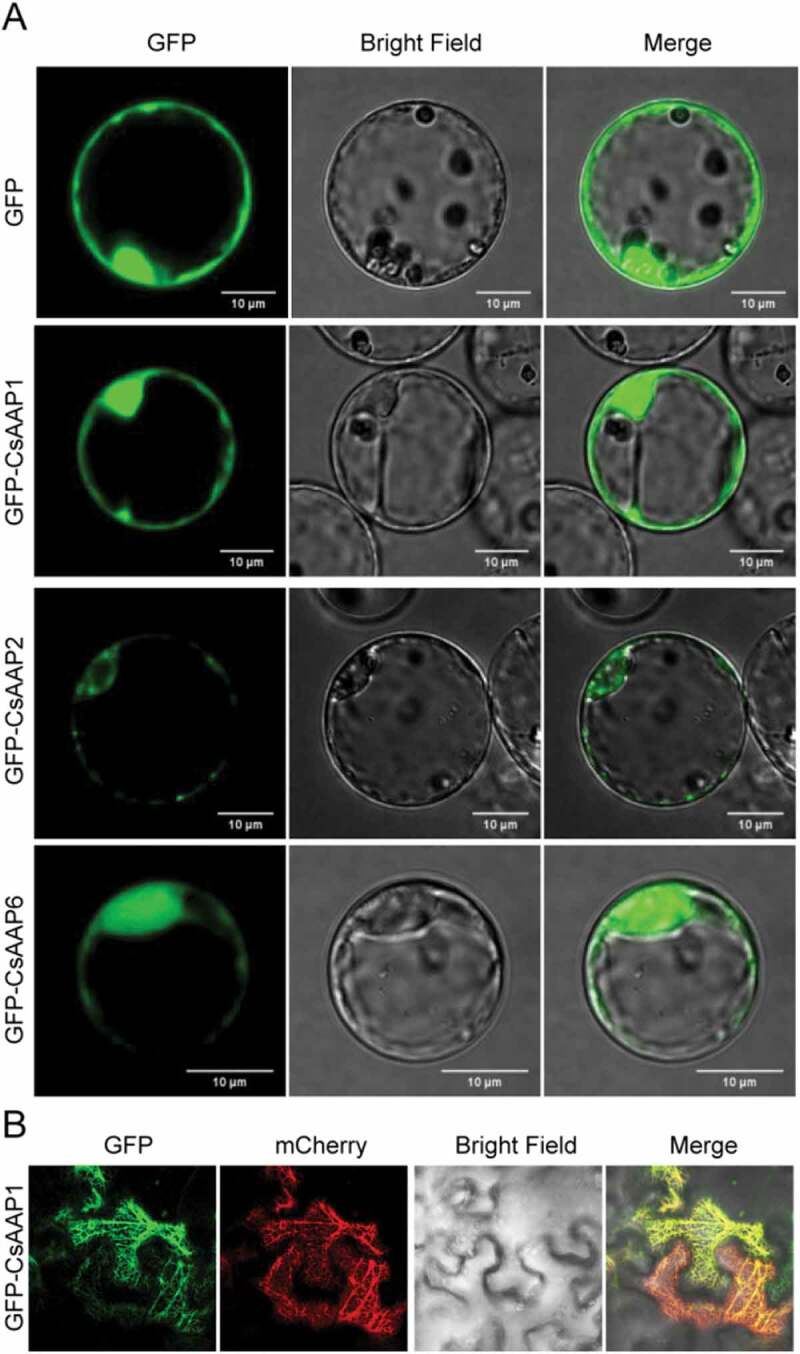
Plasma membrane localization of CsAAP in Arabidopsis protoplasts and tobacco epidermal cells. GFP-CsAAP1, GFP-CsAAP2, or GFP-CsAAP6 were transiently expressed in Arabidopsis protoplasts or expressed in tobacco epidermal cells together with AtWAK2-mCherry. (a) GFP-CsAAP1, GFP-CsAAP2 or GFP-CsAAP6 in Arabidopsis protoplasts; (b) GFP-CsAAP1 and AtWAK2-mCherry in tobacco epidermal cells.
Taken together, this study reported 6 theanine transporters also transport other amino acids including Gln, Glu, Ala, Asp and GABA. The tissue-specific induction by nitrogen deficiency suggested these CsAAPs may act tissue-specifically in mediating amino acid translocation from root to shoot and from source to sink tissues under nitrogen deficient condition. In our performing study, we are detecting CsAAPs expression in various tea plant cultivars, and we will further to evaluate whether CsAAPs highly-expressed cultivars use nitrogen more efficiently. It is also intriguing to over-express CsAAPs in tea plant in attempt to improve nitrogen use efficiency and tea quality, in future, once the gene transformation technology is overcome.
Funding Statement
This work was supported by Anhui Provincial Department of Science and Technology [grant number 17030701049]; the National Natural Science Foundation of China [grant number 31770731 to ZZ]; and Anhui Provincial Natural Science Foundation [grant number 1808085QC75 to YT].
References
- 1.Okano K, Chutani K, Matsuo K.. Suitable level of nitrogen fertilizer for tea (Camellia sinensis L.) plants in relation to growth, photosynthesis, nitrogen uptake and accumulation of free amino acids. Nippon Sakumotsu Gakkai Kiji. 1997;66:1–3. [Google Scholar]
- 2.Ruan JY, Gerendás J, Härdter R, Sattelmacher B.. Effect of root-zone pH and form and concentration of nitrogen on the accumulation of quality-related components in green tea. J Sci Food Agric. 2007;87:1505–1516. doi: 10.1002/jsfa.2875. [DOI] [Google Scholar]
- 3.Ruan L, Wei K, Wang L, Cheng H, Wu L, Li H. Characteristics of free amino acids (the quality chemical components of tea) under spatial heterogeneity of different nitrogen forms in tea (Camellia sinensis) Plants. Molecules. 2019;24:415. PMID:30678321. doi: 10.3390/molecules24030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang XD, Ni K, Shi YZ, Yi XY, Zhang QF, Fang L, Ma LF, Ruan J. Effects of long-term nitrogen application on soil acidification and solution chemistry of a tea plantation in China. Agr Ecosyst Environ. 2018;252:74–82. doi: 10.1016/j.agee.2017.10.004. [DOI] [Google Scholar]
- 5.Oh K, Kato T, Xu HL. Transport of nitrogen assimilation in xylem vessels of green tea plants fed with NH4-N and NO3-N. Pedosphere. 2008;18:222–226. doi: 10.1016/S1002-0160(08)60010-7. [DOI] [Google Scholar]
- 6.Tegeder M. Transporters involved in source to sink partitioning of amino acids and ureides: opportunities for crop improvement. J Exp Bot. 2014;65:1865–1878. PMID:24489071. doi: 10.1093/jxb/eru012. [DOI] [PubMed] [Google Scholar]
- 7.Perchlik M, Tegeder M. Improving plant nitrogen use efficiency through alteration of amino acid transport processes. Plant Physiol. 2017;175:235–247. PMID:28733388. doi: 10.1104/pp.17.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Garneau MG, Majumdar R, Grant J, Tegeder M. Improvement of pea biomass and seed productivity by simultaneous increase of phloem and embryo loading with amino acids. Plant J. 2015;81:134–146. PMID:25353986. doi: 10.1111/tpj.12716. [DOI] [PubMed] [Google Scholar]
- 9.Deng WW, Ashihara H. Occurrence and de novo biosynthesis of caffeine and theanine in seedlings of tea (Camellia sinensis). Nat Prod Commun. 2015;10:703–706. PMID:2605813. [PubMed] [Google Scholar]
- 10.Li F, Dong C, Yang T, Ma J, Zhang S, Wei C, Wan X, Zhang Z. Seasonal theanine accumulation and related gene expression in the roots and leaf buds of tea plants (Camellia Sinensis L.). Front Plant Sci. 2019;10:1397. PMID:31749819. doi: 10.3389/fpls.2019.01397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong C, Li F, Yang T, Feng L, Zhang S, Li F, Li W, Xu G, Bao S, Wan X, et al. Theanine transporters identified in tea plants (Camellia sinensis L.). Plant J. 2020;101:57–70. PMID:31461558. doi: 10.1111/tpj.14517. [DOI] [PubMed] [Google Scholar]
- 12.Rentsch D, Schmidt S, Tegeder M. Transporters for uptake and allocation of organic nitrogen compounds in plants. FEBS Lett. 2007;581:2281–2289. PMID:17466985. doi: 10.1016/j.febslet.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Nelson BK, Yuan S, Cai X, Nebenfuhr A. A multicolored set of in vivo organelle markers for co‐localization studies in Arabidopsis and other plants. Plant J. 2007;51:1126–1136. PMID:17666025. doi: 10.1111/j.1365-313.2007.03212.x. [DOI] [PubMed] [Google Scholar]
- 14.Taylor MR, Reinders A, Ward JM. Transport function of rice amino acid permeases (AAPs). Plant Cell Physiol. 2015;56:1355–1363. PMID: 25907566. doi: 10.1093/pcp/pcv053. [DOI] [PubMed] [Google Scholar]
- 15.Okumoto S, Koch W, Tegeder M, Fischer WN, Biehl A, Leister D, Stierhof YD, Frommer WB. Root phloem-specific expression of the plasma membrane amino acid proton co-transporter AAP2. J Exp Bot. 2004;55:2155–2168. PMID:15361541. doi: 10.1093/jxb/erh233. [DOI] [PubMed] [Google Scholar]
- 16.Peng B, Kong H, Li Y, Wang L, Zhong M, Sun L, Gao G, Zhang Q, Luo L, Wang G, et al. OsAAP6 functions as an important regulator of grain protein content and nutritional quality in rice. Nat Commun. 2014;5:4847. PMID:25209128. doi: 10.1038/ncomms5847. [DOI] [PMC free article] [PubMed] [Google Scholar]


