ABSTRACT
Legumes possess the autoregulation of nodulation (AON) pathway which is responsible for maintaining optimal root nodule number. In Lotus japonicus, AON comprises the CLE-HAR1-TML module, which plays an essential role in transmitting signals via root-to-shoot-to-root long-distance signaling. In addition to AON’s principal role of negatively regulating nodule number, a recent study revealed another in the systemic control of rhizobial infection. Nitrate also negatively regulates the pleiotropic phases of legume-Rhizobium symbioses, including rhizobial infection and nodule number. Nitrate signaling has recently been shown to use AON components such as CLE-RS2 and HAR1 to control nodule number. Here we consider the role of a loss-of-function mutation in CLE-RS1, -RS2 and TML in rhizobial infection in relation to nitrate. Our results agree with previous findings and support the hypothesis that AON is required for the control of rhizobial infection but not for its nitrate-induced control. Furthermore, we confirm that the tml mutants exhibit nitrate sensitivity that differs from that of cle-rs2 and har1. Hence, while the nitrate-induced control mechanism of nodule number uses AON components, an unknown pathway specific to nitrate may exist downstream of HAR1, acting in parallel with the HAR1> TML pathway.
KEYWORDS: Autoregulation of nodulation, CLE, nitrate, symbiosis
Text
Nodulation is a host specific symbiosis between legumes and rhizobia in which symbiotic organs called nodules are formed on the host roots. The host plant benefits by gaining access to atmospheric nitrogen but nodule production and rhizobial nitrogen fixation is biosynthetically costly.1,2 A mechanism called autoregulation of nodulation (AON) acts as a negative regulatory system in this symbiosis.3,4 It enables the host plant to strictly control the number of nodules and in turn control energy consumption. AON involves long-distance signaling between the root and shoot systems. The production of CLE peptides plays an important role in the initiation of AON. In Lotus japonicus, three CLE peptides (CLE-RS1/2/3) have been shown to be produced by rhizobia in the roots of host plants.5,6 The induction timing of corresponding CLE genes is different after rhizobial infection but constitutive expression of each gene strongly reduces nodule number in a shoot-acting HAR1 receptor-like kinase dependent-manner.5–7 Hence, the three root-derived CLE peptides function systemically to negatively regulate nodule number. Indeed, a mature form of CLE-RS2 has been detected in xylem sap which can physically interact with HAR1.8 As a result of this CLE-HAR1 interaction, cytokinin is produced in shoots by the upregulation of a cytokinin synthesis gene, IPT3. The resulting cytokinin is translocated from shoot to root, where it inhibits nodulation.9 A root-acting putative F-box protein, TML, is required for the action of shoot-derived cytokinin.9–11 In addition, miR2111 which is downstream of HAR1 can be translocated from shoot to root where it targets TML. Unlike shoot-derived cytokinin, miR2111 stimulates nodulation and the miR2111-TML module appears to contribute to the inhibitory effect of AON on nodulation.12
While the formation of an increased number of nodules is the most striking phenotype of AON loss-of-function mutants,10,13,14 these mutants also exhibit hyperinfected phenotypes which are identifiable by an increased number of infection threads (ITs). ITs are plant-derived structures in which the rhizobia move from the root surface to the interior of the root.15 More recently, CLE-RS1 and -RS2 have been demonstrated to systemically inhibit ITs formation in the unique nin mutant, daphne, which loses the ability to form nodules yet forms excessive numbers of ITs.16,17 HAR1 is a prerequisite for the action of these peptides, suggesting that the CLE-HAR1 pathway plays a role in negatively regulating rhizobial infection. Importantly, this is a potentially novel role of the AON pathway. Nevertheless, the detailed mechanism remains elusive. For example, the loss-of-function effects of the CLE genes on rhizobial infection are unknown.
Nitrogen sources, such as nitrate, act as another regulatory factor in legume-Rhizobium symbioses. Nodule development and rhizobial nitrogen fixation are energy-consuming processes, thus it would be advantageous to the plant to cease the symbiosis when there is sufficient available nitrogen in the soil to allow growth. Host plants respond to nitrate by negatively regulating multiple phases of the root nodule symbiosis, including rhizobial infection, nodule number, nodule growth and nitrogen fixation.2,18–20 We previously identified a nitrate unresponsive symbiosis 1 (nrsym1) mutant in L. japonicus. Phenotypic analysis provides evidence that NRSYM1 plays an essential role in the control of nitrate-induced pleiotropic regulation of symbiosis.21 NRSYM1 encodes an NLP transcription factor and regulates nitrate-inducible gene expression both in symbiotic and nonsymbiotic conditions. In the presence of nitrate, NRSYM1 uses CLE-RS2 as a direct target gene for the control of nodule number. Nitrate concentration does not affect the excessive nodule production exhibited by har1 mutants but does influence other elements of the symbiosis such as rhizobial infection, nodule growth and nitrogen fixation. These observations suggest that in NRSYM1-mediated pleiotropic nitrate regulation, the NRSYM1> CLE-RS2> HAR1 pathway predominantly has a role in the control of nodule number.21
In order to determine the precise role of CLE genes in the control of rhizobial infection, we investigated the infection phenotype of the cle-rs1, -rs2 and double mutants in conditions with and without nitrate. The cle-rs mutants were generated in a previous study using the CRISPR-Cas9 genome editing system.21 The nodulation phenotypes suggest that CLE-RS1 and -RS2 have a redundant function in the negative regulation of nodule number.21 Nodule number in the cle-rs2 mutant was unaffected by nitrate concentration suggesting that CLE-RS2 plays a role in controlling nodule production in response to nitrate. In the present study, a Mesorhizobium loti strain constitutively expressing DsRED was inoculated onto host plants and subsequent IT production was then assessed. In the absence of nitrate, each cle-rs single mutant produced a normal number of ITs at 7 days after inoculation (dai) (Figure 1a). By contrast, the number of ITs observed in the cle-rs1 -rs2 double mutants was significantly greater, compared to other genotypes. This result suggests that the two CLE genes possess a redundant function in negatively regulating rhizobial infection. The hyperinfected phenotype of the cle-rs1 -rs2 double mutant is consistent with those of other AON mutants.10,13,21 In both the cle-rs and double mutants, exposure to a high nitrate concentration (10 mM) resulted in a reduced number of ITs to a level similar to that observed in wild-type (WT) plants (Figure 1a). Therefore, the two CLE genes are dispensable for nitrate-induced control of rhizobial infection.
Figure 1.
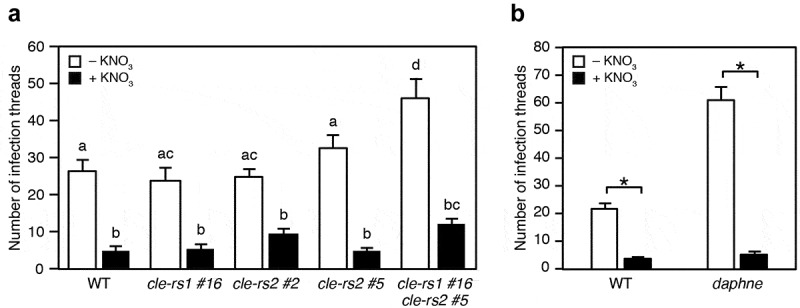
(a) The number of infection threads observed in the WT, cle-rs1 and cle-rs2 mutants and in the cle-rs1 cle-rs2 double mutant with 0 or 10 mM KNO3 at 7 days after inoculation (dai) with Mesorhizobium loti MAFF303099 constitutively expressing DsRed (n = 10–12 plants). Stable cle-rs mutants were previously created by the CRISPR-Cas9 genome editing system.21 Columns with the same lower-case letter indicate no significant difference (Tukey’s test, P < .05). (b) The number of infection threads in the WT and daphne mutants with 0 or 10 mM KNO3 at 5 dai with Mesorhizobium loti MAFF303099 constitutively expressing DsRed (n = 10–12 plants). *P < .05 by Student’s t-test. Error bars indicate SE.
In addition to its predominant role for positively regulating nodulation, NIN plays a role for negatively regulating nodulation by directly activating CLE-RS1 and -RS2.4,22 We investigated if NIN is required for nitrate response in nodulation. The excessive ITs formation in daphne was inhibited by high nitrate treatment (Figure 1b). Thus, NIN is unlikely to be involved in nitrate-induced control of rhizobial infection.
As previously mentioned, the CLE-HAR1 module is responsible for the nitrate-induced control of nodule number. In order to determine whether this is via the downstream AON factor of HAR1, we reassessed the tml mutant phenotypes in the presence of nitrate. It has previously been demonstrated that the tml mutant is more sensitive to nitrate than har1 and our findings broadly agreed with this finding;10 the number of nodules at 21 dai were significantly reduced by nitrate in tml, although they were still greater than in the WT control (Figure 2a). We next generated the double mutant nrsym1 tml and showed that it maintained increased nodule number in the presence of nitrate (Figure 2a). This result suggests that NRSYM1 is implicated in the observed nitrate-induced reduction of nodule number in tml single mutants. We next investigated the effect of nitrate on rhizobial infection in the tml mutants. The increased ITs formation was inhibited by high nitrate in an NRSYM1-dependent manner (Figure 2b), suggesting that TML is not required for nitrate-induced control of rhizobial infection.
Figure 2.
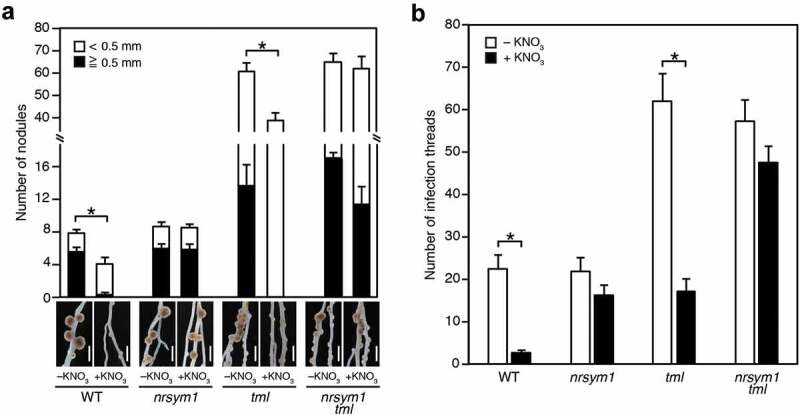
(a) Nodulation and nodule numbers in the WT, nrsym1-1 and tml-4 mutants, and the nrsym1-1 tml-4 double mutant grown in the presence of 0 or 10 mM KNO3 at 21 dai with Mesorhizobium loti MAFF303099 (n = 10–12 plants). Mature (black bars) and immature (white bars) nodules were separately counted. *P < .05 by Student’s t-test (comparison of total nodule number). Scale bars: 2 mm. (b) The number of infection threads in the WT, nrsym1-1 and tml-4 mutants, and the nrsym1-1 tml-4 double mutants with 0 or 10 mM KNO3 at 5 dai with Mesorhizobium loti MAFF303099 constitutively expressing DsRed (n = 9–12 plants). *P < .05 by Student’s t-test. Error bars indicate SE.
In this study, we determined the role of CLE-RS1 and -RS2 in the control of rhizobial infection. In the absence of nitrate, the CLE peptides have a redundant function of negatively regulating rhizobial infection. This observation provides further evidence in support of a model in which AON plays a role in regulating rhizobial infection.17 However, it is reasonable to conclude that the AON pathway is not required for nitrate-induced control of rhizobial infection because mutants of key components of AON, cle-rs1/2, har1 and tml, exhibit nitrate dependent IT formation. In addition, based on the nitrate-sensitive phenotype of daphne, NIN, an activator of CLE-RS1/2, may not be required for nitrate-induced control of rhizobial infection. The mechanism by which host plants inhibit rhizobial infection in response to nitrate is currently unclear. Given that NRSYM1 has the ability to control the nitrate-affected pleiotropic phases of root nodule symbiosis (including rhizobial infection) it could involve the targeting of unknown genes which act during rhizobial infection. Indeed, in Medicago truncatula, MtNLP1 could inhibit nodulation by targeting MtCRE1 which encodes a cytokinin receptor required for rhizobial infection and nodule organogenesis.23 We also reassessed the involvement of TML, a root-acting factor in AON, in the nitrate-induced control of nodule formation. Our results agreed with previous findings in that tml was much more sensitive to nitrate than other AON mutants.10 Therefore, in terms of the nitrate-induced control of nodule number, it is likely that NRSYM1 uses CLE-RS2 and HAR1 but not TML as downstream factors. Since nodule number in har1 is unaffected by nitrate, there might be a nitrate specific pathway downstream of HAR1, which acts in parallel with the HAR1> TML pathway (Figure 3). Future identification of such a pathway would help complete our understanding of the mechanism of nitrate-induced control of nodule number.
Figure 3.
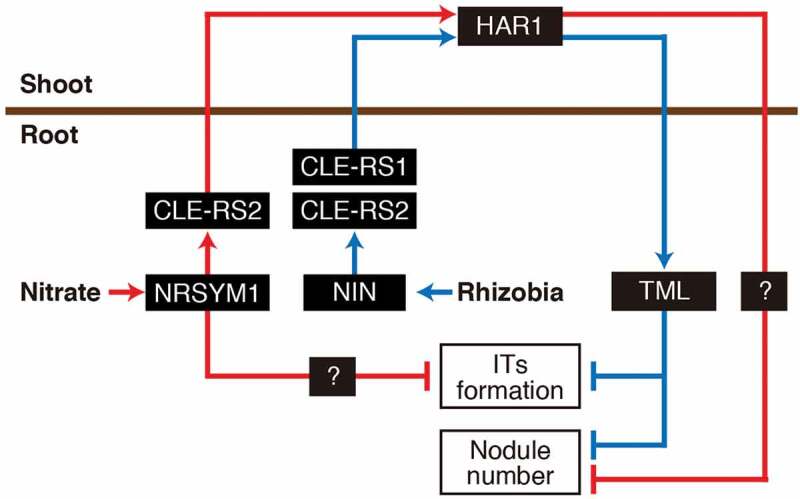
Model for the negative regulation of rhizobial infection and nodule number in L. japonicus. In response to rhizobial infection, NIN activates the CLE-RS1/2> HAR1> TML signaling pathway22 to suppress infection threads (ITs) formation and nodule number (blue lines). The nitrate-induced NRSYM1> CLE-RS2> HAR1 signaling pathway could negatively regulate nodule number by using unknown downstream factors other than TML. In the presence of nitrate NRSYM1 also controls IT formation locally in the root through a mechanism independent of AON pathway (red lines).
Funding Statement
This work was supported by Grant-in-Aid for Scientific Research from the MEXT/JSPS (18H04773 and 19H03239), by JST ERATO (JPMJER1502), by the Cooperative Research Grant of the Plant Transgenic Design Initiative by Gene Research Center, University of Tsukuba..
Acknowledgments
We thank Makoto Hayashi for providing M. loti MAFF303099 expressing DsRED; Kazuko Ito for technical support. T.S. is supported by Grant-in-Aid for Scientific Research from the MEXT/JSPS (18H04773 and 19H03239), by JST ERATO (JPMJER1502), by the Cooperative Research Grant of the Plant Transgenic Design Initiative by Gene Research Center, University of Tsukuba.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Kosslak RM, Bohlool BB.. Suppression of nodule development of one side of a split-root system of soybeans caused by prior inoculation of the other side. Plant Physiol. 1984;75:1–4. doi: 10.1104/pp.75.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishida H, Suzaki T.. Two negative regulatory systems of root nodule symbiosis: how are symbiotic benefits and costs balanced? Plant Cell Physiol. 2018;59:1733–1738. doi: 10.1093/pcp/pcy102. [DOI] [PubMed] [Google Scholar]
- 3.Reid DE, Ferguson BJ, Hayashi S, Lin Y-H, Gresshoff PM. Molecular mechanisms controlling legume autoregulation of nodulation. Ann Bot. 2011;108:789–795. doi: 10.1093/aob/mcr205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzaki T, Yoro E, Kawaguchi M. Leguminous plants: inventors of root nodules to accommodate symbiotic bacteria. Int Rev Cell Mol Biol. 2015;316:111–158. doi: 10.1016/bs.ircmb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Okamoto S, Ohnishi E, Sato S, Takahashi H, Nakazono M, Tabata S, Kawaguchi M. Nod factor/nitrate-induced CLE genes that drive HAR1-mediated systemic regulation of nodulation. Plant Cell Physiol. 2009;50:67–77. doi: 10.1093/pcp/pcn194. [DOI] [PubMed] [Google Scholar]
- 6.Nishida H, Handa Y, Tanaka S, Suzaki T, Kawaguchi M. Expression of the CLE-RS3 gene suppresses root nodulation in Lotus japonicus. J Plant Res. 2016;129:909–919. doi: 10.1007/s10265-016-0842-z. [DOI] [PubMed] [Google Scholar]
- 7.Yoro E, Nishida H, Ogawa-Ohnishi M, Yoshida C, Suzaki T, Matsubayashi Y, Kawaguchi M. PLENTY, a hydroxyproline O-arabinosyltransferase, negatively regulates root nodule symbiosis in Lotus japonicus. J Exp Bot. 2019;70:507–517. doi: 10.1093/jxb/ery364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okamoto S, Shinohara H, Mori T, Matsubayashi Y, Kawaguchi M. Root-derived CLE glycopeptides control nodulation by direct binding to HAR1 receptor kinase. Nat Commun. 2013;4:2191. doi: 10.1038/ncomms3191. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki T, Suzaki T, Soyano T, Kojima M, Sakakibara H, Kawaguchi M. Shoot-derived cytokinins systemically regulate root nodulation. Nat Commun. 2014;5:4983. doi: 10.1038/ncomms5983. [DOI] [PubMed] [Google Scholar]
- 10.Magori S, Oka-Kira E, Shibata S, Umehara Y, Kouchi H, Hase Y, Tanaka A, Sato S, Tabata S, Kawaguchi M. TOO MUCH LOVE, a root regulator associated with the long-distance control of nodulation in Lotus japonicus. Mol Plant Microbe Interact. 2009;22:259–268. doi: 10.1094/MPMI-22-3-0259. [DOI] [PubMed] [Google Scholar]
- 11.Takahara M, Magori S, Soyano T, Okamoto S, Yoshida C, Yano K, Sato S, Tabata S, Yamaguchi K, Shigenobu S, et al. TOO MUCH LOVE, a novel kelch repeat-containing F-box protein, functions in the long-distance regulation of the legume–Rhizobium symbiosis. Plant Cell Physiol. 2013;54:433–447. doi: 10.1093/pcp/pct022. [DOI] [PubMed] [Google Scholar]
- 12.Tsikou D, Yan Z, Holt DB, Abel NB, Reid DE, Madsen LH, Bhasin H, Sexauer M, Stougaard J, Markmann K. Systemic control of legume susceptibility to rhizobial infection by a mobile microRNA. Science. 2018;362:233–236. doi: 10.1126/science.aat6907. [DOI] [PubMed] [Google Scholar]
- 13.Wopereis J, Pajuelo E, Dazzo FB, Jiang Q, Gresshoff PM, De Bruijn FJ, Stougaard J, Szczyglowski K. Short root mutant of Lotus japonicus with a dramatically altered symbiotic phenotype. Plant J. 2000;23:97–114. doi: 10.1046/j.1365-313x.2000.00799.x. [DOI] [PubMed] [Google Scholar]
- 14.Oka-Kira E, Tateno K, Miura K, Haga T, Hayashi M, Harada K, Sato S, Tabata S, Shikazono N, Tanaka A, et al. klavier (klv), a novel hypernodulation mutant of Lotus japonicus affected in vascular tissue organization and floral induction. Plant J. 2005;44:505–515. doi: 10.1111/j.1365-313X.2005.02543.x. [DOI] [PubMed] [Google Scholar]
- 15.Suzaki T, Takeda N, Nishida H, Hoshino M, Ito M, Misawa F, Handa Y, Miura K, Kawaguchi M. LACK OF SYMBIONT ACCOMMODATION controls intracellular symbiont accommodation in root nodule and arbuscular mycorrhizal symbiosis in Lotus japonicus. PLoS Genet. 2019;15:e1007865. doi: 10.1371/journal.pgen.1007865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoro E, Suzaki T, Toyokura K, Miyazawa H, Fukaki H, Kawaguchi M. A positive regulator of nodule organogenesis, NODULE INCEPTION, acts as a negative regulator of rhizobial infection in Lotus japonicus. Plant Physiol. 2014;165:747–758. doi: 10.1104/pp.113.233379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoro E, Suzaki T, Kawaguchi M. CLE-HAR1 systemic signaling and NIN-mediated local signaling suppress the increased rhizobial infection in the daphne mutant of Lotus japonicus. Mol Plant Microbe Interact. 2020;33:320–327;MPMI08190223R. doi: 10.1094/MPMI-08-19-0223-R. [DOI] [PubMed] [Google Scholar]
- 18.Streeter J, Wong PP. Inhibition of legume nodule formation and N2 fixation by nitrate. Crit Rev Plant Sci. 1988;7:1–23. doi: 10.1080/07352688809382257. [DOI] [Google Scholar]
- 19.Nishida H, Suzaki T. Nitrate-mediated control of root nodule symbiosis. Curr Opin Plant Biol. 2018;44:129–136. doi: 10.1016/j.pbi.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson BJ, Mens C, Hastwell AH, Zhang M, Su H, Jones CH, Chu X, Gresshoff PM. Legume nodulation: the host controls the party. Plant Cell Environ. 2019;42:41–51. doi: 10.1111/pce.13348. [DOI] [PubMed] [Google Scholar]
- 21.Nishida H, Tanaka S, Handa Y, Ito M, Sakamoto Y, Matsunaga S, Betsuyaku S, Miura K, Soyano T, Kawaguchi M, et al. A NIN-LIKE PROTEIN mediates nitrate-induced control of root nodule symbiosis in Lotus japonicus. Nat Commun. 2018;9:499. doi: 10.1038/s41467-018-02831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soyano T, Hirakawa H, Sato S, Hayashi M, Kawaguchi M. NODULE INCEPTION creates a long-distance negative feedback loop involved in homeostatic regulation of nodule organ production. Proc Natl Acad Sci USA. 2014;111:14607–14612. doi: 10.1073/pnas.1412716111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin J-S, Li X, Luo Z, Mysore KS, Wen J, Xie F. NIN interacts with NLPs to mediate nitrate inhibition of nodulation in Medicago truncatula. Nat Plants. 2018;4:942–952. doi: 10.1038/s41477-018-0261-3. [DOI] [PubMed] [Google Scholar]


