Abstract
PURPOSE
To independently validate two biomarkers, a 44-gene DNA damage immune response (DDIR) signature and stromal tumor-infiltrating lymphocytes (sTILs), as prognostic markers in patients with triple-negative breast cancer (TNBC) treated with adjuvant doxorubicin (A) and cyclophosphamide (C) in SWOG 9313.
METHODS
Four hundred twenty-five centrally determined patient cases with TNBC from S9313 were identified. DDIR signature was performed on RNA isolated from formalin-fixed paraffin-embedded tumor tissue, and samples were classified as DDIR negative or positive using predefined cutoffs. Evaluation of sTILs was performed as described previously. Markers were tested for prognostic value for disease-free survival (DFS) and overall survival (OS) using Cox regression models adjusted for treatment assignment, nodal status, and tumor size.
RESULTS
Among 425 patients with TNBC, 33% were node positive. DDIR was tested successfully in 90% of patients (381 of 425), 62% of which were DDIR signature positive. DDIR signature positivity was associated with improved DFS (hazard ratio [HR], 0.67; 95% CI, 0.48 to 0.92; P = .015) and OS (HR, 0.61; 95% CI, 0.43 to 0.89; P = .010). sTILs density assessment was available in 99% of patients and was associated with improved DFS (HR, 0.70; 95% CI, 0.51 to 0.96; P = .026 for sTILs density ≥ 20% v < 20%) and OS (HR, 0.59; 95% CI, 0.41 to 0.85; P = .004 for sTILs density ≥ 20% v < 20%). DDIR signature score and sTILs density were moderately correlated (r = 0.60), which precluded statistical significance for DFS in a joint model. Three-year DFS and OS in a subgroup of patients with DDIR positivity and T1c/T2N0 disease were 88% and 94%, respectively.
CONCLUSION
The prognostic role of sTILs and DDIR in early-stage TNBC was confirmed. DDIR signature conferred improved prognosis in two thirds of patients with TNBC treated with adjuvant AC. DDIR signature has the potential to stratify outcome and to identify patients with less projected benefit after AC chemotherapy.
INTRODUCTION
Triple-negative breast cancer (TNBC) accounts for 15% to 20% of breast cancer and carries a poor prognosis. Despite receiving adjuvant anthracycline/taxane-based chemotherapy, approximately 20% to 40% of patients with early-stage TNBC develop metastatic disease.1-3 Biomarkers that can prospectively select patients with TNBC with good outcomes after anthracycline-based adjuvant chemotherapy, or alternatively, that identify mechanisms of resistance to this treatment strategy, will optimize personalization of adjuvant chemotherapy for TNBC.
Although TNBC is rather uniformly treated in clinical practice, distinct biologic subgroups exist.4 In particular, more than one half of TNBC tumors have molecular and genomic characteristics similar to BRCA1/2-mutant disease, described as BRCAness.5,6 The Fanconi anemia/BRCA repair pathway is required for coordination of DNA repair mechanisms, including homologous recombination, which is essential for the repair of stalled DNA replication forks.7 Loss of function of this pathway by mutation, promoter hypermethylation, or other epigenetic events results in genomic instability and DNA damage response deficiency, leading to reliance on less robust DNA repair mechanisms such as nonhomologous end joining.8 BRCAness has also been reported to be associated with increased tumor lymphocytic infiltration and upregulation of immune gene expression.6,9
The DNA damage immune response (DDIR) signature (formerly the DNA damage repair deficient signature) is a 44-gene RNA-based signature developed to identify patients with immune activation as a result of DNA damage response deficiency. Importantly, this signature represents activation of the cyclic GMP–AMP synthase (cGAS)–stimulator of interferon genes (STING) immune pathway and is characterized by increased immune gene expression.10 This functional approach allows the capture of multiple mechanisms of loss of DNA repair, resulting in a common gene expression signature. The 44-gene DDIR signature includes well-known immune checkpoint targets such as programmed death-ligand 1 and Indoleamine 2,3-dioxygenase 1, as well as several inflammatory cytokines. Constitutive activation of the cGAS-STING innate immune pathway in DDIR signature-positive cancer cells has been reported.10 Tumor-specific immune activation via the STING pathway results in infiltration by T lymphocytes and upregulation of immune checkpoints, creating an inflammatory microenvironment.11,12 In preliminary studies, when applied as a predefined binary cutoff, the DDIR signature has been shown to predict response to anthracycline-based treatment in both neoadjuvant and adjuvant settings, in hormone receptor-positive and receptor-negative breast cancer.13 However, evaluation of the DDIR signature in a large, uniformly treated patient population with TNBC with long-term outcome data has not been performed previously.
We hypothesized that a subgroup of patients with TNBC identified using the DDIR signature would derive increased benefit from adjuvant doxorubicin plus cyclophosphamide (AC)–based DNA-damaging chemotherapy by virtue of decreased repair of the DNA damage induced by the treatment. We also sought to examine the association between stromal tumor-infiltrating lymphocytes (sTILs) and loss of DNA repair capacity as measured by the DDIR signature. To test these hypotheses, we used a cohort of 425 patients with early-stage TNBC treated with uniform adjuvant AC on the SWOG S9313 protocol.
METHODS
Patients
Patient selection, signature performance, and data analysis are reported according to Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) criteria.14 Breast tumor sections from paraffin blocks collected prospectively from S9313 participants were used for this study. In S9313, patients with either high-risk (tumor size 1 cm or larger) node-negative or node-positive breast cancer were randomly assigned to one of two equivalent dose schedules of AC chemotherapy, given either sequentially or concurrently.15 There was no significant difference in disease-free survival (DFS) or overall survival (OS) between arms.15 Study population and treatment schedule details are provided in the Protocol. Investigators performed human investigations after approval by a local human investigations committee and in accord with an assurance filed with and approved by the Department of Health and Human Services. Informed consent from each participant or each participant’s guardian was obtained. The current translational work was reviewed and approved by the National Cancer Institute Cancer Therapy Evaluation Program’s Breast Cancer Steering Committee.
Estrogen receptor and progesterone receptor were determined locally and centrally (Allred scoring method; a score of 0 was considered negative). Human epidermal growth factor receptor 2 was determined centrally by immunohistochemistry and fluorescence in situ hybridization.16 TNBC was defined as estrogen receptor and progesterone receptor negative (on both local and central review) and human epidermal growth factor receptor 2–negative in accordance with 2013 ASCO–College of American Pathologists testing guidelines.17 Laboratories performing biomarker analyses were blinded to patient characteristics and outcomes. Selection of the 425 patients with TNBC has been described previously.18
Gene Expression Profiling
As described previously, microarray profiling was performed on total RNA extracted from pretreatment formalin-fixed paraffin-embedded breast tissue sections using the High Pure RNA Paraffin Kit (Roche Diagnostics, Mannheim, Germany) in a Clinical Laboratory Improvement Amendments–certified laboratory (Almac Diagnostics, Craigavon, UK).13 Tumor macrodissection per standard operating procedure for the DDIR signature was not possible; therefore, RNA was extracted from the whole tissue section.13 RNA was amplified using the WT-Ovation FFPE RNA Amplification System (NuGEN Technologies, San Carlos, CA) and hybridized to the Xcel Array (Protocol). Samples were randomly assigned to extraction and amplification batches on the basis of clinical and technical factors to ensure a balance of samples across the study while minimizing bias and batch effects.
Generation of DDIR Signature Scores
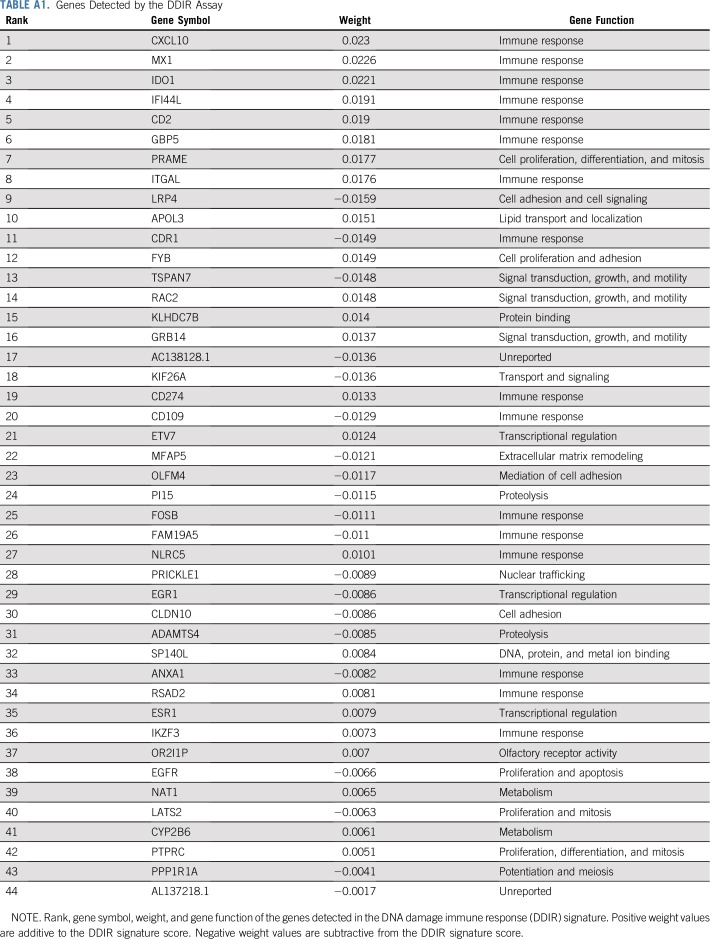
Microarray quality control analysis was performed to identify samples of suitably high quality before application of the DDIR signature, with appropriate analysis conducted to ensure no batch effects were observed. Each sample was independently corrected for background noise and normalized using a predefined quantile normalization model, and median summarization of probes to genes was calculated before applying the DDIR algorithm as described previously.13 A potential limitation of the DDIR assay published in the original manuscript was the requirement for macrodissected tissue.13 In clinical studies, such as the current one, tumor sections may have already been archived and may not be suitable for macrodissection. We therefore used an independent technical data set to identify a correction factor for non-macrodissected material (Protocol). Subsequently, DDIR scores from the S9313 cohort were adjusted for tumor percentage as defined by the central pathologist to account for non-macrodissected tissue samples and to control the proportion of tumor cells versus other cell types (Protocol). A previously published and predefined threshold of 0.3681 was used to define signature positivity.13 Appendix Table A1 (online only) lists the 44 genes composing the DDIR signature.
Immunoregulatory Gene Expression and DDIR Status
Differential gene expression analysis was performed between DDIR signature-positive and DDIR signature-negative patients. Differentially expressed genes (DEGs) are defined on the basis of a fold change > 1.5 and an adjusted P value of .05 between DDIR signature-positive and DDIR signature-negative patients. Functional analysis of the resulting gene list allowed for the identification of genes and biologic processes linked to an immune-related function. Additional information is provided in the Protocol.
sTILs Assessment
Histopathologic determination of sTILs density was assessed using a single hematoxylin and eosin–stained invasive tumor section. Slide reviews were jointly performed by two independent breast histopathologists (S.B. and Y.G.-P.), who were blinded to outcome information, according to previously described criteria.19,20 sTILs density is reported as a percentage estimate in increments of 10.
Statistical Analyses
DFS was defined as the time from registration to first invasive recurrence (local, regional, or distant), new primary invasive cancer in the contralateral breast, or death from any cause. OS was defined as the time from registration to death from any cause. Patients were censored on the date of last contact if an event had not been observed. Survival was assessed by the Kaplan-Meier method. Markers were tested for prognostic effect on DFS and OS using a Cox regression model and likelihood ratio tests adjusting for randomly assigned treatment, nodal status (positive/negative), and tumor size. The C-statistic, which is interpretable as the area under the curve in a receiver operating characteristic model and ranges from chance (0.50) to perfect (1.0), is reported. All reported P values and CIs are from two-sided tests. Statistical testing was performed in Stata version 15.1.
The estimated effect of DDIR status and sTILs density, both separately and together, was tested in a multivariable Cox model adjusted for nodal status, tumor size, and randomly assigned treatment. The Cox proportional hazards assumption was verified using a statistical test based on the Schoenfeld residuals. DDIR signature was also investigated as a continuous predictor of both DFS and OS (Protocol).
RESULTS
Identification of the Study Population
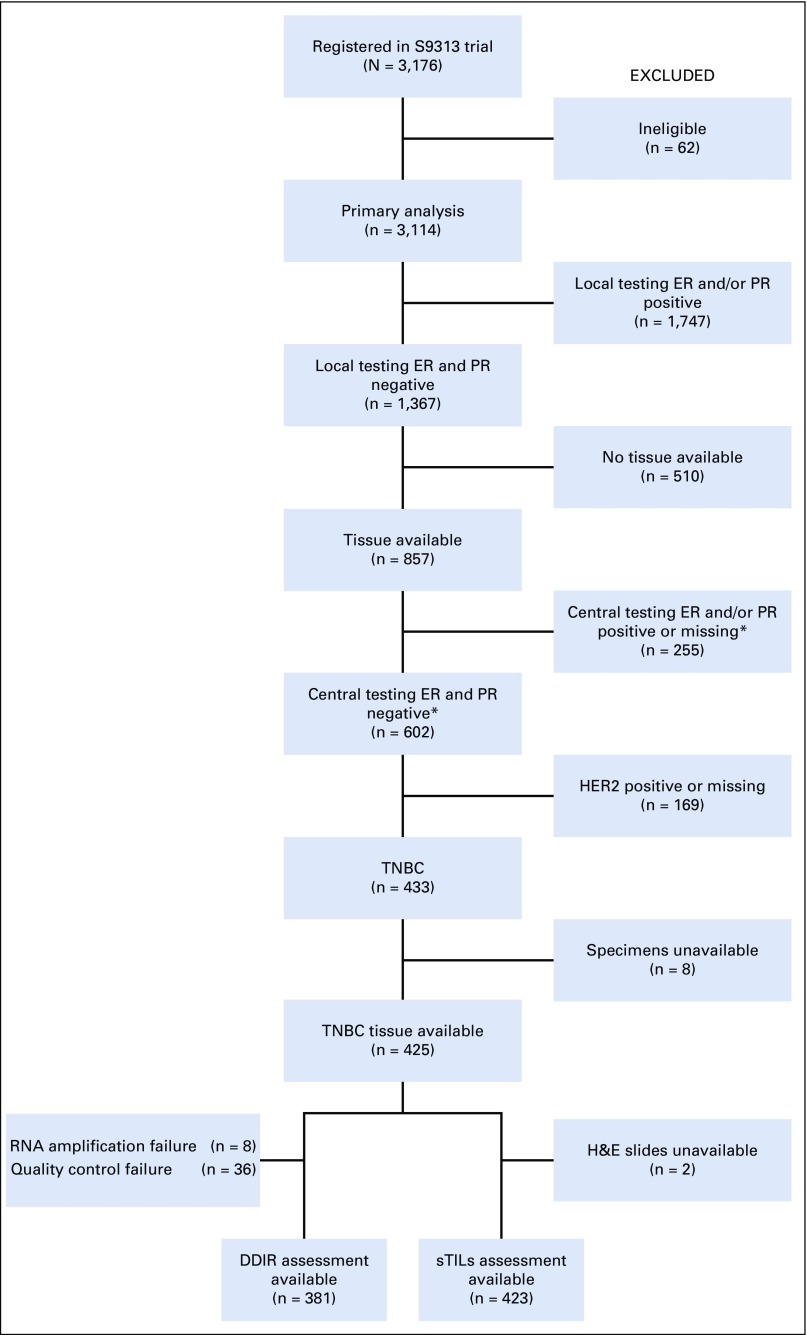
Selection of the 425 patients with TNBC from S9313 is provided in Figure 1. We have reported previously that DFS and OS were similar for participants of S9313 with and without archived tissue specimens.21
FIG 1.
Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) diagram showing biomarker study subset of SWOG S9313 trial. (*) Central ER and PR testing were performed using the Allred scoring method. For the purpose of this biomarker study, ER and/or PR score > 0 was considered positive. DDIR, DNA damage immune response; ER, estrogen receptor; H&E, hematoxylin and eosin; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor; sTILs, stromal tumor-infiltrating lymphocytes; TNBC, triple-negative breast cancer.
Patient Demographics
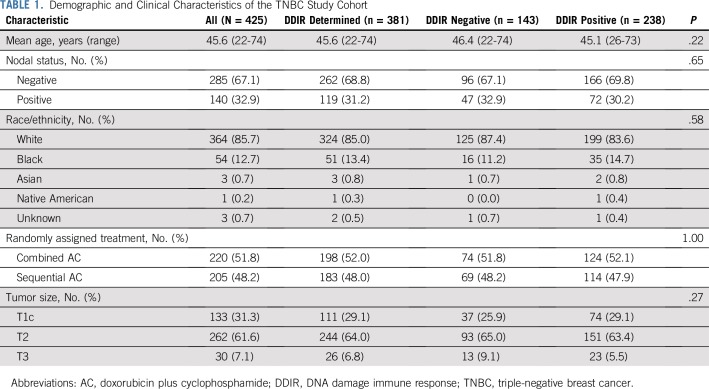
Demographic and clinical characteristics of the 425 patients with TNBC are described in Table 1. Median age at diagnosis was 46 years, and 33% of patients were node positive. At a median follow-up of 12.6 years, there were 166 DFS and 129 OS events.
TABLE 1.
Demographic and Clinical Characteristics of the TNBC Study Cohort
Biomarker Result Availability
DDIR assessment was available for 89.6% (381 of 425 patients), and sTILs assessment was available for 99.5% (423 of 425 patients; reasons for biomarker unavailability are provided in the Protocol and Fig 1). Descriptive characteristics of DDIR scores and sTILs percentages are listed in Appendix Table A2 (online only) overall and by tumor characteristics. Baseline patient characteristics were similar between the full cohort (N = 425) and the DDIR-determined cohort (n = 381; Table 1). There was no difference in DFS or OS by DDIR status known or unknown (log-rank P = .84 for DFS and P = .95 for OS).
Association of DDIR Signature Status With Patient Outcomes
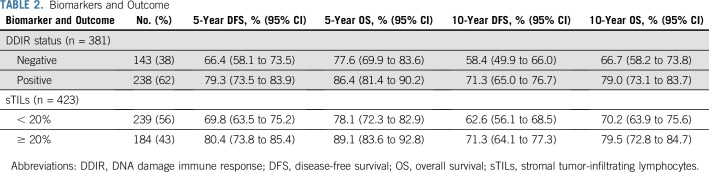
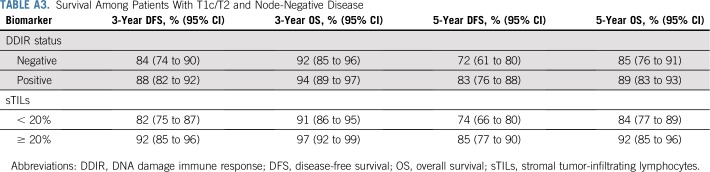
For the 381 patients with available DDIR signature results, 62% (238 of 381) were DDIR positive (on the basis of the predefined threshold of 0.3681). DDIR score was not associated with patient age, ethnicity, nodal status, tumor size, or randomly assigned treatment regimen. Five-year and 10-year DFS and OS estimates are listed in Table 2 by DDIR positivity and separately by sTILs percentage with a cutoff of 20%. When focusing on patients with T1c/T2 node-negative disease, 3- and 5-year DFS and OS estimates by DDIR status and sTILs percentage are listed in Appendix Table A3 (online only). Three and 5-year OS were 94% and 89%, respectively, for patients with T1c/T2 node-negative disease and DDIR positivity.
TABLE 2.
Biomarkers and Outcome
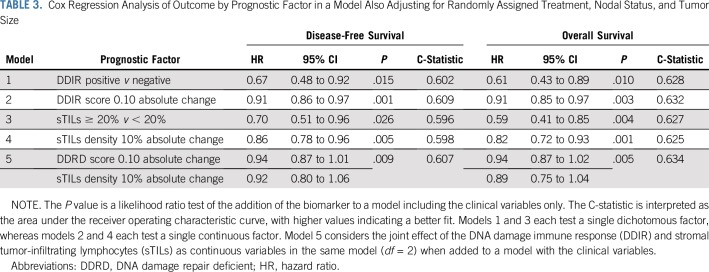
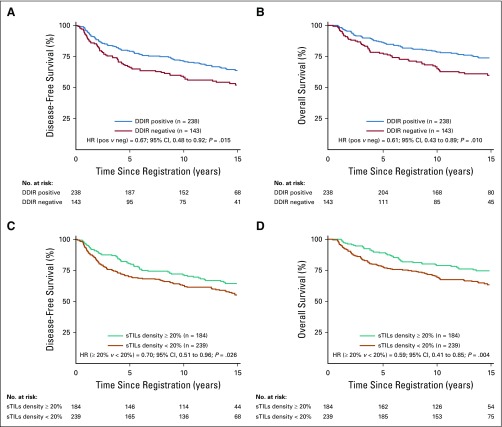
DDIR signature positivity was associated with improved DFS (hazard ratio [HR], 0.67; 95% CI, 0.48 to 0.92; P = .015) and OS (HR, 0.61; 95% CI, 0.43 to 0.89; P = .010), adjusting for treatment arm, nodal status, and tumor size (Table 3, model 1, Figs 2A and 2B). In addition, when modeled as a continuous variable, a higher DDIR score was associated with improved DFS (HR, 0.91; 95% CI, 0.86 to 0.97 for a 0.10 increase in score; P = .001) and improved OS (HR, 0.91; 95% CI, 0.85 to 0.97 for a 0.10 increase in score; P = .003; Table 3, model 2).
TABLE 3.
Cox Regression Analysis of Outcome by Prognostic Factor in a Model Also Adjusting for Randomly Assigned Treatment, Nodal Status, and Tumor Size
FIG 2.
Kaplan-Meier survival analyses predicting (A) disease-free survival and (B) overall survival by DNA damage immune response (DDIR) signature status, and (C) disease-free survival and (D) overall survival by stromal tumor-infiltrating lymphocytes (sTILs) density. DDIR status is depicted as positive (pos) versus negative (neg) on the basis of the predefined cutoff of 0.3681. sTILs density is differentiated into ≥ 20% and < 20% subgroups. HR, hazard ratio.
Association of sTILs With Patient Outcomes
For the 423 patients with available sTILs density results, 43% (184 of 423) demonstrated sTILs density ≥ 20%. sTILs density was not associated with patient age, ethnicity, T stage, nodal status, or randomly assigned treatment regimen. Increasing sTILs density was positively associated with better DFS and OS. When sTILs density was assessed as a binary variable (≥ 20% v < 20%), patients with sTILs density ≥ 20% exhibited significantly improved DFS and OS in comparison with patients with sTILs density less than 20% (HR, 0.70; 95% CI, 0.51 to 0.96; P = .026, and HR, 0.59; 95% CI, 0.41 to 0.85; P = .004, respectively; Table 3, model 3, Figs 2C and 2D). In addition, when assessed as a continuous variable, higher sTILs density was associated with improved outcomes. For every 10% increase in sTILs density, there was improvement in DFS (HR, 0.86; 95% CI, 0.78 to 0.96; P = .005) and OS (HR, 0.82; 95% CI, 0.72 to 0.93; P = .001; Table 3, model 4).
Association of DDIR Scores With sTILs
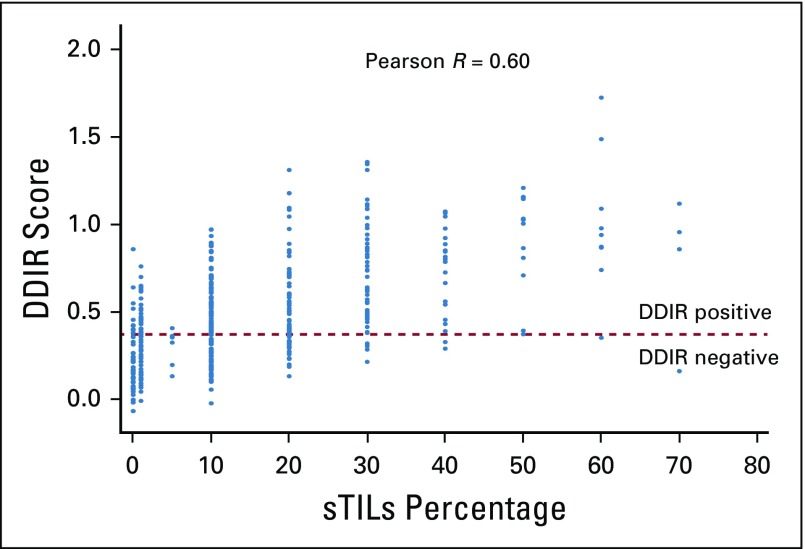
DDIR signature score and sTILs density were moderately correlated, whereby higher DDIR scores were associated with increased sTILs density (Pearson r = 0.60; Fig 3), reflecting the correlation between the underpinning biology of the two biomarkers.
FIG 3.
Scatterplot depicting the correlation of DNA damage immune response (DDIR) scores with stromal tumor-infiltrating lymphocytes (sTILs) density percentage in increments of 10 percentage points.
Joint Association of DDIR Status and sTILs With Patient Outcomes
Table 3 lists Cox regression analysis of outcome by prognostic factor in a model also adjusting for randomly assigned treatment, nodal status, and tumor size. The P value is a likelihood ratio test of the model against clinical-pathologic variables only. When both continuous DDIR and sTILs are modeled jointly in the same model, the C-statistic shows no improvement over either variable alone, likely because of the strong correlation between the two (Table 3, model 5).
Association of Immune Gene Expression With DDIR Signature Status
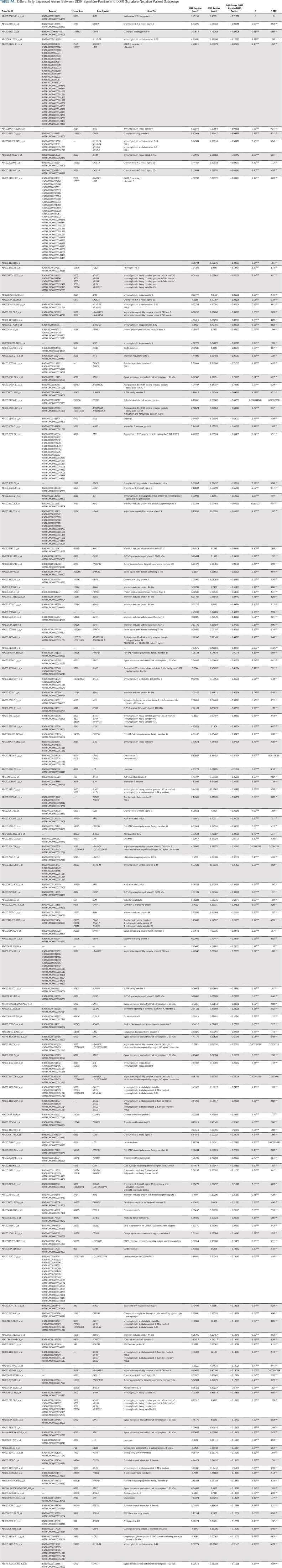
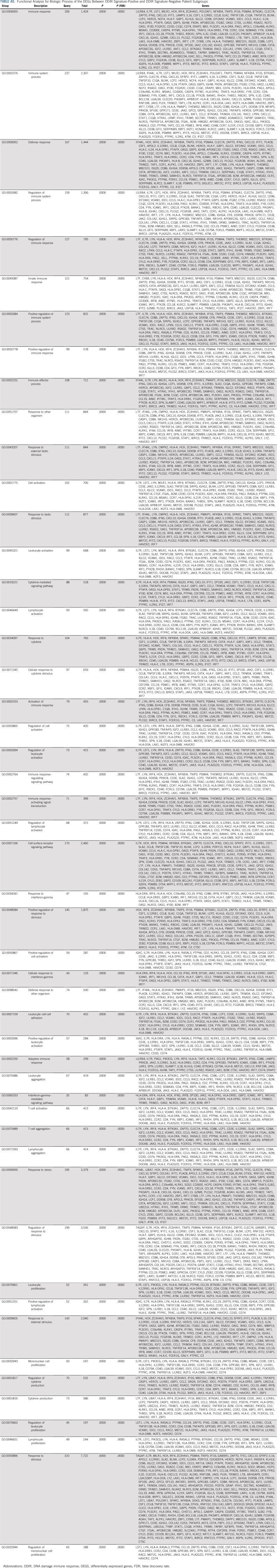
Because the DDIR score was positively correlated with sTILs density, we explored the relationship between DEGs by DDIR status. In total, 860 probe sets were differentially expressed (777 upregulated and 83 downregulated in DDIR-positive patients). This equates to 422 unique genes with significant differential expression between DDIR-positive and DDIR-negative patients (Appendix Table A4, online only). Forty-nine percent of DEGS (206 of 422; mostly upregulated in DDIR-positive patients) were linked to immune response, suggesting this to be the most significant biologic process in this cohort (Appendix Table A5, online only). Other relevant biologic processes that were upregulated in DDIR-positive tumors were activation/regulation of immune response and cytokine-mediated signaling.
DISCUSSION
In this translational study, using a prespecified cutoff, we observed that 62% of patients with TNBC treated with adjuvant AC in S9313 had DDIR signature-positive cancers. Importantly, in the context of adjuvant AC chemotherapy, DDIR positivity was strongly associated with improved DFS (HR, 0.67) and OS (HR, 0.61), independently of other clinicopathologic variables. Moreover, the presence of sTILs was associated with better outcomes, a finding consistent with several prior studies reporting a better prognosis in association with high levels of sTILs for patients with TNBC who receive adjuvant chemotherapy.19,20,22,23 A recent pooled analysis of nine adjuvant clinical trials demonstrated the prognostic role of sTILs in patients with early-stage TNBC treated with adjuvant chemotherapy.22 S9313 was not part of this pooled analysis and provides independent validation of these findings. Furthermore, in line with the immune response biology associated with the DDIR signature, a positive correlation between sTILs and DDIR score (r = 0.60) was observed, which masked detection of each variable’s independent effect on DFS in a joint model.
Our results may have clinical implications. First, we hypothesized that DDIR positivity might result in better clinical outcomes in patients with TNBC, because chemotherapy would be expected to be more active against tumors with DNA repair deficiency. Indeed, suboptimal outcomes were observed in patients with DDIR signature negativity, with almost 35% suffering a DFS event by 5 years (5-year DFS, 66%). These patients may be better served by investigation of therapies alternative to or in addition to anthracycline-based chemotherapy within clinical trials (eg, antibody-drug conjugates, drugs targeting specific genomic aberrations). Molecular drivers of tumors with low DDIR scores should be investigated further to identify better treatment strategies for these patients, although it is possible that this group could represent a heterogeneous population with several underpinning biologies. Conversely, patients with DDIR positivity and lower clinical-pathologic risk (T1c/T2N0) experienced 3-year DFS of 88% and OS of 94% when treated with AC chemotherapy alone. Identification of such patients destined to have good outcomes may pave the way for de-escalation treatment trials (shorter chemotherapy regimens, nonanthracycline regimens).
Second, the apparent importance of immune regulation in the prognosis for these chemotherapy-treated patients suggests a few important hypotheses. It is known that the presence of sTILs is associated with neo/adjuvant chemotherapy response and good prognosis in TNBC.19,20,23 Our data suggest that DDIR signature positivity may provide a more objective yet similar effect and may identify a larger proportion of patients with favorable prognosis. Sixty-two percent of patients had tumor DDIR signature positivity, compared with 43% of patients with sTILs ≥ 20 (36% of patients with sTILs ≥ 30%). Five-year DFS for patients with DDIR positivity and sTILs ≥ 20% were similar (79% and 80%, respectively) in the entire population and when evaluated in patients with node-negative T1c/T2 disease (83% and 85%, respectively). We also demonstrate that activation/regulation of immune response is the most significant biologic process in DDIR-positive tumors, suggesting that DDIR-positive tumors may represent a target population for immune checkpoint treatment.
Recently reported encouraging data support the efficacy of immune checkpoint inhibitors (ICIs) in patients with metastatic TNBC.24,25 In many cancer types, several possible predictive factors have been associated with response and favorable outcomes after ICI therapies, including programmed death-ligand 1 expression in tumor cells and/or immune cells, sTILs density, tumor mutational burden, human leukocyte antigen status, and GI microbiome diversity.25,26 DDIR signature might also serve as a predictive factor for this treatment class. Constitutive activation of the cGAS-STING innate immune pathway in DDIR-positive cancer cells has been identified previously.10 Chronic STING activation as a result of ineffective DNA repair may promote an inflamed yet immunosuppressive microenvironment that could then potentially be targeted with ICIs.11,12 Indeed, a DNA damage-sensing pathway signature was shown recently to be associated with response to neoadjuvant pembrolizumab in the I-SPY2 trial.27 Poly-(ADP-ribose)polymerase (PARP) inhibitors have demonstrated clinical efficacy in BRCA-mutant metastatic breast cancer.28,29 In BRCA wild-type tumors, the BRCAness phenotype may be associated with sensitivity to PARP inhibitor therapy, and biomarkers such as DDIR that can identify this phenotype have the potential to aid additional investigation of PARP inhibitors in BRCA wild-type cancers.30,31
Our study has certain limitations. All patients in the trial received adjuvant AC chemotherapy. It cannot be determined whether DDIR is prognostic in the absence of chemotherapy, or if it is predictive of benefit from AC chemotherapy. Previous retrospective studies have noted that DDIR signature is not prognostic in patients who did not receive neo/adjuvant chemotherapy.13 These data suggest that our observed association of DDIR positivity with better outcomes is likely indicative of the predictive nature of this signature in the context of AC chemotherapy. Details on the nature of DFS events (distant, locoregional, contralateral breast cancer, and so forth) from S9313 are not readily available; therefore, we were unable to look at other end points such as distant DFS or invasive DFS. S9313 was conducted before the demonstration of taxane activity in the adjuvant setting. Thus, we cannot speculate on the impact of DDIR status on taxane benefit, which is currently part of standard neo/adjuvant chemotherapy for TNBC.32,33 Similarly, data regarding the association of DDIR signature with response to platinum agents in breast cancer are lacking. Although the prognosis of patients with DDIR positivity is superior to those with DDIR negativity, there are currently insufficient data to clinically modify the treatment of TNBC in the adjuvant setting on the basis of DDIR status.
In summary, we have identified the DDIR signature and sTILs as predictive of improved DFS and OS for TNBC in the context of adjuvant AC chemotherapy. There is a clear need to further explore the DDIR signature in prospective stratified studies to elucidate the assay’s ability to predict response to DNA-damaging therapies, such as anthracycline, platinum agents, and PARP inhibitors, in breast cancer. Given the direct link with cGAS/STING biology, additional studies are also warranted to assess the ability of the DDIR signature to predict response to immune checkpoint-targeted therapies.
ACKNOWLEDGMENT
We gratefully acknowledge the late Robert Livingston, MD, for his important contributions to SWOG and the clinical trial study S9313.
APPENDIX
Methods
S9313 trial details.
In S9313, 3,125 women with early-stage breast cancer were recruited from April 1994 through May 1997. Patients were required to have one to three involved nodes or to have high-risk node-negative breast cancer, which was defined as a primary tumor > 2 cm in size or > 1 cm for tumors that were both estrogen and progesterone receptor negative. Patients were randomly assigned to receive either (arm I) doxorubicin and cyclophosphamide given in combination (AC; doxorubicin 54 mg/m2 and cyclophosphamide 1.2 g/m2 intravenously [IV] every 3 weeks for six cycles) or (arm II) AC in sequence (A3C; doxorubicin 40.5 mg/m2 IV days 1 and 2 of a 21-day cycle for four cycles, followed by cyclophosphamide 2.4 g/m2 IV every 2 weeks for three cycles). Granulocyte colony-stimulating factor was administered on day 3 after cyclophosphamide doses and continued until day 12 or until a postnadir count of 10,000 granulocytes.15 There was no difference in disease-free survival or overall survival for patients treated on the two arms.15
Central human epidermal growth factor receptor 2 testing.
Both human epidermal growth factor receptor 2 (HER2) fluorescence in situ hybridization and immunohistochemistry were performed using standard methodology, the details of which have already been published (Jansen MP, et al: J Clin Oncol 25:662-668, 2007).16 HER2 fluorescence in situ hybridization was performed using dual-color, direct-label fluorescent in situ hybridization using the TOP2A/CEP17 probe set (Abbott Molecular/Vysis, Des Plaines, IL). HER2 immunohistochemistry procedures were performed on a Dako Autostainer (Dako, Carpinteria, CA). A polyclonal antibody to HER2 (A0485; Dako) was applied at a 1:200 dilution in phosphate-buffered saline to sections and was incubated for 40 minutes at room temperature. With intervening wash steps in phosphate-buffered saline, slides were incubated for 30 minutes at room temperature in a rabbit-specific, labeled polymer (EnVision15; Dako), which was followed by 10 minutes at 37°C in a solution that contained 3% hydrogen peroxide and 3,3-diaminobenzidine. Slides were counterstained with hematoxylin.
Immunostained slides were scored according to a modification of the scoring system approved by the US Food and Drug Administration (Gown AM, et al: Mod Path 21:1271-1277, 2008). Only invasive carcinoma was scored among the neoplastic cells. For tumor cells only, membrane staining intensity and pattern were evaluated by using the semiquantitative scale of 0 to ≥ 3.
Gene expression profiling.
Hematoxylin and eosin (H&E)–stained sections from each patient case were centrally pathology-reviewed by a specialized breast clinical pathologist to quantify the proportion of tumor material and viable tumor cells within the specimen. Total RNA was extracted from formalin-fixed paraffin-embedded tissue curls using the High Pure RNA Paraffin Kit (Roche Diagnostics GmbH, Mannheim, Germany). RNA (required concentration, 12.5 ng/µL) was converted into complementary DNA (cDNA), amplified (required concentration, 140 ng/µL), and converted into a single-stranded form using the SPIA technology of the WT-Ovation FFPE RNA Amplification System (NuGEN Technologies, San Carlos, CA). The amplified cDNA was then fragmented, biotin labeled using the FL-Ovation cDNA Biotin Module (NuGEN Technologies), and hybridized to the Xcel. Arrays were scanned using the Affymetrix Genechip Scanner 7G (Affymetrix, Santa Clara, CA). Stratagene Universal Human Reference samples and ES-2 cell lines were used as process control measures, monitored using statistical process control charts. Quality control (QC) analysis was performed in each cohort.
Microarray QC: Microarray QC analysis was performed to identify samples of suitably high quality before application of the DNA damage immune response (DDIR) assay. Samples were preprocessed using the robust multiarray average. QC assessment comprised a combination of the following metrics:
Array image analysis: Array image analysis analyzes Affymetrix CEL files (containing raw intensity data) and assesses if large deviations in background values or unusual patterns in probe intensities exist that may indicate the presence of artifacts or problems with hybridization (such as leakage from the array or uneven washing).
GeneChip QC: GeneChip QC examines a number of control parameters from the RPT files as supplied by Affymetrix. Full descriptions of these control parameters are available from the Affymetrix Statistical Algorithms Description Document. This assessment facilitates the monitoring of profile quality and allows for the evaluation of assay and hybridization performance. Affymetrix has specified absolute thresholds (lower limit, upper limit, or both limits) for a number of these parameters. In addition, it is expected that data should be comparable for the majority of studies; therefore, an assessment of overall profile similarity is performed using thresholds based on median absolute deviation. Any values outside median ± 3.5 sigma (sigma defined as 1.4826 times the median absolute deviation) for that metric will be flagged as potential QC outliers.
Principal component analysis: Principal component analysis is used to detect outliers and (known or unknown) systematic structures using the robust multi-array average preprocessed expression data using the Hotelling T2 and residual Q method.
Intensity distribution analysis: Histogram plots of normalized expression data are constructed to visualize the distribution patterns of the expression data. Distribution similarity is assessed using the Kolmogorov-Smirnov test. Clinical samples routinely fail data analysis QC assessment if they are flagged in two or more of the microarray QC metrics outlined previously in the text or if a sample had a percent present value below 20%.
DDIR signature scoring adjusted for tumor percentage.
To use non–macro-dissected tumor material as input for the DDIR assay, a generalized linear model was developed within an independent technical cohort (n = 156 samples), in which tumor titration data using six ovarian samples were assessed at a number of titration points (0%, 25%, 50%, 75%, and 100%). This fitted model was subsequently applied to the DDIR scores of samples within the S9313 cohort to provide DDIR predictions, adjusted for the effects of tumor percentage resulting from non–macro-dissected sample material.
DDIR assessment as a continuous variable.
DDIR assay scores were transformed to a representative continuous scale of 0 to 1 and evaluated against disease-free survival and overall survival end points using hazard ratios from Cox proportional hazards regression, Harrell’s C-index, and the area under the receiver operating characteristic curve.
Tumor-infiltrating lymphocytes.
Histopathologic analysis of stromal tumor-infiltrating lymphocytes (sTILs) percentages was performed on a single full-face H&E-stained tumor section using previously described criteria.19,20,23 The density (%) of sTILs was recorded for stromal areas. sTIL density is defined as the percentage of tumor stroma containing infiltrating lymphocytes. Areas of in situ carcinoma and crush artifacts were not included. Histopathologic evaluation of sTILs was jointly performed by two breast pathologists (S.B. and P.P.), who were blinded to the clinical information, including treatment allocation and outcomes. All tumors were evaluated jointly, and the results were reported in increments of 10. A score of 0 was defined as 0% to 1%, all other estimates being rounded up to the next highest decile (ie, 11% to 20% represents a sTIL score of 20).
Differential expression analysis.
Preprocessed data were filtered to remove all Affymetrix AFFX control probe sets and uninformative probe sets within the background region (background filtering). Background filtering was performed on the basis of a combination of the expression and the variance, selecting probe sets with an average expression above the threshold defined by σBg at a significance level of α. The variance selects probe sets with a variance above that of the background. A statistical t test was performed and corrected for multiple tests applied using the false discovery rate. Differentially expressed genes were defined by a fold change > 1.5 and an adjusted P value of .05. Functional enrichment analysis was performed on the resulting gene list to provide insight into the processed biologic associated with the genes in the list.
Results
DDIR assessment was not available for 44 of the 425 patients (RNA amplification failure, eight; data QC failure, 22; and bridging samples, 14). Assessment of sTILs was not available for two of the 425 patients because of unavailability of H&E slides.
TABLE A1.
Genes Detected by the DDIR Assay
TABLE A2.
Distributions of DDIR Score and sTILs Density
TABLE A3.
Survival Among Patients With T1c/T2 and Node-Negative Disease
TABLE A4.
Differentially Expressed Genes Between DDIR Signature-Positive and DDIR Signature-Negative Patient Subgroups
TABLE A5.
Functional Analysis for Biologic Process of the DEGs Between DDIR Signature-Positive and DDIR Signature-Negative Patient Subgroups
Footnotes
Presented in part at the 2017 Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, on June 2-6, 2017.
Supported by National Cancer Institute/National Clinical Trials Network Grants U10CA180888 and U10CA180819); the American Society of Clinical Oncology Advanced Clinical Cancer Research Award (P.S.); National Cancer Institute Cancer Center Support Grant P30 CA168524 using the Biospecimen Repository Core Facility; the Breast Cancer Research Foundation (D.F.H. and P.S.); National Cancer Institute Support Grant P30 CA015704 (H.M.L.); SWOG U01 Grant (D.T.); the Innovate UK Small Business Research Initiative (971337); Amgen; the Eileen Stein Jacoby Fund (A.K.G.); Midwest Cancer Alliance Partners Advisory Board Grant from Children’s Mercy (A.K.G.); and The University of Kansas Cancer Center (P.S. and A.K.G.).
Clinical trial information: Int0137 (The trial predates Clinicaltrial.Gov website establishment).
AUTHOR CONTRIBUTIONS
Conception and design: Priyanka Sharma, William E. Barlow, Steven M. Walker, Denis P. Harkin, Peggy Porter, Debu Tripathy, Gabriel N. Hortobagyi, Daniel F. Hayes
Financial support: Denis P. Harkin, Daniel F. Hayes
Administrative support: Denis P. Harkin, Gemma E. Logan, Lajos Pusztai, Gabriel N. Hortobagyi
Provision of study material or patients: Hannah M. Linden, Gabriel N. Hortobagyi
Collection and assembly of data: Priyanka Sharma, William E. Barlow, Andrew K. Godwin, Laura A. Knight, Steven M. Walker, Sunil Badve, Yesim Gökmen-Polar, Kamilla Isakova, Peggy Porter, Daniel F. Hayes
Data analysis and interpretation: Priyanka Sharma, William E. Barlow, Andrew K. Godwin, Eileen E. Parkes, Laura A. Knight, Steven M. Walker, Richard D. Kennedy, Denis P. Harkin, Gemma E. Logan, Christopher J. Steele, Shauna M. Lambe, Sunil Badve, Harsh B. Pathak, Hannah M. Linden, Lajos Pusztai, Alastair M. Thompson, Debu Tripathy, Gabriel N. Hortobagyi, Daniel F. Hayes
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST AND DATA AVAILABILITY STATEMENT
Validation of the DNA Damage Immune Response Signature in Patients With Triple-Negative Breast Cancer From the SWOG 9313c Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Priyanka Sharma
Consulting or Advisory Role: TapImmune, Almac Diagnostics, AstraZeneca, Novartis, Pfizer, Myriad Genetics, Puma Biotechnology
Research Funding: Novartis (Inst), Celgene (Inst), Bristol-Myers Squibb (Inst), Cosmo Biosciences (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Almac Diagnostics
William E. Barlow
Research Funding: AbbVie (Inst), Merck (Inst), AstraZeneca (Inst)
Andrew K. Godwin
Honoraria: Sinochips Kansas
Consulting or Advisory Role: NanoString Technologies, Personal Genome Diagnostics
Research Funding: BioFluidica (Inst)
Travel, Accommodations, Expenses: West China Cooperative Network of Pharmacy, Personal Genome Diagnostics
Eileen E. Parkes
Consulting or Advisory Role: Almac Diagnostics
Travel, Accommodations, Expenses: Almac Diagnostics
Laura A. Knight
Employment: Almac Diagnostics
Steven M. Walker
Employment: Almac Diagnostics
Patents, Royalties, Other Intellectual Property: Named inventor on Almac Diagnostics patents
Richard D. Kennedy
Employment: Almac Diagnostics
Honoraria: AstraZeneca, Tesaro
Denis P. Harkin
Employment: Almac Diagnostics
Patents, Royalties, Other Intellectual Property: Patents
Gemma E. Logan
Employment: Almac Diagnostic Services
Christopher J. Steele
Employment: Almac Diagnostics
Shauna M. Lambe
Employment: Almac Diagnostics
Sunil Badve
Leadership: YeSSGenomics
Stock and Other Ownership Interests: YeSSGenomics
Honoraria: Athenex
Consulting or Advisory Role: ClearLight
Speakers' Bureau: Genomic Health, Targos Molecular Pathology
Research Funding: Dako/Agilent Technologies
Patents, Royalties, Other Intellectual Property: EarlyR: signature for ER+ breast cancer (Inst), E-Score for predicting recurrence of DCIS (Inst)
Yesim Gökmen-Polar
Travel, Accommodations, Expenses: Thermo Fisher Scientific
Hannah M. Linden
Leadership: Evolent (I)
Stock and Other Ownership Interests: Evolent (I)
Consulting or Advisory Role: Genomic Health, Context Therapeutics, Syndax, AstraZeneca, Eisai
Research Funding: Eisai, Sanofi/Aventis (Inst), GTx (Inst)
Lajos Pusztai
Honoraria: Merck, AstraZeneca/MedImmune, Pfizer, Syndax, Almac Diagnostics, Pieris Pharmaceuticals, Genentech, Immunomedics, Eisai, Seattle Genetics/Astellas
Consulting or Advisory Role: H3 Biomedicine, Merck, Novartis, PierianDx, Seattle Genetics, Syndax, Athenex
Speakers' Bureau: bioTheranostics
Research Funding: Merck, Genentech, Seattle Genetics, AstraZeneca
Alastair M. Thompson
Honoraria: Novartis (I), Pfizer
Research Funding: Genentech/Roche
Travel, Accommodations, Expenses: Novartis (I), Pfizer
Debu Tripathy
Consulting or Advisory Role: Novartis, Nektar, Pfizer, Sellas Life Sciences, GlaxoSmithKline, Genomic Health, Polyphor
Research Funding: Novartis (Inst), Polyphor (Inst)
Travel, Accommodations, Expenses: Novartis
Gabriel N. Hortobagyi
Consulting or Advisory Role: Novartis, Peregrine Pharmaceuticals, Agendia
Research Funding: Novartis (Inst)
Travel, Accommodations, Expenses: Novartis
Daniel F. Hayes
Stock and Other Ownership Interests: OncImmune, InBiomotion, Cepheid, Freenome, Cellworks, CVS Caremark Breast Cancer Expert Panel, Agendia
Research Funding: AstraZeneca (Inst), Pfizer (Inst), Merrimack (Inst), Menarini Silicon Biosystems (Inst)
Patents, Royalties, Other Intellectual Property: Royalties from licensed technology; Diagnosis and Treatment of Breast Cancer. Patent No. US 8,790,878 B2. Date of Patent: July 29, 2014. Applicant Proprietor: University of Michigan. Daniel F. Hayes, MD, is designated as inventor/co-inventor; Circulating Tumor Cell Capturing Techniques and Devices. Patent No. US 8,951,484 B2. Date of Patent: February 10, 2015. Applicant Proprietor: University of Michigan. Daniel F. Hayes, MD, is designated as inventor/co-inventor; Title: A method for predicting progression-free and overall survival at each follow-up time point during therapy of metastatic breast cancer patients using circulating tumor cells. Patent No. 05725638.0-1223-US2005008602.
Travel, Accommodations, Expenses: Menarini Silicon Biosystems
Other Relationship: Menarini
No other potential conflicts of interest were reported.
REFERENCES
- 1.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 2.Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 3.Tan DS, Marchió C, Jones RL, et al. Triple negative breast cancer: Molecular profiling and prognostic impact in adjuvant anthracycline-treated patients. Breast Cancer Res Treat. 2008;111:27–44. doi: 10.1007/s10549-007-9756-8. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lips EH, Mulder L, Oonk A, et al. Triple-negative breast cancer: BRCAness and concordance of clinical features with BRCA1-mutation carriers. Br J Cancer. 2013;108:2172–2177. doi: 10.1038/bjc.2013.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4:814–819. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy RD, D’Andrea AD. DNA repair pathways in clinical practice: Lessons from pediatric cancer susceptibility syndromes. J Clin Oncol. 2006;24:3799–3808. doi: 10.1200/JCO.2005.05.4171. [DOI] [PubMed] [Google Scholar]
- 8.Ceccaldi R, Rondinelli B, D’Andrea AD. Repair pathway choices and consequences at the double-strand break. Trends Cell Biol. 2016;26:52–64. doi: 10.1016/j.tcb.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nolan E, Savas P, Policheni AN, et al. Combined immune checkpoint blockade as a therapeutic strategy for BRCA1-mutated breast cancer. Sci Transl Med. 2017;9:eaal4922. doi: 10.1126/scitranslmed.aal4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parkes EE, Walker SM, Taggart LE, et al. Activation of STING-dependent innate immune signaling by S-phase-specific DNA damage in breast cancer. J Natl Cancer Inst. 2016;109:109. doi: 10.1093/jnci/djw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dou Z, Ghosh K, Vizioli MG, et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature. 2017;550:402–406. doi: 10.1038/nature24050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakhoum SF, Ngo B, Laughney AM, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553:467–472. doi: 10.1038/nature25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulligan JM, Hill LA, Deharo S, et al. Identification and validation of an anthracycline/cyclophosphamide-based chemotherapy response assay in breast cancer. J Natl Cancer Inst. 2014;106:djt335. doi: 10.1093/jnci/djt335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97:1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 15.Linden HM, Haskell CM, Green SJ, et al. Sequenced compared with simultaneous anthracycline and cyclophosphamide in high-risk stage I and II breast cancer: Final analysis from INT-0137 (S9313) J Clin Oncol. 2007;25:656–661. doi: 10.1200/JCO.2006.07.0847. [DOI] [PubMed] [Google Scholar]
- 16.Tubbs R, Barlow WE, Budd GT, et al. Outcome of patients with early-stage breast cancer treated with doxorubicin-based adjuvant chemotherapy as a function of HER2 and TOP2A status. J Clin Oncol. 2009;27:3881–3886. doi: 10.1200/JCO.2008.20.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolff AC, Hammond MEH, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 18.Sharma P, Barlow WE, Godwin AK, et al. Impact of homologous recombination deficiency biomarkers on outcomes in patients with triple-negative breast cancer treated with adjuvant doxorubicin and cyclophosphamide (SWOG S9313) Ann Oncol. 2018;29:654–660. doi: 10.1093/annonc/mdx821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 21.Porter PL, Barlow WE, Yeh IT, et al. p27(Kip1) and cyclin E expression and breast cancer survival after treatment with adjuvant chemotherapy. J Natl Cancer Inst. 2006;98:1723–1731. doi: 10.1093/jnci/djj467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loi S, Drubay D, Adams S, et al. Tumor-infiltrating lymphocytes and prognosis: A pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol. 2019;37:559–569. doi: 10.1200/JCO.18.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 24.Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34:2460–2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 26.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 27. Yau C, Wolf D, Brown-Swigart L, et al: Analysis of DNA repair deficiency biomarkers as predictors of response to the PD1 inhibitor pembrolizumab: Results from the neoadjuvant I-SPY 2 trial for stage II-III high-risk breast cancer. Cancer Res 78: 2018 (4 suppl; abstr PD6-14)
- 28.Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation N Engl J Med 377523–533.2017[Erratum: N Engl J Med 2017] [DOI] [PubMed] [Google Scholar]
- 29.Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hastak K, Alli E, Ford JM. Synergistic chemosensitivity of triple-negative breast cancer cell lines to poly(ADP-Ribose) polymerase inhibition, gemcitabine, and cisplatin. Cancer Res. 2010;70:7970–7980. doi: 10.1158/0008-5472.CAN-09-4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Severson TM, Wolf DM, Yau C, et al. The BRCA1ness signature is associated significantly with response to PARP inhibitor treatment versus control in the I-SPY 2 randomized neoadjuvant setting. Breast Cancer Res. 2017;19:99. doi: 10.1186/s13058-017-0861-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]