Abstract
Since the emergence of a novel coronavirus (severe acute respiratory syndrome coronavirus 2) in Wuhan, China, at the end of December 2019, coronavirus disease 2019 has been associated with severe morbidity and mortality and has left world governments, healthcare systems, and providers caring for vulnerable populations, such as pregnant women, wrestling with the optimal management strategy. Unique physiologic and ethical considerations negate a one-size-fits-all approach when caring for critically ill pregnant women with coronavirus disease 2019, and few resources exist to guide the multidisciplinary team through decisions regarding optimal maternal-fetal surveillance, intensive care procedures, and delivery timing. We present a case of rapid clinical decompensation and development of severe acute respiratory distress syndrome in a woman at 31 weeks’ gestation to highlight these unique considerations and present an algorithmic approach to the diagnosis and management of the disease.
Key words: acute SARS-CoV-2, ARDS, coronavirus, COVID-19, pneumonia, pregnancy, respiratory distress syndrome
As of April 3, 2020, there have been 972,303 total cases of coronavirus disease 2019 (COVID-19) with 50,322 deaths (5.2% mortality rate) worldwide, and it is spreading rapidly with a basic reproduction number (R0) of 2–2.5, suggesting that 2–3 people will become infected from an index patient.1 , 2 Although the United States now has the highest number of cases (239,279), the US mortality rate is less than half than that seen worldwide at 2.3% (5443 confirmed deaths) and the hospitalization rate remains low at 24.1 per 100,000 population.3 These statistics may encourage skeptics eager to challenge the severity of this public health crisis. However, the rates of critical illness and mortality associated with COVID-19 among pregnant women—a potentially highly vulnerable population—remain unclear.4, 5, 6, 7
We present the clinical challenges and potential strategies for optimal maternal-fetal surveillance, intensive care procedures, and delivery timing in a case of a pregnant woman at 31 weeks’ gestation who presented to a tertiary care hospital in Cincinnati, Ohio, with COVID-19 symptoms, laboratory abnormalities, and chest-imaging findings immediately before the development of rapid clinical decompensation and severe acute respiratory distress syndrome (ARDS) requiring prolonged mechanical ventilation and ultimately indicated preterm delivery.
Case Study
A 39-year-old white G6 P2031 with a 31.0-week live singleton intrauterine gestation conceived through in vitro fertilization required admission on March 24, 2020, from the emergency department of a tertiary care center in Cincinnati, Ohio, due to complaints of 5 days of worsening nonproductive cough, shortness of breath, fever, and malaise. Four days before admission (March 20, 2020; COVID-19 day 1, Figure 1 ), she had been discharged from the emergency department after workup for a milder presentation of these symptoms that included normal findings on chest x-ray examination, normal vital sign assessment, and normal results of a respiratory viral pathogen laboratory analysis including influenza A and B rapid screening. Of note, a nasopharyngeal swab for COVID-19 reverse transcriptase polymerase chain reaction (RT-PCR) was sent to the Ohio Department of Health at that time, but results did not return for 8 days and were still pending when she re-presented on March 24, 2020 (COVID-19 day 5). She had previously established early and complete prenatal care through the Maternal-Fetal Medicine (MFM) service owing to underlying mild myotonic dystrophy (without cardiomyopathy), bicuspid aortic valve (without aortic dilation, stenosis, or regurgitation), history of 2 previous low-transverse cesarean deliveries, and history of a previous mild cerebrovascular accident while on combined oral contraceptives. Her pregravid body mass index was 24.7 kg/m2, and she denied any tobacco or illicit substance abuse. Serial surveillance with obstetric ultrasound imaging and maternal echocardiography suggested that her pregnancy had thus far been without complication, and she had been compliant with prescribed daily prophylactic low-molecular-weight heparin.
Figure 1.

Clinical course, major symptoms, and outcomes from illness onset in this patient with COVID-19–related critical illness and severe ARDS
ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; ICU, intensive care unit.
Schnettler et al. Severe acute respiratory distress syndrome in COVID-19–infected pregnancy. AJOG MFM 2020.
Upon re-presentation to the emergency department (COVID-19 day 5), her symptoms had progressed to include fever at 101°F, worsening shortness of breath adversely affecting the ability to complete full sentences, and persistent nonproductive cough (Figure 1). Initial vital sign assessment identified significant tachypnea (respiratory rate of 32 breaths per minute), mild tachycardia (heart rate in the low 100’s beats per minute), low normal blood pressure (mean arterial pressure [MAP] in the low 70’s mm Hg), and mild hypoxia (SpO2 93%) despite 4 L of oxygen via nasal cannula. Physical examination was notable for rhonchi and egophony throughout all lung fields. A chest x-ray, computed tomography (CT) pulmonary angiogram, and lung ultrasound assessment were performed (Figure 2 ). The attending emergency department provider ordered the CT pulmonary angiogram to investigate the potential for pulmonary embolism, and lung ultrasound was performed at bedside by the attending MFM physician to further investigate the potential for COVID-19 pneumonia. Specifically, her chest x-ray examination identified bilateral diffuse pulmonary infiltrates, and her chest CT scan identified bilateral airspace disease characterized by ground-glass appearance with peripheral consolidations compatible with viral pneumonia. Her lung ultrasound demonstrated bilateral pleural thickening and nodularity of the visceral pleura (Figure 2). Horizontal A-lines representing normal aerated lung were absent and replaced by multiple B-lines, pleural nodularity and thickening, and an overall “white lung” appearance with focal areas of consolidation.
Figure 2.
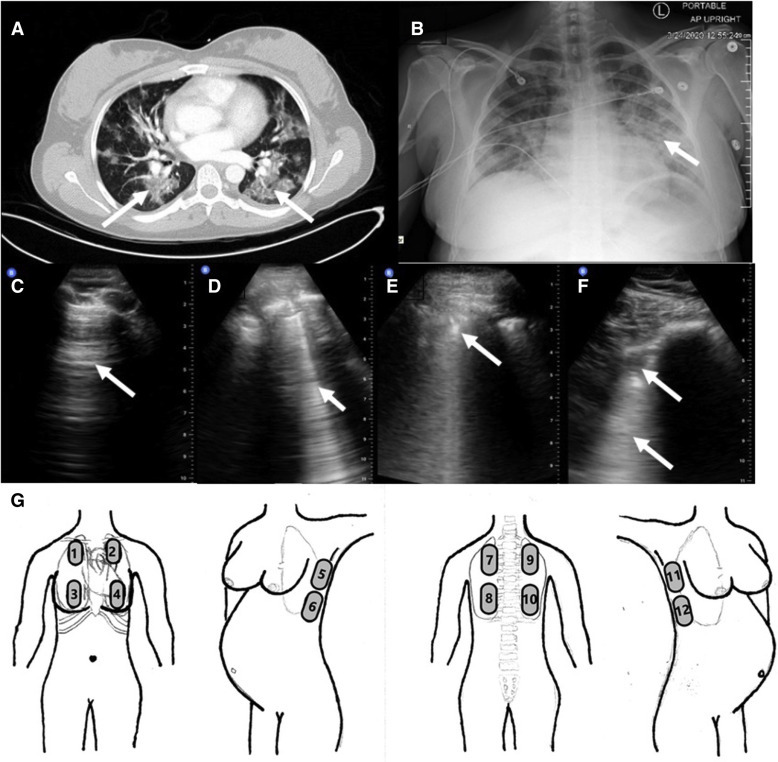
Lung imaging findings and sonography location in a case of COVID-19
Patient’s chest imaging including chest CT (A) with ground-glass opacities and peripheral consolidation bilaterally (arrows), chest x-ray film (B) indicating bilateral diffuse pulmonary infiltrates (arrow), normal (different healthy patient) lung ultrasound (C) with characteristic normal A-lines (arrow), and the patient’s abnormal lung ultrasound images (D–F) demonstrating abnormal B-lines (arrow, D), pleural thickening and nodularity (arrow, E), and focal subpleural consolidation (arrow, F) with underlying “white lung” (arrow, F), and a schematic (G) depicting the 12 anatomic locations to thoroughly evaluate the lung with sonography.
COVID-19, coronavirus disease 2019.
Schnettler et al. Severe acute respiratory distress syndrome in COVID-19–infected pregnancy. AJOG MFM 2020.
Laboratory analysis identified a normal PaO2:FiO2 ratio of >300 (suggesting no evidence of ARDS) but definite leukopenia (7700/μL), lymphopenia (800/μL), thrombocytopenia (114,000/μL), elevated transaminases (AST 65 IU/L), and a mildly elevated procalcitonin (0.33 ng/mL). Although the results from her previous COVID-19 RT-PCR were pending, her clinical presentation supported the diagnosis of COVID-19 with pneumonia and the potential for ARDS. The emergency department physician thought that her SpO2 of 93% on 4 L/min oxygen via nasal cannula and PaO2:FiO2 ratio of >300 were appropriate for admission to the hospital’s lower-acuity COVID-19 floor, and her disposition was planned to that unit. However, the attending MFM physician recognized her SpO2 as abnormal for pregnancy and redirected her care to the intensive care unit (ICU). The degree of probable COVID-19 at this point was severe.8
Upon arrival to a negative pressure room in the ICU, she was placed on strict isolation with airborne precautions. Reassessment of her SpO2 revealed worsening hypoxia (78%) despite 4 L/min of oxygen via nasal cannula. A nonrebreather mask was applied, and inhaled oxygen rate was increased to 15 L/min. Her SpO2 increased to 82%, but she complained of feeling exhausted with inspiration; hence the decision was made to proceed with rapid sequence intubation with planned mechanical ventilation for respiratory failure and critical illness severity (approximately 9.5 hours after presentation to the emergency department). Continuous electronic fetal monitoring was initiated and demonstrated a 3-minute prolonged fetal heart rate deceleration to 80 beats per minute that spontaneously resolved after intubation. During this brief period, plan for emergent delivery was considered. However, maximal ventilatory assistance was applied, and both the maternal and fetal status improved, prompting initiation of both antenatal corticosteroid administration and magnesium sulfate for the dual benefit of fetal neuroprotection and control of maternal bronchospasm (6-g bolus over 20 minutes intravenously followed by 2 g/h intravenously). She required large dosages of intravenous (IV) benzodiazepines and narcotics for sedation, and her MAP dropped below 65 mm Hg requiring continuous infusion of norepinephrine.
Over the next 8 hours, the ventilator settings were increased to 100% FiO2 and positive end-expiratory pressure (PEEP) of 10 cm H2O, without complete resolution of hypoxia (SpO2 approximately 90%–93%). A volume control ventilation modality with automatic pressure augmentation (VC+) was employed to minimize volu- and barotrauma with the following settings: respiratory rate of 20 breaths per minute, tidal volume of 6 mL/kg, FiO2 of 100%, inspiratory-to-expiratory time of 1:1.3, and PEEP of 10 cm H2O. The PaO2:FiO2 ratio remained below 150, signifying severe ARDS per American-European Consensus Conference criteria.9 Surprisingly, the fetal heart rate remained reassuring and signified a certain degree of maternal stability. Per recommendations by the infectious disease specialist, the following medications were initiated: ceftriaxone, azithromycin, oseltamivir, and hydroxychloroquine. There was no marked improvement in her oxygenation until the team manually placed her in a prone position per the PROSEVA study protocol.9 The initial plan included using a mechanical rotating bed designed for prone ventilation, but manual prone positioning was preferred due to the ability to more quickly return her to supine positioning for performance of cardiopulmonary resuscitation or emergent delivery. Manual pronation required a collaborative effort involving the intensive care and obstetric team members to establish invasive hemodynamic monitoring (central venous access and arterial line access), secure her airway, cushion and support her gravid abdomen, and maintain continuous tocodynamometry and electronic fetal heart rate monitoring (Figure 3 ). This allowed for gradual reduction of FiO2 and PEEP requirements.
Figure 3.
A depiction of pad placement and body positioning to achieve manual prone ventilation in a pregnant woman with severe coronavirus disease 2019–related acute respiratory distress syndrome
When the patient is supine, 6 to 8 standard hospital bed pillows are placed across the patient’s face, upper chest and arms, lateral abdomen on each side, pelvis, and upper legs. A bedsheet is draped over these pillows and then rolled together with the bedsheet beneath the patient’s back on each side to create a “sandwich.” The team members grasp the rolled sheets on each side and use them to roll the patient onto her side and then prone such that the pillows remain in position as shown.
Schnettler et al. Severe acute respiratory distress syndrome in COVID-19–infected pregnancy. AJOG MFM 2020.
Her care throughout the following week involved multidisciplinary “huddles” twice daily to elicit input from the Infectious Disease specialists, Pulmonology and Critical care teams, MFM teams, Anesthesia Critical Care specialists, Cardiology, Cardiothoracic surgeons, Neonatology, and Obstetric nursing teams. Plans were established regarding staff exposure mitigation, emergency preparedness, delivery timing, neonatal resuscitation, nutritional support (oral gavage feedings), venous thromboembolism prophylaxis (subcutaneous heparin twice daily), and adjunctive measures including the potential for inhaled pulmonary vasodilators (epoprostenol) and extracorporeal membrane oxygenation (ECMO). She had a positive COVID-19 RT-PCR result on hospital day 4 (COVID-19 day 8), and strict isolation with airborne droplet precautions was maintained. Consent and regulatory permission were obtained to allow for initiation of a 10-day course of remdesivir—a promising antiviral agent targeting a wide array of RNA viruses including severe acute respiratory syndrome (SARS)/Middle East respiratory syndrome coronavirus.7 Daily lung ultrasound assessments revealed a lack of visual improvement to her sonographic findings. She was rotated between the prone and left lateral decubitus positions each day with a gradual tolerance toward longer durations out of the prone position on hospital days 6 and 7. This tolerance combined with a gradual reduction in FiO2 and PEEP requirements negated the need to move forward with delivery or to institute inhaled pulmonary vasodilators or ECMO.
On hospital day 8 (COVID-9 day 13) at 32.0 weeks’ gestation, continuous tocodynamometry and fetal heart rate tracing began to demonstrate regular uterine contractions with persistent late decelerations. This prompted a “huddle” and mobilization of all teams to prepare for urgent but nonemergent delivery via repeat cesarean delivery. Her ICU ventilator circuit was maintained and transported with her to the operating suite to minimize exposure during transport. Bedside echocardiography was used to monitor her intravascular volume status to help guide fluid resuscitation. Repeat laboratory assessment revealed a mild coagulopathy (elevated international normalized ratio, 1.7; prothrombin time, 20.2 seconds; and activated partial thromboplastin time, 35.1 seconds), and preparations were made for prevention of massive hemorrhage including procurement of blood products and uterotonics (oxytocin and misoprostol) in the operating room. A vertical midline skin incision was made to optimize exposure and minimize vascular injury in case of hemorrhage. Delayed cord clamping was intentionally not employed, and the neonatology team used an adjacent operating room for neonatal resuscitation to minimize staff and newborn exposure to the mother. The patient tolerated the repeat low-transverse cesarean delivery quite well without postpartum hemorrhage or respiratory compromise. She returned to the ICU where her status continued to improve over the next several days. A “de-brief” was held among all team members to review opportunities for improvement. Umbilical cord blood gas analysis revealed a normal pH of 7.2, PCO2 of 63 mm Hg, PaO2 of 21 mm Hg, and base deficit of 3, and the male newborn transitioned to extrauterine life without complication and was extubated on day of life (DOL) 3. His amniotic fluid and nasopharyngeal swabs were sent for COVID-19 RT-PCR analysis on DOL 1 and 2 (24 hours apart), and the test results were negative. Because of the need to maintain strict isolation and airborne/droplet precautions, the patient’s husband was prohibited from visiting either his wife or his newborn son for a total of 14 days. Currently (hospital day 17/COVID-19 day 22), she is improving but continues on synchronized intermittent mandatory ventilation with FiO2 of 35%, no PEEP requirement, and daily attempts of spontaneous breathing trials.
Discussion
This case highlights the rapidity of COVID-19 in pregnancy with development of severe COVID-19–related ARDS within 10 hours of admission and the importance of considering physiologic maternal adaptations in delineating an algorithmic approach. The maternal physiologic adaptations to pregnancy not only leave the woman more vulnerable to cell-mediated viral infections such as COVID-19 but also more susceptible to rapid cardiopulmonary decompensation because of reduced cardiac and pulmonary reserves. Such considerations may not be the priority for intensive care team members, and these physiologic alterations must be emphasized by the obstetric providers because they assist in serving in a “quarter-back” role, leading the implementation of the algorithmic approach. Such approach must entail input from multiple disciplines and establish a framework for optimal team dynamics using daily “huddles” or other open means of direct communication. This planning should occur before any patient’s arrival wherein the myriad of team members establish a consensus regarding optimal imaging investigations, laboratory studies, COVID testing, fetal assessment, and admission locations for these women. The team’s safety must also remain a priority ensuring appropriate personal protective equipment, staffing (nurse-to-patient ratio), facilities equipped to minimize exposure, and mobile or handheld equipment with easy cleaning/disinfecting. An example of our management algorithm is included for reference but should be individualized to one’s own institution (Figure 4 ).
Figure 4.
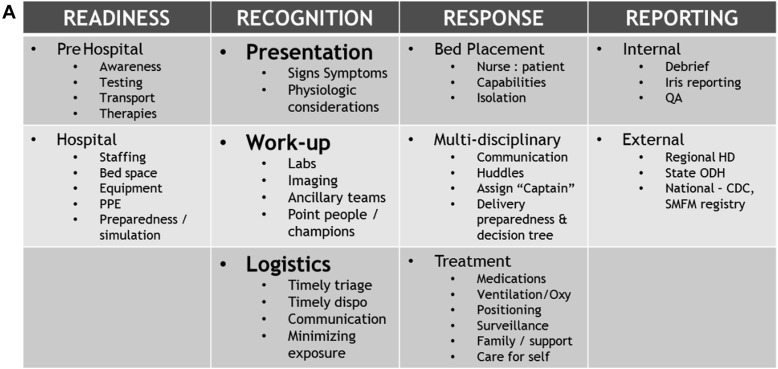
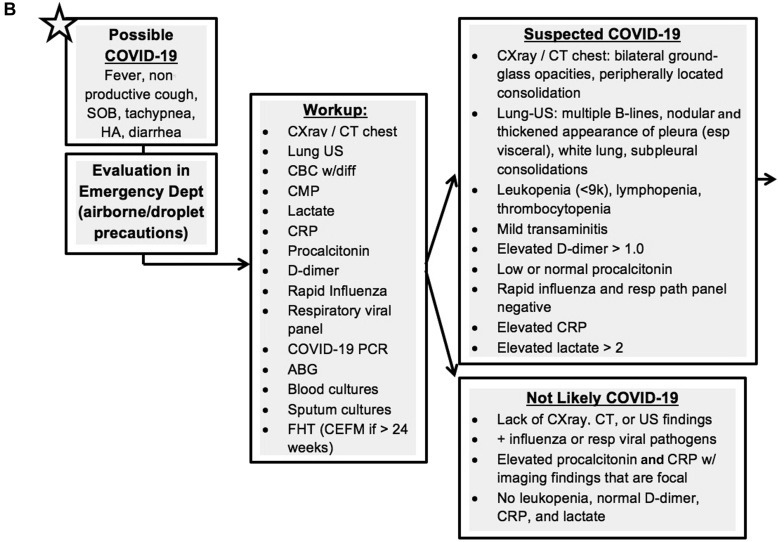
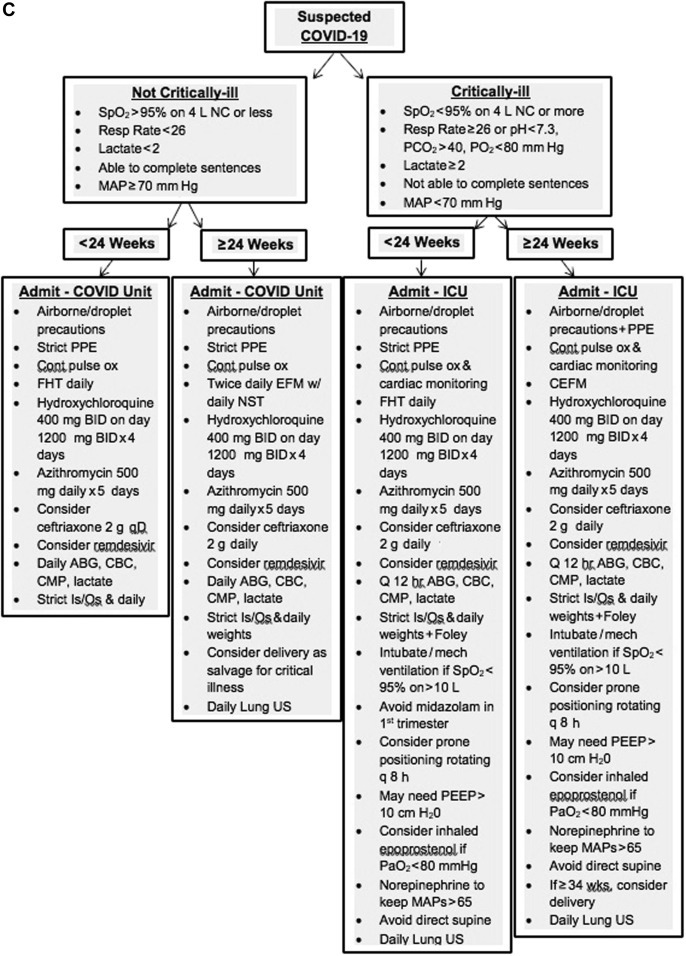
A–C, Example management algorithm for the pregnant patient with COVID-19
ABG, arterial blood gas; BID, twice daily; CBC, complete blood cell count; CDC, Centers for Disease Control and Prevention; CEFM, continuous electronic fetal monitoring; CMP, complete metabolic panel; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CT, computed tomography; D&C, dilation and curettage; EFM, electronic fetal monitoring; FHT, fetal heart tones; GA, gestational age; HA, headache; HD, health department; ICU, intensive care unit; LDR, labor, delivery, and recovery room; LR, lactated Ringer’s solution; MAP, mean arterial pressure; MgSO4, magnesium sulfate; NC, nasal cannula; NRFHT, nonreassuring fetal heart rate tracing; NST, nonstress test; ODH, Ohio Department of Health; OR, operating room; PCR, polymerase chain reaction; PEEP, positive end-expiratory pressure; PPE, personal protective equipment; PTL, preterm labor; QA, Quality Assurance committee; SMFM, Society for Maternal-Fetal Medicine; SOB, shortness of breath; SVD, spontaneous vaginal delivery, US, ultrasound.
Schnettler et al. Severe acute respiratory distress syndrome in COVID-19–infected pregnancy. AJOG MFM 2020.
One of the most difficult yet crucial aspects of the management approach is determining the delivery timing. The physiologic adaptations to labor, delivery, and immediate postpartum period include maximization of the maternal cardiac output, autotransfusion of up to 500 mL of blood back into the intravascular compartment, a catecholaminergic surge, release of inflammatory mediators within the endothelium, and considerable fluid shifts between the interstitial, intracellular, and intravascular compartments. In the setting of severe systemic infection, these physiologic changes can exacerbate dysregulated inflammatory cascade leading to a higher potential for endothelial dysfunction, pulmonary edema, myocardial edema, and cardiac dysfunction.10 Thus, the decision to proceed toward delivery should be deferred in severe and critical maternal COVID-19 cases until maternal cardiopulmonary stability can be achieved unless the pregnancy has reached full term, fetal status is nonreassuring, or maternal status is so dire that evacuation of the uterus is likely to facilitate improvement in cardiopulmonary function.4 Consideration for administration of antenatal corticosteroids before anticipated preterm birth is controversial in severe maternal COVID-19 cases. Evidence from treatment studies for SARS suggested that high dosages of corticosteroids posed a risk for severe adverse effects that drastically affected prognosis, but shorter courses of low to moderate dosages may be considered in the care for the critically ill patient with COVID-19.11 The decision regarding administration magnesium sulfate administration for fetal neuroprotection before 32 weeks’ gestation should proceed per standard indications in that this agent may provide an additional benefit of bronchodilation in the setting of bronchospasm after intubation. Caution is advised to minimize fluid overload with the administration of magnesium sulfate because of the potential for development of additional pulmonary edema, and we recommend restricting the total volume of infused IV fluids to 125 mL/h or less. Delayed cord clamping and immediate skin-to-skin maternal contact should be avoided.4 Table 1 represents our approach to delivery considerations including timing, location, and medications.
Table 1.
Delivery considerations for pregnant patients with COVID-19
| Gestational age | Illness severity | Delivery considerations |
|---|---|---|
| GA<24 wk | Noncritically ill |
|
| GA<24 wk | Critically ill |
|
| GA 24–34 wk | Severe but noncritically ill |
|
| GA 24–34 wk | Critically ill |
|
| GA≥34 wk | Severe but noncritically ill |
|
| GA≥34 wk | Critically ill |
|
COVID-19, coronavirus disease 2019; D&C, dilation and curettage; GA, gestational age; ICU, intensive care unit; L&D, Labor and Delivery; MgSO4, magnesium sulfate; NRFHT, nonreassuring fetal heart tracing; OR, operating room; PTL, preterm labor; SVD, spontaneous vaginal delivery.
Schnettler et al. Severe acute respiratory distress syndrome in COVID-19–infected pregnancy. AJOG MFM 2020.
When attempting to defer delivery and achieve resolution of acute maternal illness with supportive care, several adjunctive therapies should be considered. Emerging evidence suggests that antiviral agents such as hydroxychloroquine and remdesivir may be effective against severe acute respiratory syndrome coronavirus 2, but neuraminidase inhibitors such as oseltamivir have no proven benefit.12 , 13 Although the safety of these agents in pregnancy has not been definitely determined and their efficacy remains controversial, the pharmacokinetic properties and mechanisms of action may support their judicious use while we await further clinical trials. Noninvasive modes of ventilation such as “continuous positive airway pressure ” or “bilevel positive airway pressure” are not recommended for managing acute hypoxemic respiratory failure owing to their increased likelihood of failure with need for more urgent transition to invasive ventilation.14 Rapid sequence endotracheal intubation should be performed per routine but with consideration for a slightly smaller endotracheal tube size because of the potentially edematous and narrowed airway calibers in pregnancy. Oxygenation and ventilatory goals include consideration for physiologic mild respiratory alkalosis in pregnancy, diminished functional residual volume, higher PEEP requirement, and potential for less lung compliance with higher innate plateau pressures owing to diaphragmatic compression by the gravid uterus and chest wall compression by enlarged breast tissue. Physiologic tidal volumes in pregnancy are greater than the target value of 6 mL/kg ideal body weight used in the ARDS Network study.15 This, coupled with decreased chest wall/diaphragmatic compliance, presents a challenge to the “lung protective” strategy for mechanical ventilation in pregnant patients. Our clinical observation suggests a 5-cm H2O difference in plateau pressures before and immediately after evacuation of the gravid uterus. Therefore, it seems reasonable to increase tidal volume and/or PEEP to meet goal PCO2 and oxygenation targets, while being mindful not to allow alveolar plateau pressures to exceed 35-cm H2O (Table 2 ).
Table 2.
Critical care goals and considerations in pregnant patients with COVID-19
| Measure | Target | Considerations |
|---|---|---|
| MAP | >65 mm Hg |
|
| SpO2 | >94% |
|
| PaO2 | >80 mm Hg |
|
| PCo2 | <40 mm Hg |
|
| pH | 7.3–7.5 |
|
| Bicarb | 16–22 mm Hg |
|
| Anion gap | 6–15 |
|
| PiP | <35 mm Hg |
|
| UOP | >20 mL/kg/h |
|
| Skin | No breakdown |
|
| VTE | Prophylaxis |
|
| Peptic ulcer | Prophylaxis |
|
| CEFM | Category 1–2 |
|
| Sedation | Lowest achievable |
|
CEFM, continuous electronic fetal monitoring; I:E ratio, inspiratory:expiratory ratio; GA, gestational age; LR, lactated Ringer’s solution; MAP, mean arterial pressure; PEEP, positive end-expiratory pressure; PiP, peak inspiratory pressure; RASS, Richmond Agitation and Sedation Scale; UOP, urine output; VC, volume control; VTE, venous thromboembolism.
Schnettler et al. Severe acute respiratory distress syndrome in COVID-19–infected pregnancy. AJOG MFM 2020.
The prone position can help overcome some of these issues. Prone ventilation has been found to significantly improve oxygenation in the setting of ARDS, and its feasibility and safety in pregnancy have been documented.16 , 17 Finally, veno-venous ECMO is a proven life-saving salvage therapy for severe, reversible respiratory failure, and its benefit among critically ill pregnant women has been reported.18 Consideration for ECMO cannulation should be entertained among a multidisciplinary team of experienced providers in situations where the patient’s oxygenation is so severely compromised as to require maximal ventilatory support early in the disease process (less than 7 days of mechanical ventilatory support). Therapeutic anticoagulation is often required, and the postpartum period appears to be a potentially tenuous time point for initiation of ECMO with 100% maternal mortality in a recent case series.18
In summary, this case of rapid clinical decompensation and development of severe (PaO2:FiO2<150) COVID-19–related ARDS in a woman at 31 weeks’ gestation highlights many physiologic and management considerations for the care of critically ill pregnant women with COVID-19. Few contemporary resources exist to guide the multidisciplinary team through decisions regarding optimal maternal-fetal surveillance, intensive care procedures, and delivery timing. This detailed case reviews the thought process, team-based strategy, and algorithmic approach to this emerging disease’s diagnosis and management.
Footnotes
This paper is part of a supplement that represents a collection of COVID-related articles selected for publication by the editors of AJOG MFM without additional financial support.
The authors report no conflict of interest.
Cite this article as: Schnettler WT, Al Ahwel Y, Suhag A. Severe acute respiratory distress syndrome in COVID-19-infected pregnancy. Am J Obstet Gynecol MFM 2020;2:100120.
References
- 1.World Health Organization Coronavirus disease 2019 (COVID-19) Situation Report – 74. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200403-sitrep-74-covid-19-mp.pdf?sfvrsn=4e043d03_14 Available at: Accessed April 3, 2020.
- 2.Salata C., Calistri A., Parolin C., Palù G. Coronaviruses: a paradigm of new emerging zoonotic diseases. Pathog Dis. 2019;77:ftaa006. doi: 10.1093/femspd/ftaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Cases of Coronavirus Disease (COVID-19) in the U.S. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html Available at: Accessed April 3, 2020.
- 4.Dashraath P, Jing Lin Jeslyn W, Mei Xian Karen L, et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol [In press]. [DOI] [PMC free article] [PubMed]
- 5.Schwartz D.A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 2020 doi: 10.5858/arpa.2020-0901-SA. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Di Mascio D., Khalil A., Saccone G., et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID1-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100107. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breslin N., Baptiste C., Gyamfi-Bannerman C., et al. COVID-19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100118. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 9.Guérin C., Reignier J., Richard J.C., et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 10.Juusela A., Nazir M., Gimovsky M. Two cases of COVID-19 related cardiomyopathy in pregnancy. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100113. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yi Y., Lagniton P.N.P., Ye S., Li E., Xu R.H. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16:1753–1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M., Cao R., Zhang L., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H., Wang Y.M., Xu J.Y., Cao B. Potential antiviral therapeutics for 2019 novel coronavirus. Zhonghua Jie He Hu Xi Za Zhi. 2020;43:170–172. doi: 10.3760/cma.j.issn.1001-0939.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Arabi Y.M., Fowler R., Hayden F.G. Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Med. 2020;46:315–328. doi: 10.1007/s00134-020-05943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisner M.D., Thompson T., Hudson L.D., et al. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:231–236. doi: 10.1164/ajrccm.164.2.2011093. [DOI] [PubMed] [Google Scholar]
- 16.Dennis A.T., Hardy L., Leeton L. The prone position in healthy pregnant women and in women with preeclampsia—a pilot study. BMC Pregnancy Childbirth. 2018;18:445. doi: 10.1186/s12884-018-2073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akatsuka M., Tatsumi H., Yama N., Masuda Y. Therapeutic evaluation of computed tomography findings for efficacy of prone ventilation in acute respiratory distress syndrome patients with abdominal surgery. J Crit Care Med (Targu Mures) 2020;6:32–40. doi: 10.2478/jccm-2020-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webster C.M., Smith K.A., Manuck T.A. Extracorporeal membrane oxygenation in pregnant and postpartum women: a ten-year case series. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100108. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


