We have read with great interest the work from Zhang W et al. reviewing their experience in the treatment of COVID-19 patients with anti-inflammatory therapies [1] The immune dysregulation and cytokine storm observed in severe COVID-19 cases has led to the trial of licensed RA drugs such as Chloroquine, IL-1/IL-6 blockers, TNF or Janus kinase inhibitors in COVID-19 patients [1] In addition we propose that blockade of granulocyte macrophage-colony stimulating factor (GM-CSF) may be an effective strategy to prevent pulmonary complications and fatality in SARS-Cov2 infection.
Clinical findings in severe COVID-19 cases indicate a dysregulated innate immune response with an overexuberant inflammation, characterized by a cytokine storm syndrome that is responsible for the associated respiratory failure, multiorgan failure and lethality. Analysis of cytokine profiles in COVID-19 patients shows some similarities to secondary haemophagocytic syndrome (sHPS), with increased IL-2, IL-6, IL-7, GM-CSF, IP-10, MCP-1, MIP-1α and TNF-α [2]. In this uncommon and potentially fatal disorder, severe hyperinflammation is caused by uncontrolled proliferation and activation of macrophages, which secrete high amounts of inflammatory cytokines and show increased phagocytic activity [3]. Causes for this pathological immune activation can be genetic or secondary under sporadic conditions such as viral infection. This virus-associated hemophagocytic syndrome (VAHS) has been extensively studied, with severe complications often resulting in multiorgan failure and death. During several influenza pandemics such as 2009 influenza A H1N1, 1918 H1N1 and 1998 H5N1, VAHS was shown to represent an important contributor to associated respiratory failure and high lethality rates [4,5]. Findings from these cases showed involvement of a massive macrophage activation and rapid occurrence of multi-organ failure.
Research on SARS-Cov2 pathogenesis indicates that infection induces morphological and inflammation-related phenotypic changes in peripheral blood monocytes, and correlation with acute respiratory distress syndrome (ARDS) in severe patients [6] Furthermore, single-cell RNA sequencing of lung bronchoalveolar immune cells pointed to peripheral blood monocyte-derived macrophages as the predominant macrophage subset in most severe COVID-19 patients. Conversely, in mild disease, alveolar macrophages were predominant along with highly expanded clonal CD8+ T cells, suggesting a well-orchestrated adaptive immune response to a COVID-19 infection [7].
If these findings are confirmed, they would indicate that in SARS-Cov2, similarly to SARS-Cov1, acute lethal disease is produced by delayed and dysregulated type I interferon response and pulmonary accumulation of inflammatory monocyte-macrophages, which are mainly responsible for immunopathology [8,9]. This would identify these cells as potential therapeutic targets in severe patients. Furthermore, SARS-Cov1 has demonstrated ability to infect primary human monocyte-derived macrophages in vitro; antibody-dependent enhancement (ADE) of macrophages by non-neutralizing antiviral antibodies has been shown during other coronavirus infections [10], skewing macrophages to a hyper-activated pathogenic response.
During infection and inflammatory response, bloodstream monocytes derived from precursors in the bone marrow are recruited and stimulated to differentiate into macrophage cell population. This recruitment is essential for an effective control and clearance of viral infection, but it also contributes to the pathogenesis and degenerative disease in an uncontrolled immune response [11]. GM-CSF is the main cytokine implicated in recruitment, activation and monocyte-macrophage differentiation and polarization to a M1 macrophage pro-inflammatory phenotype, in detriment of a regulatory-wound healing M2 phenotype [12]. Several pre-clinical models and clinical trials have demonstrated that harmful over-inflammation can be controlled by targeting the action of this cytokine [13].
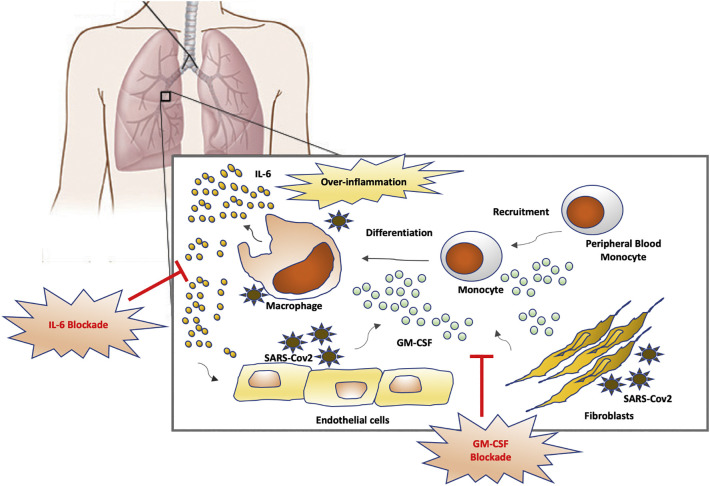
Preliminary results indicate that therapeutic blockade of interleukin-6 (IL-6), another macrophage related-cytokine involved in RA pathogenesis, is also effective in severe COVID-19 patients [14]. IL-6 is a potent pro-inflammatory cytokine mainly produced by inflammatory macrophages and a key mediator of pathogenesis in chronic inflammation. Hence, a therapeutic combination of GM-CSF and IL-6 blockade in severe COVID-19 patients could avoid pulmonary complications and respiratory failure by inhibiting monocyte-macrophage recruitment/differentiation to the lung and blocking the main mediator of inflammatory response. Blockade of GM-CSF could even be delivered at initial phases of severe disease (upon admission) to avoid hyperinflammatory response and prevent the need of intensive care unit (ICU) admission for mechanic ventilation (Fig. 1 ).
Fig. 1.
Proposed strategy of IL-6 and GM-CSF blockade to avoid pulmonary complications in SARS-Cov2 infection. SARS-Cov2 replication in pulmonary tissues activates production of GM-CSF by endothelial cells and fibroblast. This produces a chemoattractant gradient that recruits peripheral blood monocytes to lungs, promoting activation and differentiation to inflammatory macrophages and production of an over-exuberant inflammatory response with increased levels of IL-6 and tissue destruction.
GM-CSF: granulocyte and monocyte-colony stimulating factor; IL-6: interleukin-6.
There is currently no licensed drug for inhibition of GM-CSF. However, there are several drugs currently in clinical development phase being assayed in RA and other inflammatory conditions: lenzilumab, namilumab and otilimab. Lenzilumab, is a humanized monoclonal antibody developed by Humanigen, that targets GM-CSF originally designed for the treatment of chronic myelomonocytic leukaemia and currently under clinical trial for refractory large B-cell lymphoma. Namilumab is a monoclonal antibody that targets the GM-CSF ligand, developed by Takeda Pharmaceuticals currently in phase II for treatment in axial spondyloarthritis and with good phase II results in RA and plaque psoriasis. Otilimab, a fully human antibody against GM-CSF, developed by biotechnology company MorphoSys in cooperation with GlaxoSmithKline, is currently in phase III start in patients with rheumatoid arthritis. Otilimab has shown promising results during initial developmental phases and might constitute a good therapeutic candidate in COVID-19, alone or in combination with other immunosuppressive drugs such as IL-6 blockaders and anti-viral regimes. Given the circumstances, these drugs might be also considered in COVID-19 patients therapy, leveraging their application on the limited but already available safety profile from their use in the performed and ongoing clinical trials.
References
- 1.Zhang W. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the Perspectives of clinical immunologists from China. Clin. Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta P. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan M.B. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118(15):4041–4052. doi: 10.1182/blood-2011-03-278127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beutel G. Virus-associated hemophagocytic syndrome as a major contributor to death in patients with 2009 influenza A (H1N1) infection. Crit. Care. 2011;15(2):R80. doi: 10.1186/cc10073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsieh Y.C. Influenza pandemics: past, present and future. J. Formos. Med. Assoc. 2006;105(1):1–6. doi: 10.1016/S0929-6646(09)60102-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhang D. COVID-19 infection induces readily detectable morphological and inflammation-related phenotypic changes in peripheral blood monocytes, the severity of wich correlate with patient outcome. medRxiv. 2020 doi: 10.1101/2020.03.24.20042655. [DOI] [Google Scholar]
- 7.Liao M. The landscape of lung bronchoalveolar immune cells in COVID-19 revealed by single-cell RNA sequencing. medRxiv. 2020 doi: 10.1101/2020.02.23.200266908. [DOI] [Google Scholar]
- 8.Channappanavar R. Dysregulated type I interferon and Inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19(2):181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung C.Y. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 2005;79(12):7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4(4) doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi C., Pamer E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011;11(11):762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacey D.C. Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. J. Immunol. 2012;188(11):5752–5765. doi: 10.4049/jimmunol.1103426. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton J.A. GM-CSF-Dependent Inflammatory Pathways. Front. Immunol. 2019;10:2055. doi: 10.3389/fimmu.2019.02055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents. 2020:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]