Abstract
Objective
Colchicine has been utilized safely in a variety of cardiovascular clinical conditions. Among its potential mechanisms of action is the non-selective inhibition of NLRP3 inflammasome which is thought to be a major pathophysiologic component in the clinical course of patients with COVID-19. GRECCO-19 will be a prospective, randomized, open-labeled, controlled study to assess the effects of colchicine in COVID-19 complications prevention.
Methods
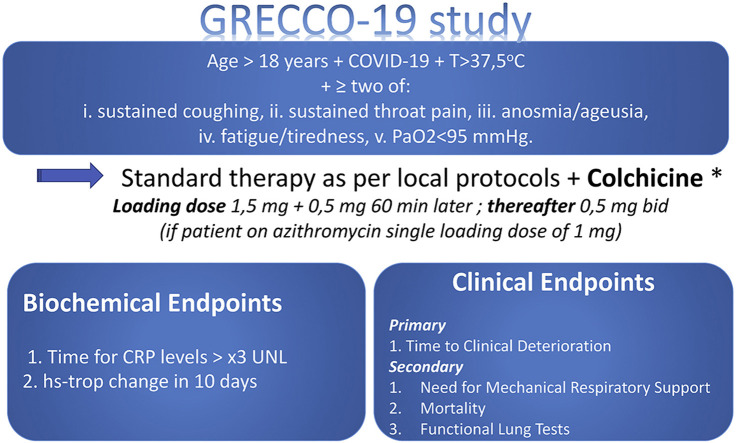
Patients with laboratory confirmed SARS-CoV-2 infection (under RT PCR) and clinical picture that involves temperature >37.5 oC and at least two out of the: i. sustained coughing, ii. sustained throat pain, iii. Anosmia and/or ageusia, iv. fatigue/tiredness, v. PaO2<95 mmHg will be included. Patients will be randomised (1:1) in colchicine or control group.
Results
Trial results will be disseminated through peer-reviewed publications and conference presentations.
Conclusion
GRECCO-19 trial aims to identify whether colchicine may positively intervene in the clinical course of COVID-19. (ClinicalTrials.gov Identifier: NCT04326790).
Keywords: COVID, Cardiac, Troponin, CRP, Complications
Graphical abstract
1. Introduction
The COVID-19 pandemic crisis has literally altered the way scientific knowledge is to be discovered and diffused. It is our strong belief that moving into this new era, we should also implement a paradigm shift in research: we should move from open-data to open-research-ideas. In fact, in a time when reliable “cross-checked” results are needed in a time-efficient manner, multiple-input multimodal collaborative projects (a medical research GitHub, if one dares the simile) may be the answer. Therefore, we opted to publish the rationale and design of the present study prior to obtaining its first results.
2. Background
Colchicine is a drug administered for years in various patient settings. It is a classic treatment for acute pericarditis1. Moreover, in the recent ESC guidelines it is suggested that it may be administered for the prevention of post-pericardiotomy syndrome1 and in a consensus document it is suggested that it may be used for prevention of atrial fibrillation recurrence after cardiac surgery or ablation procedures2. Beyond these, colchicine has been administered in numerous research protocols with various clinical settings (including acute myocardial infarction) showing a favorable safety profile and promising results3 , 4.
As far as patients with COVID-19 are concerned, first reports indicate that myocardial injury is frequently present. In a study from Wuhan, China, in 191 patients, cardiac necrosis biomarkers' elevation was found to be an independent predictor of need for mechanical respiratory support5. In another study from Wuhan, which included 150 patients with COVID-19, 7% of deaths was attributed in myocarditis with circulatory collapse, while in 33% myocarditis had played a crucial role in the final adverse outcome6.
Patients with COVID-19 often develop acute respiratory distress syndrome and/or acute lung injury (ARDS/ALI). Fulminant pulmonary inflammation provokes diffuse alveolar injury resulting in pulmonary infiltrations and, clinically, in acute respiratory failure and it has been reported that myopericarditis is observed in a substantial proportion of hospitalized patients5.
In experimental models it has been shown that inflammasome NLRP3 is a major pathophysiological component in the development of ARDS/ALI7, 8, 9, 10, while structural dry-lab models have shown that in the new SARS-CoV-2 proteins such as viroporins E, 3a and 8A play a substantial role in viral replication and pathogenetic sequelae11. Additionally, there are data supporting that these three proteins provoke the activation of inflammasome NLP312, 13, 14, 15, 16.
Furthermore, SARS-CoV-2 entry in cells is dependent on the connection of viral proteins S with cellular receptors and activation of viral proteins by proteases of host-cells17 , 18. Therefore, factors that may have an effect on clathrin-mediated endocytosis (a procedure that is – in part – regulated by microtubules remodeling19) would potentially decelerate viral infection of cells20.
Colchicine is a lipid-soluble alkaloid21, which after oral administration is absorbed in the jejunum and Ileum with maximum plasma concentrations achieved after 1-2 hours (after a single p.o. dose). Maximum anti-inflammatory effect develops within a time frame of 24-48h. This time period is required for drug accumulation in granulocyes and monocytes, in which concentrations are several times higher than plasma concentrations22. Indeed, it remains there for several days after last administration23.
Colchicine binds to unpolymerized tubulin heterodimers, forming a stable complex that effectively inhibits microtubule dynamics upon binding to microtubule ends24. Moreover, colchicine is a non-selective inhibitor of NLRP3 inflammasome25. While initially it has been thought of merely as an inhibitor of microtubule polymerization and leucocyte infiltration, it is now presumed that a significant part of colchicine anti-inflammatory action is attributed to inhibition of the NLRP3 inflammasome26. Colchicine inhibits inflammasome on two levels: it inhibits P2X7 receptor activation and ASC polymerization, thereby inhibiting interaction between pyrin-like domains27. Additionally, colchicine suppresses the transport of mitochondria and subsequent approximation of ASC to NLRP3, indicating that microtubules mediated the transport of mitochondria to create optimal sites for activation of the NLRP3 inflammasome28. Colchicine has been shown to limit IL-1b production as a response to various NLRP3 inflammasome inducers in a dose-dependent fashion. For example, in the setting of acute coronary syndrome, colchicine was effective in suppressing interleukin IL-1b, IL-18 and IL-6, which was attributed to inflammasome inhibition29 , 30.
In terms of side-effects, colchicine has been safely administered to patients with myocardial infarction both in the acute phase and later on, in the chronic post-myocardial infarction period4 , 31.
3. Research hypothesis
Based on the aforementioned data, the question which arises is whether colchicine, administered in a relatively low dose, could potentially have an effect the patients’ clinical course by limiting the myocardial necrosis and pneumonia development in the context of COVID-19. If present, this effect would be attributed to its potential to inhibit inflammasome and (less probably) to the process of SARS-CoV-2 endocytosis in myocardial and endothelial respiratory cells.
4. Patient population
Patients with laboratory confirmed SARS-CoV-2 infection (under RT PCR) and clinical picture that involves body temperature >37.5 oC and at least two out of the: i. sustained coughing, ii. sustained throat pain, iii. anosmia and/or ageusia, iv. fatigue/tiredness, v. PaO2<95 mmHg.
5. Exclusion criteria
Pregnancy; known hypersensitivity to colchicine; known hepatic failure; eGFR<20 ml/min; clinical estimation that the patient will require mechanical respiratory support in less than 24 hours; any clinical estimation of the attending physician under which the patient shall be excluded; QTc >450 msec.
6. Study design
Prospective, randomized, open labeled, controlled study. Patients will be randomized in two groups (A and B). Patients of group-A will be treated under optimal treatment based on the algorithm proposed in Greece (as per Ministerial Committee decisions) while in group-B patients on top of this colchicine will be also administered.
6.1. Treatment Arm
On top of usual medical treatment, a loading dose (p.o) of colchicine 1.5 mg (followed 60 min later by 0.5 mg if no adverse gastrointestinal effects are observed) will be administered followed by 0.5 mg colchicine twice a day (except for patients weighting <60 kg in whom 0.5 mg colchicine will be administered once daily). In the case of concurrent treatment with azithromycin as per local protocol then the loading dose of colchicine will be reduced to 1 mg and the maintenance dose will remain unchanged.
6.2. Control Arm
Usual medical treatment only.
7. Endpoints
BIOCHEMICAL “PHASE”
-
-
Time for CRP levels that exceeds > 3xUNL
-
-
Difference in maximum high-sensitivity troponin within 10 days from treatment
8. Clinical “PHASE”
8.1. Primary
Time to clinical deterioration (criterion: 2 levels in WHO R&D Blueprint scale32, 33, 34).
8.2. Secondary
-
-
Percentage of patients who will require mechanical ventilation
-
-
Mortality
-
-
Function Lung Tests in healed patients
9. Sample size
For the clinical phase time-dependent univariate COX regression analysis (power 0.8, alpha 0.05) a total of 180 patients was found to be required to detect a hazard reduction of 50% (i.e. hazard ratio = 2 for the group A)35.
Due to the pandemic crisis, data will be analyzed every 20 patients who complete the study.
10. Ethics and dissemination
Local Ethics approval has already been obtained in Greece and submission is in process in other participating countries. Trial results will be disseminated through peer-reviewed publications and conference presentations.
11. Trial registration number
ClinicalTrials.gov Identifier: NCT04326790
12. Conclusion
GRECCO-19 trial aims to identify whether colchicine may positively intervene in the clinical course of COVID-19.
Conflict of interest
No conflict of interest exists.
Acknowledgements
DV is personally supported by a scholarship from Hellenic Society of Cardiology (Athens, Greece)
JSS is personally supported by a grant from the Fundación Alfonso Martin Escudero (Madrid, Spain)
The study is funded by ELPEN Pharmaceutical Co. Inc. and sponsored by Hellenic Society of Rhythmology.
Footnotes
Peer review under responsibility of Hellenic Society of Cardiology.
References
- 1.The 2015 ESC Guidelines on the diagnosis and management of pericardial diseases. https://www.ncbi.nlm.nih.gov/pubmed/26547486 PubMed - NCBI. [DOI] [PubMed]
- 2.Calkins H., Hindricks G., Cappato R. HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. J Arrhythmia. 2017;33(5):369–409. doi: 10.1016/j.joa.2017.08.001. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemkens L.G., Ewald H., Gloy V.L. Cardiovascular effects and safety of long-term colchicine treatment: Cochrane review and meta-analysis. Heart. 2016;102(8):590–596. doi: 10.1136/heartjnl-2015-308542. [DOI] [PubMed] [Google Scholar]
- 4.Tardif J.C., Kouz S., Waters D.D. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381(26):2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. March 2020 doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. March 2020 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grailer J.J., Canning B.A., Kalbitz M. Critical Role for the NLRP3 Inflammasome during Acute Lung Injury. J Immunol. 2014;192(12):5974–5983. doi: 10.4049/jimmunol.1400368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li D., Ren W., Jiang Z., Zhu L. Regulation of the NLRP3 inflammasome and macrophage pyroptosis by the p38 MAPK signaling pathway in a mouse model of acute lung injury. Mol Med Rep. 2018;18(5):4399–4409. doi: 10.3892/mmr.2018.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones H.D., Crother T.R., Gonzalez-Villalobos R.A. The NLRP3 inflammasome is required for the development of hypoxemia in LPS/mechanical ventilation acute lung injury. Am J Respir Cell Mol Biol. 2014;50(2):270–280. doi: 10.1165/rcmb.2013-0087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolinay T., Kim Y.S., Howrylak J. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med. 2012;185(11):1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castaño-Rodriguez C., Honrubia J.M., Gutiérrez-Álvarez J. Role of Severe Acute Respiratory Syndrome Coronavirus Viroporins E, 3a, and 8a in Replication and Pathogenesis. MBio. 2018;9(3) doi: 10.1128/mBio.02325-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nieto-Torres J.L., DeDiego M.L., Verdiá-Báguena C. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 2014;10(5) doi: 10.1371/journal.ppat.1004077. e1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieto-Torres J.L., Verdiá-Báguena C., Jimenez-Guardeño J.M. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology. 2015;485:330–339. doi: 10.1016/j.virol.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi C.-S., Nabar N.R., Huang N.-N., Kehrl J.H. SARS-Coronavirus Open Reading Frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell death Discov. 2019;5(1):101. doi: 10.1038/s41420-019-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siu K.-L., Yuen K.-S., Castaño-Rodriguez C. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J. 2019;33(8):8865–8877. doi: 10.1096/fj.201802418R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen I.-Y., Moriyama M., Chang M.-F., Ichinohe T. Severe Acute Respiratory Syndrome Coronavirus Viroporin 3a Activates the NLRP3 Inflammasome. Front Microbiol. 2019;10(JAN):50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. March 2020 doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morales L., Oliveros J.C., Fernandez-Delgado R., tenOever B.R., Enjuanes L., Sola I. SARS-CoV-Encoded Small RNAs Contribute to Infection-Associated Lung Pathology. Cell Host Microbe. 2017;21(3):344–355. doi: 10.1016/j.chom.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaksonen M., Roux A. Mechanisms of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2018;19(5):313–326. doi: 10.1038/nrm.2017.132. [DOI] [PubMed] [Google Scholar]
- 20.Stebbing J., Phelan A., Griffin I. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. February 2020 doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerquaglia C., Diaco M., Nucera G., La Regina M., Montalto M., Manna R. Pharmacological and clinical basis of treatment of Familial Mediterranean Fever (FMF) with colchicine or analogues: An update. Curr Drug Targets - Inflamm Allergy. 2005;4(1):117–124. doi: 10.2174/1568010053622984. [DOI] [PubMed] [Google Scholar]
- 22.Chappey O.N., Niel E., Wautier J.L. Colchicine disposition in human leukocytes after single and multiple oral administration. Clin Pharmacol Ther. 1993;54(4):360–367. doi: 10.1038/clpt.1993.161. [DOI] [PubMed] [Google Scholar]
- 23.Molad Y. Update on colchicine and its mechanism of action. Curr Rheumatol Rep. 2002;4(3):252–256. doi: 10.1007/s11926-002-0073-2. [DOI] [PubMed] [Google Scholar]
- 24.Deftereos S., Giannopoulos G., Papoutsidakis N. Colchicine and the heart: pushing the envelope. J Am Coll Cardiol. 2013;62(20):1817–1825. doi: 10.1016/j.jacc.2013.08.726. [DOI] [PubMed] [Google Scholar]
- 25.Toldo S., Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol. 2018;15(4):203–214. doi: 10.1038/nrcardio.2017.161. [DOI] [PubMed] [Google Scholar]
- 26.Leung Y.Y., Yao Hui L.L., Kraus V.B. Colchicine-Update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 2015;45(3):341–350. doi: 10.1016/j.semarthrit.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marques-da-Silva C., Chaves M.M., Castro N.G., Coutinho-Silva R., Guimaraes M.Z.P. Colchicine inhibits cationic dye uptake induced by ATP in P2X2 and P2X7 receptor-expressing cells: implications for its therapeutic action. Br J Pharmacol. 2011;163(5):912–926. doi: 10.1111/j.1476-5381.2011.01254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misawa T., Takahama M., Kozaki T. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol. 2013;14(5):454–460. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- 29.Martínez G.J., Robertson S., Barraclough J. Colchicine Acutely Suppresses Local Cardiac Production of Inflammatory Cytokines in Patients With an Acute Coronary Syndrome. J Am Heart Assoc. 2015;4(8) doi: 10.1161/JAHA.115.002128. e002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson S., Martínez G.J., Payet C.A. Colchicine therapy in acute coronary syndrome patients acts on caspase-1 to suppress NLRP3 inflammasome monocyte activation. Clin Sci (Lond) 2016;130(14):1237–1246. doi: 10.1042/CS20160090. [DOI] [PubMed] [Google Scholar]
- 31.Deftereos S., Giannopoulos G., Angelidis C. Anti-Inflammatory Treatment With Colchicine in Acute Myocardial Infarction: A Pilot study. Circulation. 2015;132(15):1395–1403. doi: 10.1161/CIRCULATIONAHA.115.017611. [DOI] [PubMed] [Google Scholar]
- 32.WHO | Coronavirus disease (COVID-2019) R&D. https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus/en/
- 33.Wang Y., Fan G., Salam A. Comparative Effectiveness of Combined Favipiravir and Oseltamivir Therapy Versus Oseltamivir Monotherapy in Critically Ill Patients With Influenza Virus Infection. J Infect Dis. December 2019 doi: 10.1093/infdis/jiz656. [DOI] [PubMed] [Google Scholar]
- 34.Cao B., Wang Y., Wen D. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. March 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chow S., Shao J., Wang H. 2nd Ed. Chapman & Hall/CRC Biostatistics Series.; 2008. No Title. (Sample Size Calculations in Clinical Research). [Google Scholar]