Abstract
Aims
In December 2019, the Coronavirus disease-2019 (COVID-19) virus has emerged in Wuhan, China. In this research, the first resolved COVID-19 crystal structure (main protease) was targeted in a virtual screening study by of FDA approved drugs dataset. In addition, a knowledge gap in relations of COVID-19 with the previously known fatal Coronaviruses (CoVs) epidemics, SARS and MERS CoVs, was covered by investigation of sequence statistics and phylogenetics.
Materials and methods
Molecular modeling, virtual screening, docking, sequence comparison statistics and phylogenetics of the COVID-19 main protease were investigated.
Key findings
COVID-19 Mpro formed a phylogenetic group with SARS CoV that was distant from MERS CoV. The identity% was 96.061 and 51.61 for COVID-19/SARS and COVID-19/MERS CoV sequence comparisons, respectively. The top 20 drugs in the virtual screening studies comprised a broad-spectrum antiviral (ribavirin), anti-hepatitis B virus (telbivudine), two vitamins (vitamin B12 and nicotinamide) and other miscellaneous systemically acting drugs. Of special interest, ribavirin had been used in treating cases of SARS CoV.
Significance
The present study provided a comprehensive targeting of the first resolved COVID+19 structure of Mpro and found a suitable save drugs for repurposing against the viral Mpro. Ribavirin, telbivudine, vitamin B12 and nicotinamide can be combined and used for COVID treatment. This initiative relocates already marketed and approved safe drugs for potential use in COVID-treatment.
Abbreviations: COVID-19, Coronavirus disease 2019; 2019-nCoV, 2019 New Coronavirus; MERS CoV, Middle East Respiratory Syndrome Coronavirus; ORF, open reading frames; S, spike protein; E, envelope; M, membrane protein; N, nucleocapsid; 3CLpro, 3-C-like protease; SARS, Severe Acute Respiratory Syndrome CoV
Keywords: COVID-19, 2019-nCoV; 2019-Novel Coronavirus; Wuhan Coronavirus; Main protease; Molecular modeling
1. Introduction
Recently, a severe and highly contagious viral disease was evolved in Wuhan, China [1]. The causative agent was detected to be a new coronavirus and termed as Coronavirus disease 2019 (COVID-19).
Coronavirus has been associated with several infectious epidemics with major health hazard concerns. In 2000–2004, the Severe Acute Respiratory Syndrome CoV (SARS CoV) was evolved [2]. The source of the infection was recorded from animal origins, including a bat as an intermediate host [3]. About a decade later, the Middle East Respiratory Syndrome Coronavirus (MERS CoV) was diagnosed in Saudi Arabia [4]. Soon after the emergence of SARS and MERS CoVs, they spread to outside of the countries of its origin, causing major world-wide concerns [5,6]. Lastly, COVID-19 emerged in Wuhan, China. The first isolation was related to an animal market and human-to-human transmission was recorded [7].
The coronavirus polyprotein encodes two proteases, which share in its processing and release of the translated non-structural proteins (NSPs), the main protease is called 3-C-like protease (M-pro) and a papain-like protease (Plpro) [8]. Both Plpro and Mpro were vital targets for drug discovery studies against the recent coronavirus epidemics including SARS and MERS CoV [[9], [10], [11], [12]].
The first and the only available crystal structure of COVID-19 proteins is Mpro, which was published in February 2020 (PDB ID 6lu7). In this study, the first virtual screening study against the first known COVID-19 was performed. The obtained results will help in the repurposing of already approved drugs to combat the recent dangerous COVID-19.
2. Methods
2.1. Retrieval of Mpro sequences
The NCBI GenBank or GISAID (https://www.gisaid.org/) were used to obtain the COVID-19 sequences. SARS CoV and MERS CoV sequences were obtained from the GenBank.
2.2. Sequence alignment and multiple sequence comparisons
Pairwise and multiple sequence comparisons of Mpro were done using CLC genomics software (Qiagen Inc., USA). The sequence comparison matrix was generated, including the number of gaps, number of different residues and identity %.
2.3. Construction of phylogenetic tree
The aligned sequences file was used to generate, a phylogenetic tree by using the neighbor joining method implemented in CLC genomics workbench software.
2.4. Construction of drugs dataset and ligand preparation
FDA approved drugs dataset was retrieved from Selleckchem Inc. (WA, USA). All compounds were imported to Ligprep software, deslated and 3D optimized using OPLS2005 force field.
2.5. COVID-19 Mpro protein preparation
Virtual screening was implemented by using the published crystal structure of COVID-19 Mpro (PDB ID 6lu7). The protein preparation module in Maestro software package (Schrodinger LLC, NY, USA) was used to optimize the protein structure for docking. Water and other nonspecific molecules were removed, the protein was protonated to add polar hydrogens, the structure was optimized at cellular pH conditions, and the structure energy minimized using OPLS2005 force field. The docking grid was generated by using the co-crystalised ligand as the center for docking box of 20 Å size.
2.6. Virtual screening
The Schrodinger glide docking module was used for virtual screening. In order to get accurate results, the standard precision (SP docking) was selected [27,28]. The output results were ordered by docking score. In this set, the curcumin was found to be a strong inhibitor of SARS Mpro [13]. The measured IC50 was 0.0235 μM. The obtained docking results were reanalyzed to include a comparison with curcumin. The relative docking scores were calculated for every compound by dividing its score with the docking score curcumin.
3. Results and discussion
Molecular modeling, virtual screening and other computational techniques are widely used methods to understand the phylogenetics, molecular aspects of proteins and protein-ligand interactions during drug discovery process [[14], [15], [16], [17], [18]]. Virtual screening has been used in drug discovery against emerging and fatal diseases including SARS CoV proteases [19,20], Hepatitis C virus RNA-dependent RNA polymerase [21], dengue virus [22] and Ebola virus [23].
In this work, we cover a knowledge gap about the COVID-19 Mpro and its comparison with the two most dangerous coronavirus epidemics, SARS and MERS CoVs. In addition, we used the first resolved COVID-19 crystal structure of Mpro to find suitable binding agents with proved safety. In this context, the FDA approved drugs were the best starting point in the way of repurposing approved and safe drugs against COVID-19.
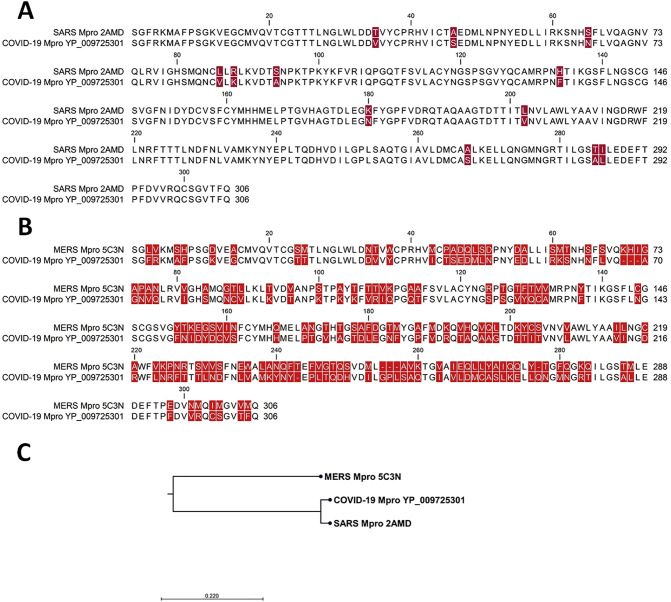
At first, the Mpro from COVID-19 was compared with SARS and MERS CoVs (Fig. 1 ). Comparing COVID-19 Mpro with SARS Mpro (Fig. 1A) and MERS CoV Mpro (Fig. 1B) revealed high degree of conservation between COVID-19 and SARS CoV Mpros and little number of different residues. However, MERS CoV Mpro showed large number of differences and low conservation with COVID-19 Mpro. This was evidenced by the phylogenetic relations (Fig. 1C), which showed close relation of SARS and COVID-19 Mpros and distant relation to MERS CoV.
Fig. 1.
Sequences alignment and phylogenetic relations of Mpro from SARS CoV, MERS CoV and COVID-19. A) Pairwise sequence alignment of COVID-19 and SARS CoVs Mpro. Conserved residues are not highlighted and displayed in black colour. The different residues are highlighted in by red colour B) pairwise sequence comparison of COVID-19 and MERS CoVs. Conserved residues are not highlighted and displayed in black colour. The different residues are highlighted in by red colour. C) the phylogenetic relations of Mpro, the tree was generated after the maximum likelihood. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Statistics of sequences comparison was generated in a pairwise sequence comparison matrix (Table 1 ). There was 96.08% identity between COVID-19 and SARS CoV Mpro, while low identity% of 51.61 was detected for COVID-19 and MERS CoV Mpro. The number of COVID-19 amino acid differences was 12 and 153 for SARS CoV and MERS CoV, respectively.
Table 1.
Sequence comparison matrix of COVID-19, SARS and MERS CoVs Mpro. The upper diagonal panel shows the number of amino acids differences. The lower diagonal panel shows the identity%.
| COVID-19 | SARS CoV | MERS CoV | |
|---|---|---|---|
| COVID-19 | 12 | 150 | |
| SARS CoV | 96.08 | 153 | |
| MERS CoV | 51.61 | 50.65 |
The results of virtual screening of FDA approved drugs against COVID-Mpro is presented in Supplementary Table 1. The presented parameters include the docking scores, ligand efficiency, lipophilic and hydrogen bonding interactions. The top 20 hits showing the highest docking score are provided in Table 2. The data were presented as a relative value to curcumin (a previously approved SARS Mpro inhibitor). Curcumin docking score was ranked at the 334 position, implying the presence of wide range of expected more powerful binding drugs. Within the top 20 drugs there was 2 antivirals, 2 antituberculous agents, 2 vitamins, 1 anticancer and other miscellaneous systemically acting drugs.
Table 2.
The top 20 drugs showing the highest docking score.
| Item name | Formula | MW | Relative docking score | Relative ligand efficiency | Relative glide lipo | Relative glide hbond | Clinical uses |
|---|---|---|---|---|---|---|---|
| Chromocarb | C10H6O4 | 190 | 2.08 | 4.03 | 0.92 | 4.80 | Vasoprotective |
| Ribavirin | C8H12N4O5 | 244 | 2.01 | 3.21 | 0.37 | 4.36 | Antiviral |
| Telbivudine | C10H14N2O5 | 242 | 2.00 | 3.19 | 0.44 | 6.12 | Hepatitis B virus |
| Vitamin B12 | C63H88CoN14O14P | 1355 | 1.99 | 0.60 | 0.49 | 9.70 | Vitamin |
| Aminophylline | C16H24N10O4 | 420 | 1.92 | 4.00 | 0.46 | 3.20 | Bronchodilator |
| Nicotinamide | C6H6N2O | 122 | 1.91 | 5.76 | 0.71 | 5.76 | Vitamin |
| Triflusal | C10H7F3O4 | 248 | 1.87 | 2.99 | 1.05 | 2.53 | Cardiovascular |
| Bemegride | C8H13NO2 | 155 | 1.83 | 4.52 | 0.68 | 3.88 | CNS stimulant |
| Aminosalicylate Sodium | C7H6NNaO3 | 175 | 1.80 | 4.44 | 0.76 | 1.60 | Antituberculosis agents |
| Pyrazinamide | C5H5N3O | 123 | 1.80 | 5.42 | 0.52 | 4.29 | Antituberculosis agents |
| Temozolomide | C6H6N6O2 | 194 | 1.79 | 3.47 | 0.12 | 3.20 | Anticancer |
| Methazolamide | C5H8N4O3S2 | 236 | 1.78 | 3.45 | 0.00 | 5.76 | Glaucoma |
| Tioxolone | C7H4O3S | 168 | 1.78 | 4.38 | 0.92 | 3.20 | Anti-acne |
| Propylthiouracil | C7H10N2OS | 170 | 1.77 | 4.38 | 0.56 | 3.20 | Antithyroid agent |
| Cysteamine HCl | C2H8ClNS | 114 | 1.77 | 12.01 | 0.00 | 2.00 | Nephropathic cystinosis |
| methoxamine hydrochloride | C11H18ClNO3 | 248 | 1.77 | 3.20 | 0.10 | 5.09 | Alpha-adrenergic agonist |
| Zonisamide | C8H8N2O3S | 212 | 1.76 | 3.42 | 0.47 | 3.20 | Anticonvulsant |
| (+,-)-Octopamine HCl | C8H12ClNO2 | 190 | 1.76 | 4.33 | 0.09 | 5.09 | Adrenergic agonist |
| Amiloride hydrochloride | C6H9Cl2N7O | 266 | 1.76 | 3.18 | 0.30 | 6.69 | Diuretic |
Among antivirals, ribavirin and telbivudine were ranked at the second and third positions. They showed 2 folds increase in the docking scores, compared with curcumin. Vitamin B12 and nicotinamide were ranked at the 4th and 6th positions.
Ribavirin is abroad spectrum antiviral agent acting by induction of mutations in the viral genome, especially in RNA viruses [24]. Ribavirin is officially approved for treating respiratory syncytial virus (RSV) infection and, in combination with interferon α2b for hepatitis C virus [25]. In addition, it was used for treating SARS CoV infections [25]. Given the high similarity of SARS and COVID-19 Mpros, ribavirin might be of a value in treating COVID-19.
Telbivudine is approved for treating hepatitis B virus [26]. The drug is nucleoside analogue and best fits the viral polymerase. The high rank of telbivudine in this study suggests its repurposing for COVID-19 treatment by its binding to Mpro.
In comparison with curcumin, the top 10 ranked compounds showed improved hydrogen bonding profile. There was 1.6–9.7 fold increase in hydrogen bonding interactions. In contrast, the hydrophobic interactions were kept constant or lowered.
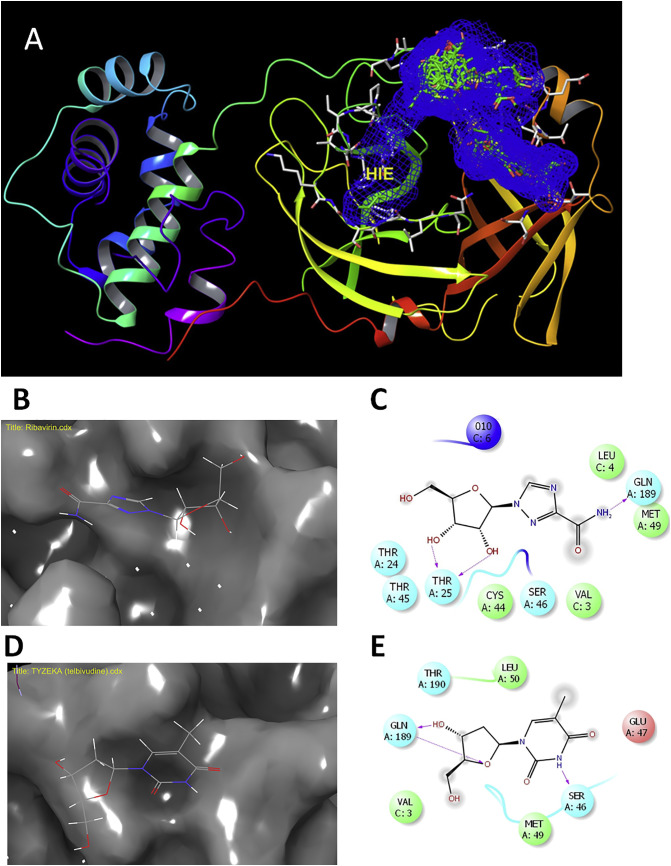
By inspection of the binding mode of top ranked drugs, hydrogen bonding and hydrophobic interactions were the driving force for binding (Fig. 2 ). Ribavirin formed two hydrogen bonds with the backbone of THR25 and side chain of GLN189 (Fig. 2B and C). Telbivudine formed two hydrogen bonds with SER49 and GLV189 (Fig. 2D and E).
Fig. 2.
Virtual screening and docking of FDA drugs with COVID-19 Mpro. A) The top 40 compounds docked into the Mpro binding site. B) The docking site of ribavirin C) The ligand interactions of ribavirin with Mpro D) The site of docking of telbivudine E) The ligand interactions of telbivudine with Mpro. Hydrogen bonds are shown in purple arrows, hydrophobic interactions in grey circles. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In conclusion, molecular modeling tools were used to compare the Mpro of the recently evolved COVID-19 and the previous two coronavirus epidemics SARS and MERS CoV. COVID-19 Mpro was more phylogenetically related to SARS Mpro. After a virtual screening capmain against COVID-19 Mpro, a set of antivirals, vitamins, antimicrobials, and other systemically acting drugs were more potent binding with COVID-19 Mpro, compared with curcumin (known Mpro inhibitor). The repurposing of ribavirin, telbivudine, vitamin B12 and nicotinamide is suggested.
The following is the supplementary data related to this article.
The docking output parameters for FDA approved drugs dataset against SARS-CoV-2 Mpro.
Acknowledgments
Acknowledgments
The authors acknowledge the Deanship of Scientific Research, King Faisal University for the financial support under Research Groups track (Grant No. 1811016).
Data availability
All data are within the manuscript and the supplementary materials.
Declaration of competing interest
The authors declare no conflict of interest.
References
- 1.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020:1–4. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peiris J., Lai S., Poon L., Guan Y., Yam L., Lim W. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W., Wong S.-K., Li F., Kuhn J.H., Huang I.-C., Choe H. Animal origins of the severe acute respiratory syndrome coronavirus: insight from ACE2-S-protein interactions. J. Virol. 2006;80:4211–4219. doi: 10.1128/JVI.80.9.4211-4219.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 5.Desenclos J.-C., Van der Werf S., Bonmarin I., Levy-Bruhl D., Yazdanpanah Y., Hoen B. Introduction of SARS in France, March–April, 2003. Emerg. Infect. Dis. 2004;10:195. doi: 10.3201/eid1002.030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guarner J. Three emerging coronaviruses in two decades: the story of SARS, MERS, and now COVID-19. Am. J. Clin. Pathol. 2020;153(4):420–421. doi: 10.1093/ajcp/aqaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J. Virol. 2020;94(7):e00127–20. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilgenfeld R. From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 2014;281:4085–4096. doi: 10.1111/febs.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kandeel M., Altaher A., Alnazawi M. Molecular dynamics and inhibition of MERS CoV papain-like protease by small molecule imidazole and aminopurine derivatives. Lett. Drug Des. Discov. 2019;16:584–591. [Google Scholar]
- 10.Li Y.-H., Hu C.-Y., Wu N.-P., Yao H.-P., Li L.-J. Molecular characteristics, functions, and related pathogenicity of MERS-CoV proteins. Engineering. 2019;5(5):940–947. doi: 10.1016/j.eng.2018.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pillaiyar T., Meenakshisundaram S., Manickam M. Recent discovery and development of inhibitors targeting coronaviruses. Drug Discov. Today. 2020 doi: 10.1016/j.drudis.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.-Y. Coronaviruses—drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15:327. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen C.-C., Kuo Y.-H., Jan J.-T., Liang P.-H., Wang S.-Y., Liu H.-G. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007;50:4087–4095. doi: 10.1021/jm070295s. [DOI] [PubMed] [Google Scholar]
- 14.Altaher Y., Kandeel M. Molecular analysis of some camel cytochrome P450 enzymes reveals lower evolution and drug-binding properties. J. Biomol. Struct. Dyn. 2016;34:115–124. doi: 10.1080/07391102.2015.1014423. [DOI] [PubMed] [Google Scholar]
- 15.Altaher Y., Nakanishi M., Kandeel M. Annotation of camel genome for estimation of drug binding power, evolution and adaption of cytochrome P450 1a2. Int. J. Pharmacol. 2015;11:243–247. [Google Scholar]
- 16.Kandeel M., Ando T., Kitamura Y., Abdel-Aziz M., Kitade Y. Mutational, inhibitory and microcalorimetric analyses of Plasmodium falciparum TMP kinase. Implications for drug discovery. Parasitology. 2009;136:11–25. doi: 10.1017/S0031182008005301. [DOI] [PubMed] [Google Scholar]
- 17.Murgueitio M.S., Bermudez M., Mortier J., Wolber G. In silico virtual screening approaches for anti-viral drug discovery. Drug Discov. Today Technol. 2012;9 doi: 10.1016/j.ddtec.2012.07.009. (e219-e25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheikh A., Al-Taher A., Al-Nazawi M., Al-Mubarak A.I., Kandeel M. Analysis of preferred codon usage in the coronavirus N genes and their implications for genome evolution and vaccine design. J. Virol. Methods. 2020;277 doi: 10.1016/j.jviromet.2019.113806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu B., Zhou J. SARS-CoV protease inhibitors design using virtual screening method from natural products libraries. J. Comput. Chem. 2005;26:484–490. doi: 10.1002/jcc.20186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sirois S., Wei D.-Q., Du Q., Chou K.-C. Virtual screening for SARS-CoV protease based on KZ7088 pharmacophore points. J. Chem. Inf. Comput. Sci. 2004;44:1111–1122. doi: 10.1021/ci034270n. [DOI] [PubMed] [Google Scholar]
- 21.ElHefnawi M., ElGamacy M., Fares M. Multiple virtual screening approaches for finding new Hepatitis c virus RNA-dependent RNA polymerase inhibitors: structure-based screens and molecular dynamics for the pursue of new poly pharmacological inhibitors. BMC Bioinf. BioMed Central. 2012:S5. doi: 10.1186/1471-2105-13-S17-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Z., Khaliq M., Suk J.-E., Patkar C., Li L., Kuhn R.J. Antiviral compounds discovered by virtual screening of small− molecule libraries against dengue virus E protein. ACS Chem. Biol. 2008;3:765–775. doi: 10.1021/cb800176t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raj U., Varadwaj P.K. Flavonoids as multi-target inhibitors for proteins associated with Ebola virus: in silico discovery using virtual screening and molecular docking studies. Interdiscip. Sci. Comput. Life Sci. 2016;8:132–141. doi: 10.1007/s12539-015-0109-8. [DOI] [PubMed] [Google Scholar]
- 24.Crotty S., Maag D., Arnold J.J., Zhong W., Lau J.Y., Hong Z. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat. Med. 2000;6:1375–1379. doi: 10.1038/82191. [DOI] [PubMed] [Google Scholar]
- 25.Koren G., King S., Knowles S., Phillips E. Ribavirin in the treatment of SARS: a new trick for an old drug? CMAJ. 2003;168:1289–1292. [PMC free article] [PubMed] [Google Scholar]
- 26.McKeage K., Keam S.J. Telbivudine. Drugs. 2010;70:1857–1883. doi: 10.2165/11204330-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Friesner R.A., Jay J., Murphy T.A., Halgren J.J., Klicic D.T., Mainz M.P., Repasky et al. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004;47(7):1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 28.Halgren T.A., Murphy R.B., Friesner R.A., Beard H.S., Frye L.L., Pollard W.T., Banks J.L. Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004;47(7):1750–1759. doi: 10.1021/jm030644s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The docking output parameters for FDA approved drugs dataset against SARS-CoV-2 Mpro.
Data Availability Statement
All data are within the manuscript and the supplementary materials.