Abstract
Background
Coronavirus disease 2019 (COVID-19) has spread outside the initial epicenter of Wuhan. We compared cases in Guangzhou and Wuhan to illustrate potential changes in pathogenicity and epidemiological characteristics as the epidemic has progressed.
Methods
We studied 20 patients admitted to the Third Affiliated Hospital of Sun Yat-Sen University in Guangzhou, China from January 22 to February 12, 2020. Data were extracted from medical records. These cases were compared with the 99 cases, previously published in Lancet, from Wuhan Jinyintan Hospital from January 1 to January 20, 2020.
Results
Guangzhou patients were younger and had better prognosis than Wuhan patients. The Wuhan patients were more likely to be admitted to the ICU (23% vs 5%) and had a higher mortality rate (11% vs 0%). Cases in Guangzhou tended to be more community clustered. Diarrhea and vomiting were more common among Guangzhou patients and SARS-CoV-2 RNA was found in feces. Fecal SARA-CoV-2 RNA remained positive when nasopharyngeal swabs turned negative in some patients.
Conclusions
This study indicates possible diminishing virulence of the virus in the process of transmission. Yet persistent positive RNA in feces after negative nasopharyngeal swabs suggests a possible prolonged transmission period that challenges current quarantine practices.
Keywords: Coronavirus disease 2019, Severe acute respiratory syndrome-related coronavirus 2, Epidemiology, Clinical features, Virulence, Transmission
List of Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; CDC, Centre for Disease Control and Prevention; RNA, ribonucleic acid; ICU, intensive care unit; ESR, erythrocyte sedimentation rate; CRP, C-reaction protein; PCT, Procalcitonin; CT, computerized tomography
1. Introduction
Coronaviruses are enveloped non-segmented positive-sense RNA viruses belonging to the family Coronaviridae, with a genome ranging from 26 to 32 kilobases in length [1]. Among the several coronaviruses that are pathogenic to humans, most are associated with mild clinical symptoms, with two notable exceptions: severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) [2,3], which have caused more than 10000 cumulative cases in the past two decades with mortality rates of 9.5% and 34.4% respectively [[4], [5], [6]]. In December 2019, a novel pneumonia, termed coronavirus disease 2019 (COVID-19), emerged in Wuhan, China and has been proven to be caused by a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [7]. All the twenty-seven first cases reported by December 31, 2019 were closely related to exposure with the Huanan Seafood Market in Wuhan [8]. By analyzing the genome sequence of SARS-CoV-2, it was speculated that the pathogen originated in bat, the natural reservoir host, and then transmitted to humans via an uncertain wild animal intermediate host [9]. There is convincing evidence that the human-to-human transmission has occurred since the end of December 2019 or the early January 2020 [10]. To control the spread of COVID-19, the Wuhan authority has sealed off the entire city from outside contact since Jan 23, 2020. However, it's estimated that five million people have left Wuhan before the “lockdown” and travelled throughout China [11]. By April 1, 2020, the disease has affected over 200 countries, with over 880,000 confirmed cases and 44220 deaths [[12], [13], [14], [15], [16], [17], [18]]. In China, substantial attention has focused mainly on Wuhan, whereas the detailed information regarding the disease outside Wuhan is rarely reported [19,20]. Furthermore, little is known about the changes in pathogenicity, clinical manifestations and mode of transmission of SARS-CoV-2 when the epidemic expanded beyond Wuhan.
Here, we summarized the epidemiologic and clinical characteristics of 20 laboratory-confirmed cases of COVID-19 in Guangdong who hospitalized in the 3rd affiliated hospital of Sun Yat-sen University from January 22 to February 18, 2020, and compared these cases with previous reported cases in Wuhan, aiming to provide information for understanding the changes in clinical manifestations and transmission model.
2. Methods
2.1. Study design and participants
The study was approved by the Third Affiliated Hospital of Sun Yat-Sen University Ethics Committee, and oral consent was obtained from patients. All confirmed patients with COVID-19 admitted to the Third Affiliated Hospital of Sun Yat-Sen University from Jan 22 to Feb 12, 2020 were enrolled and followed-up until February 18, 2020. All patients with COVID-19 enrolled in this study were diagnosed according to the WHO interim guidance [21]. To compare the epidemiological and clinical characteristics between patients in Guangdong and Wuhan (epidemic area), we included previously published data on 99 cases diagnosed in Wuhan Jinyintan Hospital from January 1 to January 20, 2020. Wuhan patients were followed-up until January 25, 2020 [22].
2.2. Data collection
The epidemiological characteristics (including recent exposure history), clinical symptoms and signs, radiologic and laboratory findings were extracted from electronic medical records. Radiologic assessments included chest X-ray or computed tomography (CT). Laboratory assessments consisted of complete blood count, blood chemistry, coagulation test, liver and renal function, electrolytes, C-reactive protein (CRP), procalcitonin (PCT), lactate dehydrogenase and creatine kinase. ARDS was defined according to the Berlin definition [23].
2.3. Laboratory confirmation of SARS-Cov-2 infection
The viral nucleic acid testing-based laboratory confirmation of SARS-CoV-2, performed by the hospital's laboratory and the key laboratory of Guangdong Centre for Disease Control and Prevention (CDC), China, was determined by real-time RT-PCR according to the Chinese national CDC recommended protocol. Firstly, the nasopharyngeal swab was collected every 2 days during hospitalization. Then the RNA samples from the nasal swab specimens were extracted and subjected to the real-time RT-PCR test using SARS-Cov-2 specific primers and probes. Specifically, the primers for the open reading frame 1 ab (ORF1ab) are 5′-CCCTGTGGGTTTTACACTTAA-3' (Forward) and 5′-ACGATTGTGCATCAGCTGA-3' (Reverse); the corresponding probe is 5′-CY3-CCGTCTGCGGTATGTGGAAAGGTTATGG-BHQ1-3′. Primers for nucleocapsid protein (N) are 5′-GGGGAACTTCTCCTGCTAGAAT-3' (Forward) and 5′-CAGACATTTTGCTCTCAAGCTG-3' (Reverse); the corresponding probe is 5′-FAM-TTGCTGCTGCTTGACAGATT-TAMRA-3′. The clearance of SARS-CoV-2 was defined as two consecutive negative results with qPCR detection at an interval of 24 h.
2.4. Statistical analysis
Continuous variables were expressed as mean (SD) or median (IQR) and compared with the t-test or Mann-Whitney U test; categorical variables were expressed as frequency (%) and compared by χ2 test or Fisher's exact test between the cases in Guangzhou and Wuhan. For laboratory indicators, we categorized the results into normal or abnormal (increased or decreased). We used SPSS (IBM, version 26.0) for all analyses.
2.5. Role of the funding source
Funding sources had no role in this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Demographics, baseline characteristics and clinical characteristics
All the COVID-2019 patients (N = 20) admitted in the Third Affiliated Hospital of Sun Yat-sen University were enrolled, including 10 males and 10 females. Patients in Guangzhou were younger than those in Wuhan (43.2 vs 55.5 years, P < 0.001). Among Guangzhou patients, 14 had a history of living or travelling in Wuhan, and 6 were local incident patients with a history of close contact with confirmed cases. 10 Guangzhou patients were from seven clusters, including five family clusters, one cruise ship cluster and one driving tour cluster to Wuhan, while only two shoppers were community clustered among Wuhan patients.
Patients in both cities mainly manifested fever, cough and myalgia. Cough (82% vs 55%, P = 0.017) and shortness of breath (31% vs 10%, P = 0.052) were more prevalent among patients in Wuhan, while patients in Guangzhou had a higher prevalence of diarrhea (2% vs 25%, P = 0.001) and vomiting (1% vs 15%, P = 0.015). Higher percentages of patients were admitted to the ICU (23% vs 5%, P = 0.072) and complications (33% vs 15%, P = 0.118), such as ARDS (17% vs 1%, P = 0.302) in Wuhan (Table 1 ).
Table 1.
Baseline characteristics and epidemiology of patients with COVID-19 within and outside Wuhan.
| Guangzhou#1 (N = 20) | Wuhan#2 (N = 99) | P values | |
|---|---|---|---|
| Characteristics | |||
| Age, years, Mean (SD) | 43.2 (14.0) | 55.5 (13.1) | <0.001 |
| Range | 25–64 | 21–82 | |
| ≤39 | 9 (45.0%) | 10 (10.1%) | 0.002 |
| 40-49 | 4 (20.0%) | 22 (22.2%) | |
| 50-59 | 2 (10.0%) | 30 (30.3%) | |
| 60-69 | 5 (25.0%) | 22 (22.2%) | |
| ≥70 | 0 (0.0%) | 15 (15.2%) | |
| Sex | 0.198 | ||
| Female | 10 (50.0%) | 32 (32.3%) | |
| Male | 10 (50.0%) | 67 (67.7%) | |
| Chronic medical illness | 7 (35.0%) | 50 (50.5%) | 0.229 |
| Cardiovascular and cerebrovascular diseases | 5 (25.0%) | 40 (40.4%) | 0.209 |
| Digestive system disease | 3 (15.0%) | 11 (11.1%) | 0.703 |
| Endocrine system disease | 1 (5.0%) | 13 (13.1%) | 0.460 |
| Malignant tumor | 0 (0.0%) | 1 (1.0%) | 1.000 |
| Respiratory system disease | 1 (5.0%) | 1 (1.0%) | 0.309 |
| Admission to intensive care unit |
1 (5.0%) |
23 (23.2%) |
0.072 |
|
Signs and symptoms | |||
| Fever | 16 (80.0%) | 82 (82.8%) | 0.752 |
| Cough | 11 (55.0%) | 81 (81.8%) | 0.017 |
| Shortness of breath | 2 (10.0%) | 31 (31.3%) | 0.052 |
| Myalgia | 7 (35.0%) | 11 (11.1%) | 0.013 |
| Sore throat | 4 (20.0%) | 5 (5.1%) | 0.043 |
| Diarrhea | 5 (25.0%) | 2 (2.0%) | 0.001 |
| Nausea and vomiting | 3 (15.0%) | 1 (1.0%) | 0.015 |
| More than one sign or symptom | 18 (90.0%) | 89 (89.9%) | 1.000 |
| Fever, cough, and shortness of breath |
2 (10.0%) |
15 (15.2%) |
0.734 |
|
Comorbid conditions | |||
| Any | 3 (15.0%) | 33 (33.3%) | 0.118 |
| ARDS | 1 (5.0%) | 17 (17.2%) | 0.302 |
| Acute renal injury | 0 (0.0%) | 3 (3.0%) | 1.000 |
| Acute respiratory injury | 1 (5.0%) | 8 (8.1%) | 1.000 |
| Septic shock | 1 (5.0%) | 4 (4.0%) | 1.000 |
| Ventilator-associated pneumonia |
0 (0.0%) |
1 (1.0%) |
1.000 |
|
Epidemiological survey | |||
| Exposure to Huanan seafood market | 0 (0.0%) | 49 (49.5%) | <0.001 |
| Live history in epidemic areaa | 14 (70.0%) | 99 (100.0%) | <0.001 |
| Community cluster outbreak | 10 (50.0%) | 2 (2.0%)#3 | <0.001 |
| Close contacts with COVID-19 patient | 6 (30.0%) | NA | NA |
Data are n (%) unless specified otherwise. N is the total number of patients with available data. ARDS = acute respiratory distress syndrome. COVID-19 = coronavirus disease −19. P values for comparing two groups were derived using Fisher's exact test for categorized variables and t-test for continuous variables.
#1 Admitted to the Third Affiliated Hospital of Sun Yat-Sen University in Guangzhou from January 22 to February 12, 2020, the last follow-up was on February 18, 2020.
#2 Diagnosed in Wuhan Jinyintan Hospital from January 1 to January 20, 2020, the last follow-up was on January 25, 2020. #3 Only include the cases without long-term exposure to Huanan seafood market.
NA, not available.
Epidemic area refers to Wuhan and other epidemic areas in Hubei Province.
3.2. Laboratory, imaging, and pathogenic characteristics of patients with COVID-19
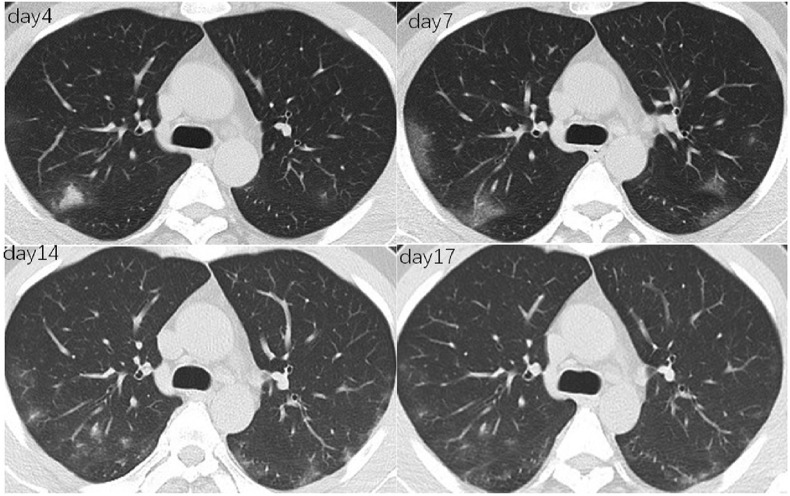
Blood routine and biochemical data of the patients in Guangzhou were basically normal, and only 25% of the patients had a lymphocyte count of less than 1.0 × 109/L. The lymphocyte count, hemoglobin and albumin of patients in Wuhan were significantly lower, while inflammatory biomarkers, blood erythrocyte sedimentation rate (ESR), CRP and PCT were significantly higher than those in Guangzhou (all P values < 0.05). Chest CT scan indicated bilateral involvement in both cities. Based on Guangzhou data, the main manifestations were multiple patches (90%), ground-glass opacity (80%) and interstitial lesions (80%). Invasive lesions were presented in 55% of patients (Fig. 1 ). Multiple mottling and ground-glass opacity were more common in Guangzhou patients (14% vs 60%, P < 0.001) (Table 2 ).
Fig. 1.
Dynamic changes of CT images in a 37-year-old man who confirmed COVID-19. Axial thin-section non-contrast CT scan shows bilateral multiple lobular ground-glass opacities progressed from day 4 to day 7 from onset. The non-contrast CT of day 14 and day 17 shows that bilateral lobular ground-glass opacities resolved gradually.
Table 2.
Comparison of Laboratory data and Outcome of Patients with COVID-19 Within and Outside Wuhan.
| Guangzhou#1 (N = 20) | Wuhan#2 (N = 99) | P values | |
|---|---|---|---|
|
Blood routine | |||
| Leucocytes ( × 109/L, normal range 3.5–9.5) | 5.2 (1.6) | 7.5 (3.6) | 0.006 |
| Lymphocytes ( × 109/L, normal range 1.1–3.2) | 1.4 (0.7) | 0.9 (0.5) | 0.003 |
| Platelets ( × 109/L, normal range 125.0–350.0) | 205.0 (62.6) | 213.5 (79.1) | 0.652 |
| Hemoglobin (g/L, normal range 130.0–175.0) |
139.3 (15.3) |
129.8 (14.8) |
0.010 |
|
Blood biochemistry | |||
| Increased alanine aminotransferase | 4 (20.0%) | 28 (28.3%) | 0.584 |
| Increased aspartate aminotransferase | 3 (15.0%) | 35 (35.4%) | 0.113 |
| Increased total bilirubin | 1 (5.0%) | 18 (18.2%) | 0.192 |
| Albumin (g/L) | 44.8 (5.6) | 31.6 (4.0) | <0.001 |
| Decreased | 1 (5.0%) | 97 (98.0%) | <0.001 |
| Increased serum creatinine | 1 (5.0%) | 3 (3.0%) | 0.526 |
| Increased creatine kinase | 5 (25.0%) | 13 (13.1%) | 0.183 |
| Increased myoglobin |
2/17 (11.8%) |
15 (15.2%) |
1.000 |
|
Infection-related biomarkers | |||
| Erythrocyte sedimentation rate (mm/h, normal range 0–15) | 19.3 (13.2) | 49.9 (23.4) | <0.001 |
| C-reaction protein (mg/L, normal range 0–6) | 25.0 (29.3) | 51.4 (41.8) | 0.002 |
| Procalcitonin (ng/mL, normal range 0–0.05) |
0.08 (0.09) |
0.5 (1.1) |
<0.001 |
|
Chest X-ray and CT finding | |||
| Unilateral pneumonia | 2 (10.0%) | 25 (25.3%) | 0.239 |
| Bilateral pneumonia | 18 (90.0%) | 74 (74.7%) | 0.239 |
| Multiple mottling and ground-glass opacity | 12 (60.0%) | 14 (14.1%) | <0.001 |
| Peripheral pneumonia | 17 (85.0%) | NA | NA |
| interstitial lesions | 16 (80.0%) | NA | NA |
| Ground-glass opacity | 16 (80.0%) | NA | NA |
| Multiple patches | 18 (90.0%) | NA | NA |
| Multiple Infiltration | 11 (55.0%) | NA | NA |
| Nodule | 4 (20.0%) | NA | NA |
| Lung consolidation | 4 (20.0%) | NA | NA |
| Pleural effusion | 2 (10.0%) | NA | NA |
Data are n (%) and mean (SD). N is the total number of patients with available data. COVID-19 = coronavirus disease −2019 P values for comparing two groups were derived using Fisher's exact test for categorized variables and t-test for continuous variables.
#1 Admitted to the Third Affiliated Hospital of Sun Yat-Sen University in Guangzhou from January 22 to February 12, 2020, the last follow-up was on February 18, 2020.
#2 Diagnosed in Wuhan Jinyintan Hospital from January 1 to January 20, 2020, the last follow-up was on January 25, 2020.
NA, not available.
3.3. Treatment and prognosis of patients with COVID-19
In both cities, most patients were mainly treated with antibiotics or antivirus therapy, including Lopinavir and Ritonavir (Kaletra), arbidol, ribavirin and aerosol inhalation of interferon alpha, 13 (65%) patients were given combination antivirus therapy (data only available in Guangzhou patients) (Table 3 ). 27%–30% of patients were treated with immunoglobulin and 20% with thymosin alpha-1 (data only available in Guangzhou patients) to regulate immunity. 25% of patients in Guangzhou, compared to 19.2% in Wuhan, with severe pulmonary inflammation and decreased oxygenation index received short-term corticosteroids treatment, with methylprednisolone 1 mg/kg/day for 3 days. More patients in Wuhan needed oxygen treatment than those in Guangzhou (75% vs 40%, P = 0.001). By the last follow-up date, the patients in Guangzhou have a higher discharged rate and lower mortality rate than those in Wuhan (P = 0.005).
Table 3.
Treatment and prognosis of patients with COVID in Guangzhou and Wuhan.
| Guangzhou#1 (N = 20) | Wuhan#2 (N = 99) | P value | |
|---|---|---|---|
|
Treatment | |||
| Oxygen therapy | 8 (40.0%) | 75 (75.8%) | 0.001 |
| Mechanical ventilation | 2 (10.0%) | 17 (17.2%) | 0.738 |
| Non-invasive (ie, face mask) | 1 (5.0%) | 13 (13.1%) | 0.460 |
| Invasive | 1 (5.0%) | 4 (4.0%) | 1.000 |
| CRRT | 0 (0.0%) | 9 (9.1%) | 0.354 |
| ECMO | 0 (0.0%) | 3 (3.0%) | 1.000 |
| Antiviral therapy | 16 (80.0%) | 75 (76.0%) | 0.781 |
| Kaletra* | 16 (80.0%) | NA | NA |
| Arbidol | 13 (65.0%) | NA | NA |
| Interferon alpha atomized inhalation | 5 (25.0%) | NA | NA |
| Ribavirin | 1 (5.0%) | NA | NA |
| Combination of two antiviral drugs | 9 (45.0%) | NA | NA |
| Combination of three antiviral drugs | 4 (20.0%) | NA | NA |
| Antibiotic therapy | 17 (85.0%) | 70 (70.7%) | 0.271 |
| Glucocorticoids | 5 (25.0%) | 19 (19.2%) | 0.550 |
| Intravenous immunoglobulin therapy | 6 (30.0%) | 27 (27.3%) | 0.789 |
| Thymosin alpha1 | 4 (20.0%) | NA | NA |
| Chinese traditional medicine |
20 (100.0%) |
NA |
NA |
|
SARS-CoV-2 RNA positive at admission | |||
| Nasopharyngeal swabs | 20 (100.0%) | 99 (100.0%) | 1.000 |
| Feces (n/N, %) |
4/7 (57.1%) |
NA |
NA |
|
Duration of SARS-CoV-2 RNA positive | |||
| Nasopharyngeal swabs (N = 20) (days) | 12.0 (7.5, 15.5) | NA | NA |
| Feces (N = 4 of 7) (range, days) |
4.0–16.0 |
NA |
NA |
|
Clinical outcome | |||
| Outcome | 0.005 | ||
| Remained in hospital | 6 (30.0%) | 57 (57.6%) | |
| Discharged | 14 (70.0%) | 31 (31.3%) | |
| Died | 0 (0.0%) | 11 (11.1%) | |
Data are n (%) and mean (SD). N is the total number of patients with available data. COVID-19 = coronavirus disease-2019. SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2. CRRT = continuous renal replacement therapy. ECMO = extracorporeal membrane oxygenation. P values for comparing two groups were derived using Fisher's exact test for categorized variables and t-test for continuous variables. *Kaletra: Lopinavir and ritonavir.
#1 Admitted to the Third Affiliated Hospital of Sun Yat-Sen University in Guangzhou from January 22 to February 12, 2020, the last follow-up was on February 18, 2020.
#2 Diagnosed in Wuhan Jinyintan Hospital from January 1 to January 20, 2020, the last follow-up was on January 25, 2020.
NA, not available.
3.4. SARS-CoV-2 RNA in nasopharyngeal swabs and feces
Among Guangzhou patients, SARS-CoV-2 RNA in nasopharyngeal swabs disappeared at an average of 12 days (maximum, 22 days). SARS-CoV-2 RNA in feces turned negative in 4–16 days. In two cases, the fecal RNA did not turn negative until 5 and 6 days after negative nasopharyngeal swab.
4. Discussion
By comparing cases from the Third Affiliated Hospital of Sun Yat-sen University between January 22 to February 18, 2020 and Wuhan Jinyintan Hospital between January 1 to January 25, 2020, this study revealed the changes in pathogenicity and epidemic characteristics of COVID-19 in the epidemic process. Both hospitals are designated hospital for COVID-19. Although small in number, the Guangzhou patients were a random sample at an early stage when cases started being imported into a major city. The different admission time in the two hospitals could represent two stages of the epidemic, while treatment and follow-up time are the same, which may well reflect the changing clinical and epidemiological characteristics of the disease.
The median ages of cases in Wuhan reported by Cao and Zhang were 49.0 and 55.5 years respectively8,22, while patients in Guangzhou were relatively younger, with the median age of 43.2 years. There have been reports of cases in children, indicating all ages are susceptible [24]. Compared with patients in Guangzhou, patients in Wuhan were more severe, reflected by that Wuhan patients had a higher incidence of dyspnea and ARDS, higher levels of inflammatory biomarkers and worse prognosis. These differences could be due to age and pre-existing conditions [8,25,26]. However, in the present study, we did not observe an evident difference in underlying disease between Guangzhou patients and Wuhan patients. We speculate that the differences may be related to that the diminishing pathogenicity of the virus after transmission of multiple generations [5,27], however, further studies are required to confirm this hypothesis. Besides, patients in Guangzhou were more aware of the disease; thus, they visited the hospital at the earlier stage with less severe symptoms. Furthermore, given the experience in Wuhan, the health authorities in Guangzhou were well-prepared for the epidemic and were able to carry out comprehensive screening, early case identification and contact tracing and timely treatment.
In terms of clinical characteristics, we observed that the gastrointestinal symptoms such as diarrhea and vomiting were more prevalent in patients with later-onset in Guangzhou. Meanwhile, fecal RNA was positive in 4 out of 7 patients, indicating the possibility of gastrointestinal transmission. Xiao Fei also confirmed gastrointestinal infection by detecting SARS-CoV-2 in feces and gastrointestinal tissues [28]. These findings have important implications for patient triage and hospital risk zoning. Gastroenterology outpatient medical staff could be at higher risk when encountering COVID patients, thus more aggressive PPE protection and proactive patient screening are necessary. In addition, sufficient education and protocols should be given to caregivers who could be exposed to the patients' vomits and feces directly, and feces disposal should be managed properly to reduce environmental harzards. More importantly, we found that two patients’ fecal virus RNA turned negative 5 and 6 days later than the nasopharyngeal swab respectively, suggesting that transimission of virus is possibly prolonged, and fecal virus RNA may be an indispensable indicator for lifting quarantines. Given that some “recovered” cases being tested positive again a few days after discharged have been reported recently [29], our findings highlight the importance of fecal virus test for patient management and quarantines. The health authority may also have to re-consider quarantine strategy for possible extended case management time and burden when distributing health resources.
Our findings also suggest that the modes of transmission have changed considerably with the spread of the disease. A large fraction of early reported cases in Wuhan were linked to Huanan seafood markets, whereas none of cases in Guangzhou had a history of exposure to wildlife markets. Furthermore, 10 patients from Guangzhou were involved in seven cases of clustering. This finding suggests strong community transmission poses a great challenge to the entire prevention and control. It resonates with the concept that the transmissibility increases while virulence decreases as virus spread [30]. As indicated by Tang's research, the L type of SARS-CoV-2 prevalent in the early stages of the outbreak in Wuhan, decreased after early January 2020, while the S type, which is less aggressive, have increased in relative frequency due to relatively weaker selective pressure [31]. Thus early detection and timely isolation is vital before a case becomes a cluster. It also raises the concern that the risk and benefit should be balanced in home quarantine for confirmed cases which could result in family case clusters.
At present, symptomatic, support treatment and airway maintenance are the main treatments, as there is no proven effective antiviral therapy. It is claimed that kaletra, remdesivir and chloroquine phosphate have antiviral effects from preliminary studies [32,33], but the results of clinical trials are yet to be released. Patients in Guangzhou mainly use kaletra + arbidol, some with severe pulmonary inflammation added interferon atomized inhalation, which has also achieved good treatment effect. Nevertheless, further research with control groups would be helpful to differentiate whether the effect is caused by drugs or patients’ self-recovery of the disease.
Our study has limitations. First, the number of patients was small. Second, Wuhan cases are from published data, and more detailed information, such as the final prognosis of patients, could not be obtained.
In summary, our results suggest that the virulence of SARA-CoV-2 is possibly waning in the process of transmission and the clustering occurrence is becoming the primary model of transmission. In addition, our observation that fecal virus RNA turned negative later than the nasopharyngeal swab justifies that the concern of faecal-oral transmission and suggest that fecal virus RNA should be assessed before lifting quarantine.
Funding
This work was supported by the National Science and Technology Major Project, China [Bing-Liang Lin, 2018ZX10302204, Bing-Liang Lin, 2017ZX10203201003], Emergency special program for 2019-nCoV of Guangdong province science and technology project, China (GDSTP-ESP) [Zhiliang Gao, 2020B111105001] and Tackling of key scientific and emergency special program of Sun Yat-sen University, China [SYSU-TKSESP, Bing-Liang Lin].
CRediT authorship contribution statement
Ziying Lei: Conceptualization, Investigation, Data curation, Formal analysis, Writing - original draft. Huijuan Cao: Formal analysis, Writing - original draft. Yusheng Jie: Investigation, Formal analysis. Zhanlian Huang: Investigation, Formal analysis. Xiaoyan Guo: Writing - original draft. Junfeng Chen: Investigation. Liang Peng: Resources. Hong Cao: Resources. Xiaoling Dai: Resources. Jing Liu: Formal analysis. Xuejun Li: Investigation. Jianyun Zhu: Investigation. Wenxiong Xu: Investigation. Dabiao Chen: Investigation. Zhiliang Gao: Resources, Writing - review & editing, Funding acquisition, Supervision. Jian-Rong He: Conceptualization, Methodology, Data curation, Formal analysis, Writing - review & editing. Bing-Liang Lin: Conceptualization, Methodology, Data curation, Formal analysis, Writing - review & editing, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
We thank all patients involved in the study. We thank Mr. Jonathan Hsu and Mrs. Xulan Fu for proofreading this manuscript. Jian-Rong He was supported by China Scholarship Council-University of Oxford Joint Scholarship.
Contributor Information
Jian-Rong He, Email: jianrong.he@gtc.ox.ac.uk, hjr0703@163.com.
Bing-Liang Lin, Email: linbingl@mail.sysu.edu.cn.
References
- 1.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 Feb 3 doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuiken T., Fouchier R.A.M., Schutten M. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 4.WHO Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Dec 31. 2003. https://www.who.int/csr/sars/country/table2004_04_21/en/
- 5.WHO Middle East respiratory syndrome coronavirus (MERS-CoV) November, 2019. http://www.who.int/emergencies/mers-cov/en/
- 6.Munster V.J., Koopmans M., van Doremalen N., van Riel D., de Wit E. A novel coronavirus emerging in China - key questions for impact assessment. N Engl J Med. 2020;382:692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 7.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q., Guan X., Wu P. Early transmission dynamics in wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020. Jan 29 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X.1, J2 Zai, Wang X.1, Li Y.1. Potential of large "first generation" human-to-human transmission of 2019-nCoV. J Med Virol. 2020 Apr;92(4):448–454. doi: 10.1002/jmv.25693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.http://www.chinanews.com/gn/2020/01-27/9070527.shtml
- 12.COVID-19 coronavirus – update. https://virusncov.com/
- 13.Benvenuto D., Giovanetti M., Salemi M., etal The global spread of 2019-nCoV: a molecular evolutionary analysis. Pathog Glob Health. 2020 Feb 12:1–4. doi: 10.1080/20477724.2020.1725339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holshue M.L., DeBolt C. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020 Jan 31 doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giovanetti M., Benvenuto D., Angeletti S., Ciccozzi M. The first two cases of 2019-nCoV in Italy: where they come from? J Med Virol. 2020 Feb 5 doi: 10.1002/jmv.25699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernard Stoecklin S., Rolland P., Silue Y. First cases of coronavirus disease 2019 (COVID-19) in France: surveillance, investigations and control measures, January 2020. Euro Surveill. 2020 Feb;(6):25. doi: 10.2807/1560-7917.ES.2020.25.6.2000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothe C., Schunk M., Sothmann P. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020 Jan 30 doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J.Y., Ko J.H., Kim Y. Viral load kinetics of SARS-CoV-2 infection in first two patients in korea. J Kor Med Sci. 2020 Feb 24;35(7):e86. doi: 10.3346/jkms.2020.35.e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang D., Lin M., Wei L. Eepidemiologic and clinical characteristics of novel coronavirus infection involving 13 patients outside wuhan, China. J Am Med Assoc. 2020 Feb 7 doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu X.W., Wu X.X., Jiang X.G. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020 Feb 19;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infectionis suspected: interim guidance. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Published January 28. accessed Feb 29, 2020.
- 22.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan E., Brodie D., Slutsky A.S. Acute respiratory distress syndrome: advances in diagnosis and treatment. J Am Med Assoc. 2018 Feb 20;319(7):698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 24.Wang X.F., Yuan J., Zheng Y.J. Clinical and epidemiological characteristics of 34 children with 2019 novel coronavirus infection in Shenzhen. Zhonghua Er Ke Za Zhi. 2020 Feb 17;58:E008. doi: 10.3760/cma.j.issn.0578-1310.2020.0008. 0. [DOI] [PubMed] [Google Scholar]
- 25.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. 2020 Feb 17;41(2):145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. Zhonghua Liu Xing Bing Xue Za Zhi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. J Am Med Assoc. 2020 Feb 7 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim K.H., Tandi T.E., Choi J.W., Moon J.M., Kim M.S. Middle East respiratory syndrome coronavirus (MERS-CoV) outbreak in South Korea, 2015: epidemiology, characteristics and public health implications. J Hosp Infect. 2017;95:207–213. doi: 10.1016/j.jhin.2016.10.008. pmid:28153558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao Fei, Tang Meiwen, Zheng Xiaobin. Evidence for gastrointestinal infection of SARS-CoV-2.[J] Gastroenterology. 2020 doi: 10.1101/2020.02.17.20023721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.https://baijiahao.baidu.com/s?id=1658435841330054154&wfr=spider&for=pc
- 30.Hui D.S., Perlman S., Zumla A. Spread of MERS to South Korea and China. Lancet Respirat. Med. 2015;3(7):509–510. doi: 10.1016/S2213-2600(15)00238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang X, Wu C, Li X, et al. On the origin and continuing evolution of SARS-CoV-2. Nat Sci Rev nwaa036, 10.1093/nsr/nwaa036 (accessed Mar 8, 2020). [DOI] [PMC free article] [PubMed]
- 32.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020 Feb 19 doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 33.Yao Xueting, Ye Fei, Zhang Miao. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237/5801998. [DOI] [PMC free article] [PubMed] [Google Scholar]