The rapid spread of COVID19 infection across the globe is causing a health care emergency.
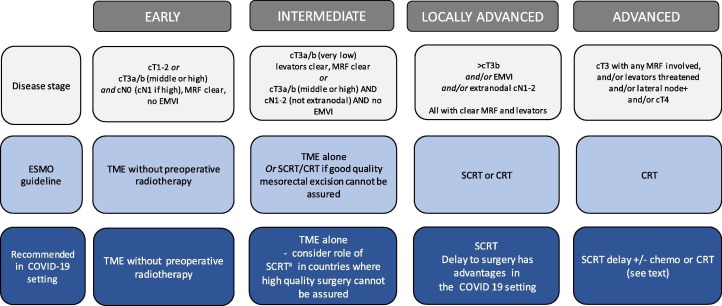
Our aim is to assist discussion about the risks and benefits to facilitate decision-making regarding radiotherapy for rectal cancer patients. In our roles as clinicians and as experts who have conducted clinical trials evaluating the role of radiotherapy in rectal cancer, we present our assessment of treatment options that should be considered by health care professionals in the setting of the COVID 19 pandemic. We want to minimize the risks to our patients whilst aiming to maintain cancer outcomes. We have used the European Society for Medical Oncology (ESMO) rectal cancer guidelines as a framework to describe our recommendations [1] (Fig. 1 ).
Fig. 1.
Treatment recommendations in the COVID-19 setting.
Early subgroup
ESMO Guideline: TME without pre-operative radiotherapy is recommended in most cases.
Recommendation: We strongly support the use of TME without pre-operative radiotherapy.
Intermediate subgroup
ESMO Guideline: TME alone or SCRT/CRT if good quality mesorectal excision cannot be assured.
Recommendation: In countries where high quality surgery is performed, we strongly recommend TME alone. Careful discussion of the use of radiotherapy in this group is needed in the COVID 19 setting where the benefits of preoperative radiotherapy are likely to be small. If radiotherapy is to be used, SCRT should be the preferred option rather than CRT (see below).
Locally advanced subgroup
ESMO Guideline: Pre-operative SCRT or CRT is recommended.
Recommendation: We strongly recommend the use of SCRT.
Two phase III trials have compared SCRT and CRT and demonstrate comparable outcomes for local recurrence, disease free survival (DFS), overall survival (OS) and late toxicity [2], [3]. Both approaches are widely used. In the COVID 19 setting there are some important factors to consider.
When the use of SCRT is compared with CRT there are many advantages of SCRT:- less acute toxicity; fewer radiotherapy treatment attendances; substantial reduction in travel and contact with other patients and staff; avoidance of any detrimental effect of concurrent chemotherapy on immune function; and thus significantly reduced risk of COVID 19 infection during treatment. The greater social distancing achieved with SCRT is a major advantage. An additional benefit is that the use of SCRT instead of CRT in this setting will have a substantial reduction in linear accelerator usage, will help avoid waiting time to start treatment and increase the ability of departments to treat all their patients in the setting of reduced staffing levels.
Timing of surgery after SCRT
ESMO Guideline: Not stated as the Stockholm III trial was published post guideline.
Recommendation: SCRT and a delay to surgery has advantages that may be beneficial in both routine clinical practice and in the COVID 19 setting.
The Dutch TME and MRC CR07 trials as well as the previous Swedish trials recommended that surgery should be performed within three to seven days of completion of SCRT [4], [5], [6]. The recently reported Stockholm III trial compared surgery performed within one week with 4–8 weeks after SCRT [7]. There was no difference in local recurrence, DFS and OS. A longer delay to surgery was associated with a reduction in post-operative and surgical morbidity but no difference in severe complications or re-operations. An admission rate of 6% was observed for the management of diarrhoea for patients who received SCRT and delay. 3D conformal radiotherapy techniques with a superior border of mid L5 were used. The use of SCRT and delay will result in approximately 10% of patients achieving a complete clinical response who may be offered an organ preservation strategy. If complete response is actively monitored, then further delay or even avoidance of surgery may be safely achieved (see below). Conversely, we note that this approach will delay the time to commencement of adjuvant chemotherapy, if considered indicated.
Advanced subgroup
ESMO Guideline: Pre-operative CRT or SCRT followed by neo-adjuvant chemotherapy is recommended. CRT is given as a fluoropyrimidine (usually capecitabine) combined with radiotherapy, commonly 45–50.4 Gy given over 5–5.5 weeks. The role of adjuvant chemotherapy is then considered with wide international variation in its use. The Polish-2 randomized phase III trial comparing CRT with SCRT followed by three two-weekly cycles of neoadjuvant chemotherapy reported similar cancer outcomes for local recurrence, DFS and OS [8]. The results of the phase III RAPIDO trial that compared CRT with pre-operative SCRT and 18 weeks of capecitabine+oxaliplatin chemotherapy are awaited. In this trial, only patients with very high-risk criteria for recurrence were included. There is currently no published level I evidence that demonstrated improvements in DFS or OS using neoadjuvant chemotherapy.
Recommendation: Based on the current evidence two options can be considered in the context of the COVID 19 pandemic:
-
1)
Pre-op CRT – this is the most established standard of care and the duration of concurrent capecitabine chemotherapy is limited to 5–5.5 weeks. It involves the use of long course of radiotherapy.
-
2)
SCRT +/− neoadjuvant chemotherapy – here the duration of radiotherapy is substantially less and the advantages of this approach when compared to CRT are described above.
We consider both options to be acceptable but note the advantages of using SCRT in the COVID 19 setting. The decision to use neoadjuvant chemotherapy in option 2 will reflect the attitudes to neoadjuvant and adjuvant chemotherapy in each country, the assessment of the risk–benefit ratio, considering the risk factors for COVID 19 increased mortality, and the capacity and prioritisation of chemotherapy delivery. The choice of chemotherapy regimen and duration is outside the scope of this document but should broadly align with the Polish trial with a preference for capecitabine-based chemotherapy.
In elderly patients, patients with poorer performance status, or patients not fit for chemotherapy or standard CRT, SCRT with a delay is strongly recommended.
Organ preservation
The use of an organ preserving strategy is increasingly considered when a complete clinical response is observed following CRT or SCRT and delay [9].
In some countries, radiotherapy is used in early stage disease to avoid the need for radical surgery. However, there is limited evidence for this approach, and it is not recommended outside clinical trials in several countries. In the context of COVID 19, if radiotherapy is used, we consider SCRT a preferred option rather than CRT for the reasons described above. This option should be considered in the context of surgical and radiotherapy capacity, and where possible in clinical studies.
An organ preservation approach may be considered during the COVID-19 period providing that resources for an adequate surveillance including imaging and endoscopy are available to detect local failures that require salvage surgery.
Conflict of interest
All authors have no conflicts of interest.
Footnotes
The Editors of the Journal, the Publisher and the European Society for Radiotherapy and Oncology (ESTRO) cannot take responsibility for the statements or opinions expressed by the authors of these articles. Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds or experiments described herein. Because of rapid advances in the medical sciences, in particular, independent verification of diagnoses and drug dosages should be made. For more information see the editorial “Radiotherapy & Oncology during the COVID-19 pandemic”, Vol. 146, 2020.
References
- 1.Glynne-Jones R., Wyrwicz L., Tiret E. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28 doi: 10.1093/annonc/mdx224. iv22-iv40. [DOI] [PubMed] [Google Scholar]
- 2.Ngan S.Y., Burmeister B., Fisher R.J. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol. 2012;30:3827–3833. doi: 10.1200/JCO.2012.42.9597. [DOI] [PubMed] [Google Scholar]
- 3.Bujko K., Nowacki M.P., Nasierowska-Guttmejer A., Michalski W., Bebenek M., Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215–1223. doi: 10.1002/bjs.5506. [DOI] [PubMed] [Google Scholar]
- 4.van Gijn W., Marijnen C.A., Nagtegaal I.D. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–582. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 5.Sebag-Montefiore D., Stephens R.J., Steele R. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–820. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folkesson J., Birgisson H., Pahlman L., Cedermark B., Glimelius B., Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005;23:5644–5650. doi: 10.1200/JCO.2005.08.144. [DOI] [PubMed] [Google Scholar]
- 7.Erlandsson J., Holm T., Pettersson D. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017;18:336–346. doi: 10.1016/S1470-2045(17)30086-4. [DOI] [PubMed] [Google Scholar]
- 8.Ciseł B., Pietrzak L., Michalski W. Long-course preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer:long-term results of the randomized Polish II study. Ann Oncol. 2019;30:1298–1303. doi: 10.1093/annonc/mdz186. [DOI] [PubMed] [Google Scholar]
- 9.van der Valk M.J.M., Hilling D.E., Bastiaannet E. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391(10139):2537–2545. doi: 10.1016/S0140-6736(18)31078-X. [DOI] [PubMed] [Google Scholar]