Abstract
Infectious bronchitis is caused by a coronavirus, infectious bronchitis virus (IBV). Infectious bronchitis is an acute and highly contagious disease of economic importance due to the reduction in weight gain observed with infected broilers and the drop in egg quality and production associated with infected laying hens. The presence of deep pectoral myopathy has been associated with IBV variants. This lesion is detected at slaughterhouses and is characterized by paleness and atrophy of the deep pectoral muscle, including necrosis of the region, leading to condemnations of the breast muscle, a valuable meat cut in the market. This work aimed to study the relationship between deep pectoral myopathy and IBV by describing tracheal and muscle lesions and comparing the frequency of IBV detection via reverse-transcription (RT) PCR in muscle, tracheal, and cecal tonsil samples from broilers with and without myopathy. A case-control study was conducted in 40 broiler flocks vaccinated with the Massachusetts strain. The case group consisted of 23 flocks that presented myopathic lesions under sanitary inspection and a control group of 17 flocks without myopathic lesions. The tracheal, cecal tonsil, and supracoracoid muscle (with and without lesions) samples from the 40 broiler flocks were screened by RT-PCR to detect IBV. Histopathology of muscle and tracheal tissue was carried out. Upon microscopic examination, the muscle samples from the case group presented extensive necrosis, intense mononuclear inflammatory infiltration, muscle fiber fragmentation, and fibrotic tissue, confirming myopathy, whereas muscles from the control group showed no alterations. The tracheal samples presented a large number of infiltrated mononuclear inflammatory cells that in some areas formed submucosal nodules. A total of 25 flocks tested IBV positive by RT-PCR: 14 from the case group and 11 from the control group. The IBV was detected by RT-PCR directly in muscle samples. Despite that, the relationship between deep pectoral myopathy and IBV was not established. The higher positive IBV RT-PCR percentage noted in the cecal tonsil samples demonstrates how important the choice of organs is for diagnostic purposes.
Key words: infectious bronchitis, deep pectoral myopathy, reverse-transcription polymerase chain reaction, broiler
INTRODUCTION
Infectious bronchitis (IB) is an acute and highly contagious disease that affects laying hen and broiler production (Cavanagh and Naqui, 2003). Infectious bronchitis is caused by a coronavirus, the IB virus (IBV), which primarily induces respiratory disease. Nevertheless, the virus is able to replicate in nonepithelial respiratory tissues, where it can cause disease (Dhinakar Raj and Jones, 1997; Cavanagh, 2003, 2007). The clinical signs of respiratory infection include coughing, sneezing, and nasal discharge. However, the economic importance of IB is that infection decreases the weight gain of infected broilers as well as the egg quality and production of infected laying hens (Cavanagh and Naqui, 2003; Alvarado et al., 2006). In addition, IB can lead to secondary infections by Escherichia coli and Mycoplasma gallisepticum (Matthijs et al., 2003). Infections by different IBV strains have been reported worldwide, mainly in regions with heavy poultry industry activity, such as the United States and Europe (Zanella et al., 2003).
The IBV genome codes for 4 structural proteins: N (nucleocapsid), M (membrane), E (envelope), and S (glycoprotein). The serologic or genotypic classification of IBV is based on characteristics of its glycoprotein S. As suggested by De Wit (2000), there are diverse methods to classify viral strains, including reverse-transcription (RT) PCR.
It is difficult to efficiently control IB through vaccination due to the existence of the many serotypes and strains of the virus, most of which induce little or no cross-protection toward each other (Liu and Kong, 2004; Zhou et al., 2004). In Brazil, there is great IBV variability; therefore, IBV is considered one of the largest flock health issues in the Brazilian poultry industry (Montassier et al., 2004).
In the early 1990s, a new type of IB presentation appeared in England. This new form presented with sizable chicken mortality and some birds presented with deep pectoral myopathy. These outbreaks were associated with IBV strains that were known at that time as 793B (Gough et al., 1992) and 4/91 (Parsons et al., 1992), which later came to be studied by Cook et al. (1996) and Dhinakar Raj and Jones (1997). The pectoral lesion is characterized by paleness and atrophy of the deep pectoral muscle (supracoracoid), and in acute cases, necrosis and compromising of the whole breast region is noted (Dhinakar Raj and Jones, 1997; Vieira et al., 2006).
However, this lesion does not cause major clinical issues in chickens and is only detected at slaughterhouses. There are no reports of this lesion being associated with the presence of bacteria or being harmful to human health. The main issue is that the repugnancy of the lesion makes it impossible to use this meat for in natura consumption or processed products.
Economically, the discarding of the breast muscle, which is considered one of the most valuable chicken cuts, leads to considerable economic loss. It is also greatly exported for in natura consumption and use in processed meats. In 2009 Brazil exported 2 million tons of chicken meat worth $3 billion, whereas the processed meat sector exported 500,000 tons worth $1.2 billion (ABEF, 2010).
Several studies have been conducted in Brazil to examine the relationship between the presence of IBV and myopathy in chickens (Brentano et al., 2005, 2006; Trevisol et al., 2006, 2009; Gomes and Brito, 2007). Because the relationship has yet to be fully elucidated, the present study aimed to carry out a case-control study to understand the relationship between deep pectoral myopathy and IBV. To this end we examined tracheal and breast muscle lesions and compared the frequency of IBV detection by RT-PCR in breast muscles, tracheas, and cecal tonsils of chickens with and without myopathy.
MATERIALS AND METHODS
Sampling
A case-control study was conducted in which the case group comprised broiler flocks that presented deep pectoral myopathy as defined by the Sanitary Inspection, that is, macroscopic evidence. The control was formed of flocks lacking myopathic lesions under the same conditions. The material was collected at a slaughterhouse under Federal Sanitary Inspection located in the state of Santa Catarina, Brazil. A total of 40 broiler flocks vaccinated with the Massachusetts strain on their day of birth were studied. From the total population, 17 flocks were assigned to the control group and 23 to the case group. In each flock, 3 chickens were taken randomly and necropsied, in which the trachea, cecal tonsils, and deep pectoral muscles with and without myopathy were sampled according to the group. In a third cranial portion of the trachea, approximately 3 cm of trachea was collected. As to the muscle samples, it was obtained from the supracoracoid muscle, limited to the lesion area in myopathy cases and in the central muscle area for controls. About 3 cm3 of muscle were collected. Muscles samples were collected by federal inspectors on the slaughter line. Sampling was performed in duplicate. All samples were properly labeled and immediately stored at −20°C, until analysis. The samples for histopathology were stored in 10% formaldehyde.
Tissue Processing and Viral Extraction
Histopathology samples were fixed, dehydrated, cleared, infiltrated, embedded in paraffin, sectioned, and then stained with hematoxylin-eosin.
Frozen samples were sent to a laboratory and then thawed under proper conditions. A swab and a scrape were obtained from 3 tracheal samples, collected from each flock. These samples, the swab and scrape, were stored in PBS, pH 7.2. The cecal tonsils were macerated using Trizol Reagent (Invitrogen, Carlsbad, CA) and the muscles in PBS (pH 7.2). The viral RNA was extracted using Trizol Reagent (Invitrogen) according to the manufacturer's recommended protocol. Briefly, nucleic acid was extracted by Trizol and chloroform (Merck, Darmstadt, Germany), precipitated with isopropanol (Merck) and ethanol (Merck), and subsequently resuspended in 25 µL of nuclease-free water (Ludwig Biotec, Alvorada, Brazil). A 3-µL aliquot was immediately used in RT-PCR.
RT-PCR
The reaction was carried out using an AccessQuick RT-PCR System (Promega, Madison, WI) and following the manufacturer's recommended protocol. For the detection of IBV, the following primers (MWG-Biotech AG, Storrs, CT) were used: IBV5GL533 reverse 5′ GCCATGTTGTCACTGTCTATT 3′ and IBV5GU391 forward 5′ GCTTTTGAGCCTAGCGTT 3′. These primers amplify a 143-bp segment of the 5′ untranslated region (UTR) gene of the avian coronavirus (Callison et al., 2006). In a thermo cycler (PX-2, Thermo, Waltham, MA), the RT was conducted at 40°C for 50 min. The PCR included an initial denaturation at 95°C for 2 min followed by 35 cycles of denaturation at 95°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 30 s. The final extension was conducted at 72°C for 7 min. The amplified products were analyzed under UV light after agarose gel electrophoresis and staining with ethidium bromide.
Statistical Analysis
Chi-square testing was used to evaluate the association between deep pectoral myopathy and IBV in the studied groups, the association between deep pectoral myopathy and IBV detection in muscle samples by RT-PCR, and the influence of the sampling method in RT-PCR positivity. A 95% CI was generated for all statistical tests (Trhusfield, 2003).
RESULTS
Histopathology
Macroscopically, muscles with deep pectoral myopathy presented alterations such as atrophy, paleness, edema, and nonstandard coloration that varied from red purple to green. Extensive necrosis was noted independent of any other macroscopic alteration. In some of the samples, there was intense mononuclear inflammatory infiltration, muscle fiber fragmentation, and fibrotic tissue in the lesion area (Figure 1 ). The control group muscles presented without alteration at either the macroscopic or microscopic level. Histopathological examination of the tracheal samples revealed a large number of infiltrated mononuclear inflammatory cells that in some areas formed submucosal nodules (Figure 2 ). Some tracheal samples presented with cilia loss, epithelial hyperplasia, and loss of alveolar mucus glands.
Figure 1.
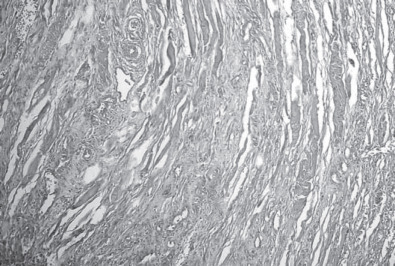
Extensive necrosis among muscle fibers. Hematoxylin and eosin 100×.
Figure 2.
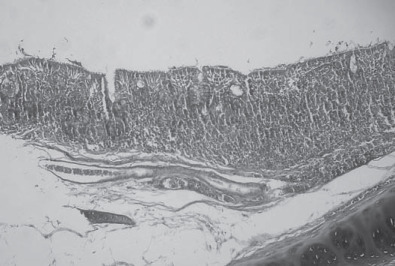
Intense mononuclear inflammatory infiltration in trachea, including occasional formation of nodules. Hematoxylin and eosin 60×.
RT-PCR
Twenty-five flocks tested IBV positive by RT-PCR independent of the type of sample used in the analysis: tracheal, cecal tonsil, or muscle. The used primers amplified a 143-bp fragment of the 5′UTR region of avian coronavirus (Figure 3 ). There was no statistically significant association between deep pectoral myopathy and IBV positivity as determined by RT-PCR (Table 1 ).
Figure 3.
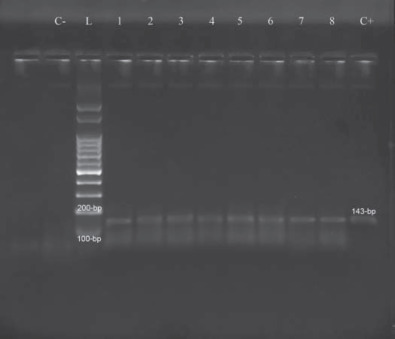
Reverse-transcription PCR amplified products from muscles samples. L = 100-bp ladder (Ludwig Biotec, Alvorada, Brazil), 1 to 8 = samples, C+ = reaction positive control (amplicon of 143 bp), C− = reaction negative control (absence of amplicon).
Table 1.
Relationship between the detection of infectious bronchitis virus (IBV) by reverse-transcription PCR in broilers with and without myopathy lesions1
| Group |
|||
|---|---|---|---|
| IBV | Case2 (%) | Control3 (%) | Total (%) |
| Positive | 60.85 (14/23) | 64.70 (11/17) | 62.5 (25/40) |
| Negative | 39.15 (9/23) | 35.30 (6/17) | 37.5 (15/40) |
| Total | 100 (23/23) | 100 (17/17) | 100 (40/40) |
χ2, CI = 95% (P > 0.05).
Case group: chickens with deep pectoral myopathy.
Control group: chickens without deep pectoral myopathy.
Nevertheless, when viral RNA was extracted from supracoracoid muscle macerate, RT-PCR indicated positive samples in both the case and control groups. Overall, 11 of 23 flocks from the case group and 7 from the control group (Table 2 ) tested positive. Moreover, when positivity was examined by sample type (tracheal swab, tracheal scrape, cecal tonsils, and muscles), a higher positive percentage was seen with cecal tonsil samples (Table 3 ).
Table 2.
Relationship between the presence of infectious bronchitis virus (IBV), as detected by reverse-transcription PCR, in breast muscles and deep pectoral myopathy in broilers1
| Group |
|||
|---|---|---|---|
| IBV | Case2 (%) | Control3 (%) | Total (%) |
| Positive | 47.83 (11/23) | 43.75 (7/17) | 47.5 (18/40) |
| Negative | 52.17 (12/23) | 56.25 (10/17) | 52.5 (22/40) |
| Total | 100 (23/23) | 100 (17/17) | 100 (40/40) |
χ2, CI = 95% (P > 0.05).
Case group: chickens with deep pectoral myopathy.
Control group: chickens without deep pectoral myopathy.
Table 3.
Relationship between infectious bronchitis virus (IBV) positivity by reverse-transcription PCR and sample type1
| IBV |
|||
|---|---|---|---|
| Material | Positive (%) | Negative (%) | Total (%) |
| Tracheal swab | 32.5 (13/40) | 67.5 (27/40) | 25 (40) |
| Tracheal scrape | 42.5 (17/40) | 57.5 (23/40) | 25 (40) |
| Cecal tonsil | 50 (20/40) | 50 (20/40) | 25 (40) |
| Muscle | 45 (18/40) | 55 (22/40) | 25 (40) |
| Total | 42.5 (68/160) | 57.5 (92/160) | 100 (160/160) |
χ2, CI = 95% (P > 0.05).
DISCUSSION
The lesions found in the supracoracoid muscles noted in this work were also noticed by Vieira et al. (2006) and Gomes and Brito (2007). The lesions characterize the macroscopic aspects of deep pectoral myopathy. However, Dhinakar Raj and Jones (1997) and Gomes and Brito (2007) did not observe major microscopic alterations with the exception of edema among muscle fibers. This edema was also observed in some of the samples studied in the present study, but most muscles presented obvious necrotic lesions as described by Vieira et al. (2006).
Microscopic examination of the trachea was considered an auxiliary tool for the diagnosis of IB. The lesions described herein were similar to those found by Brentano et al. (2005) and were characterized by mononuclear infiltration and epithelial hyperplasia.
Although no relationship was established between the presence of IBV and deep pectoral myopathy, the virus was directly detected in muscle samples by RT-PCR. Brentano et al. (2005) were among the first researchers to study the association between IBV and deep pectoral myopathy. They were able to diagnose, by viral isolation and RT-PCR, IBV in broilers with myopathy but could not confirm a relationship between them. Brentano et al. (2005) examined samples submitted for viral isolation in embryonated eggs and subjected to RT-PCR using primers for the IBV S1 gene of IBV. These were different from the primers used in the present study. The current study chose not to conduct a preliminary viral isolation stage. Instead, an RNA extraction was performed directly from muscle samples because that material is not routinely used for viral isolation in embryonated eggs (De Wit, 2000; Trevisol et al., 2009).
The direct detection of IBV in myopathic and normal muscle suggests that this lesion might not be related to the virus presence. IBV muscle detection has not been accomplished by others, as to the literature. In previous work, Trevisol et al. (2009) did not have success in viral recovery from muscle tissue either by viral isolation or PCR. It is possible that the particular primers used in this paper were more sensitive than those used by those authors.
Dhinakar Raj and Jones (1997) alleged that the virus is not directly involved in the pathogenesis of muscle lesions. Instead, they suggest the virus is involved in the formation and deposition of immunocomplexes in the muscle capillary walls. Techniques such as immunohistochemistry could complement the detection of IBV and confirm the muscle capillary wall immunocomplex hypothesis.
Gomes and Brito (2007) also detected IBV in tracheal and cecal tonsil samples from broilers with myopathy. However, they did not detect IBV in their muscle tissue samples. The authors concluded that atypical cases of IB are caused by different viral strains. This hypothesis has also been suggested by Gough et al. (1992), Parsons et al. (1992), and Brentano et al. (2005), but was not investigated in the present work. Methodologies that may help answer the question of the true nature of the association between IBV and myopathy include amplification of the S1 gene directly from muscle samples, complemented by sequencing and phylogenetic analysis of these amplicons, in addition to attempts to reproduce myopathic lesions in broilers affected by IBV.
The higher RT-PCR positive detection percentage associated with the cecal tonsil samples does not agree with the work of Alvarado et al. (2006), which also had good detection in cecal tonsil samples, but a higher positive detection percentage was noted in tracheal samples. Dhinakar Raj and Jones (1997) concluded that in 6-wk-old broilers, viral persistency is longer in the cecal tonsils compared with others organs. In addition, a review by De Wit (2000) cited the kidneys and cecal tonsils as superior choices when sampling chronic infections. Furthermore, De Wit (2000) reported that detection success is affected by several factors, including the time between the beginning of infection and sampling, quality and choice of organs, and chicken immunity levels. Such factors might better justify the cecal tonsil and muscle detection results. The higher detection rates observed for IBV when performing RT-PCR of the cecal tonsil samples demonstrate the importance of organ selection when conducting diagnostic tests.
REFERENCES
- ABEF. 2010. Brazilian Chicken Producers and Exporters Association. Annual Report. Accessed Jan. 2010. http://www.abef.com.br/noticias_portal/exibenoticia.php?notcodigo=2264.
- Alvarado I.R., Villegas P., El-Attrache J., Jackwood M.W. Detection of Massachusetts and Arkansas serotypes of infectious bronchitis virus in broilers. Avian Dis. 2006;50:292–297. doi: 10.1637/7458-101805R.1. [DOI] [PubMed] [Google Scholar]
- Brentano L., Esteves P.A., Trevisol I.M., Hayashi M.M., Luciano R.L., Castro A.G.M., Klein T.A.P., Molinari M. Sequenciamento do gene S1 de vírus de bronquite infecciosa (IBV) isolados de surtos da doença associada a lesões de miopatia peitoral. Braz. J. Poult. Sci. 2006;(Suppl. 8):241. [Google Scholar]
- Brentano L., Klein T.A.P., Jaenisch F.R., Back A., Castro A.G.M. Isolamento do vírus de bronquite infecciosa das aves de surtos da doença associada a lesões atípicas de miopatia de músculo peitoral. Braz. J. Poult. Sci. 2005;(Suppl. 7):232. [Google Scholar]
- Callison S.A., Hilt D.A., Boyton T.O., Sample B.F., Robison R., Swayne D.E., Jackwood M.W. Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. J. Virol. Methods. 2006;138:60–65. doi: 10.1016/j.jviromet.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Severe acute respiratory syndrome vaccine development: Experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32:567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007;38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Naqui S.A. Infectious bronchitis. In: Saif Y.M., Barnes H.J., Glisson J.R., Fadly A.M., McDougald L.R., Swayne D.E., editors. Diseases of Poultry. 11th ed. Iowa State University Press; Ames.: 2003. pp. 101–120. [Google Scholar]
- Cook J.K.A., Orbell S.J., Woods M.A., Huggins M.B. A survey of the presence of a new infectious bronchitis virus designated 4/91 (793B) Vet. Rec. 1996;138:178–180. doi: 10.1136/vr.138.8.178. [DOI] [PubMed] [Google Scholar]
- De Wit J.J. Detection of infectious bronchitis virus. Avian Pathol. 2000;29:71–93. doi: 10.1080/03079450094108. [DOI] [PubMed] [Google Scholar]
- Dhinakar Raj G., Jones R.C. Infectious bronchitis virus: Immunopathogenesis of infection in the chicken. Avian Pathol. 1997;26:677–706. doi: 10.1080/03079459708419246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes L.M., Brito B.G. Genotipagem do vírus de bronquite infecciosa relacionado com quadro de miopatia do músculo peitoral em frangos de corte. Braz. J. Poult. Sci. 2007;(Suppl. 9):243. [Google Scholar]
- Gough R.E., Randall C.J., Dagless M., Alexander D.J., Cox W.J., Pearson D. A “new” strain of infectious bronchitis virus infecting domestic fowl in Great Britain. Vet. Rec. 1992;130:493–494. doi: 10.1136/vr.130.22.493. [DOI] [PubMed] [Google Scholar]
- Liu S., Kong X. A new genotype of nephropathogenic infectious bronchitis virus circulating in vaccinated and non-vaccinate flocks in China. Avian Pathol. 2004;33:321–327. doi: 10.1080/0307945042000220697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijs M.G.R., Van Eck J.H.H., Landman W.J.M., Stegeman J.A. Ability of Massachusetts-type infectious bronchitis virus to increase colibacilosis susceptibility in commercial broilers: A comparison between vaccine and virulent field virus. Avian Pathol. 2003;32:473–481. doi: 10.1080/0307945031000154062. [DOI] [PubMed] [Google Scholar]
- Montassier M.F.S., Caetano A.G., Brentano L., Piza V.M.T., Furuyama C.R.G., Richtzenhain L.J., Montassier H.J. Caracterização de variantes genéticas do vírus da bronquite infecciosa isoladas no Brasil. Arq. Inst. Biol. (Sao Paulo) 2004:71. (Suppl.) [Google Scholar]
- Parsons D., Ellis M.M., Cavanagh D., Cook J.K. Characterization of an infectious bronchitis virus isolated from vaccinated broiler breeder flocks. Vet. Rec. 1992;131:408–411. doi: 10.1136/vr.131.18.408. [DOI] [PubMed] [Google Scholar]
- Trevisol I.M., Brentano L., Esteves P.A., Schaefer R., Luciano R.L., Castro A.G.M., Klein T.A.P. Teste de proteção vacinal para uma amostra de bronquite infecciosa isolada de caso de miopatia frente amostra de vacina comercial H-120. Braz. J. Poult. Sci. 2006;(Suppl. 8):240. [Google Scholar]
- Trevisol I.M., Esteves P.A., Schaefer R., Jaenisch F.R.F., Di Fabio J., Brentano L. Associação entre o vírus da bronquite infecciosa das galinhas e alterações da musculatura peitoral. Rev. Bras. Cienc. Avic. Suppl. 2009:11. [Google Scholar]
- Trhusfield M. Epidemiologia Veterinária. 2nd ed. Roca; São Paulo, Brazil.: 2003. [Google Scholar]
- Vieira T.B., Almeida D.O., Alves F.M.X., Franco R.M., Andrade C.L., Tortelly R. Aspectos anatomopatológicos da miopatia peitoral profunda em frangos de corte abatidos sob inspeção sanitária. Brazilian J. Vet. Sci. 2006;13:144–146. [Google Scholar]
- Zanella A., Lavazza A., Marchi R., Moreno Martin A., Paganelli F. Avian infectious bronchitis: Characterization of new isolates from Italy. Avian Dis. 2003;47:180–185. doi: 10.1637/0005-2086(2003)047[0180:AIBCON]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Zhou J.Y., Zhang D.Y., Ye J.X., Cheng L.Q. Characterization of infectious bronchitis virus isolated in China from chickens with nephritis. J. Med. Vet. B Infect. Dis. Vet. Public Health. 2004;51:147–152. doi: 10.1111/j.1439-0450.2004.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


