The International Organization for the Study of Inflammatory Bowel Diseases (IOIBD) is the only global organization devoted to the study of and management of the inflammatory bowel diseases (IBDs), namely, Crohn’s disease and ulcerative colitis. Membership is composed of physician-scientists who have established expertise in these diseases. The organization hosts an annual meeting and a number of working groups addressing issues of the epidemiology of IBD, diet and nutrition, and the development and use of treatments for IBD. There are currently 89 members of IOIBD representing 26 different countries. The organization has taken particular interest in the coronavirus disease-2019 (COVID-19) pandemic and how it may affect the IBD patient population. This document summarizes the results of 2 recent virtual meetings of the group and subsequent expert guidance for patients and providers.
Severe Acute Respiratory Syndrome Coronavirus-2 and COVID-19
In December 2019, health officials in Wuhan, China, described a series of unexplained pneumonias and shortly thereafter identified the causative agent as a novel coronavirus (named Severe Acute Respiratory Syndrome Coronavirus-2 [SARS-CoV-2]). It is believed that SARS-CoV-2 entered the human population from animals and that the exposure may have been a live food market in that province of China. SARS-CoV-2 can result in a mild or severe respiratory illness called COVID-19. COVID-19 rapidly spread throughout the world and on March 11, 2020, the World Health Organization declared it a pandemic.1 At the time of this writing, it has affected >800,000 people worldwide, and accounted for >38,700 deaths, and these numbers continue to rise sharply.2 COVID-19 has now been reported to affect individuals of all ages, with a slight predominance in men. Mortality from COVID-19 is estimated variably from 1.5% to as high as 3.0%, with identified risk factors of older age and comorbid illnesses including hypertension, diabetes, and other cardiovascular diseases. The risk of COVID-19 or death from COVID-19 in patients treated for immune-mediated diseases is unknown at this time, but it has been presumed that patients who are immunosuppressed are at higher risk for infection with SARS-CoV-2 and may be at increased risk for COVID-19.
IBD and COVID-19
Treatment of IBD is aimed at controlling an overactive immune response, and currently involves use of a number of well-studied classes of immune modifying therapies (Supplementary Table 1). Many of these treatments are associated with known increased risks of infections, so the IBD population has been considered an at-risk population for infection with SARS-CoV-2. However, the actual risks of infection or of development of COVID-19 in patients with IBD are not known, nor are the appropriate adjustments to treatments to mitigate such risks or reduce complications from the disease.
The IOIBD Meeting on COVID-19
The 2020 annual meeting for IOIBD was scheduled to occur in March, but was cancelled owing to the emergence of COVID-19. Given how this disease might affect patients with IBD and the absence of data, members of the organization recognized the need for rapid international collaboration and held 2 IBD-COVID-19 webinars, the details of which are summarized in this Commentary.
The overall goal of these webinars was to develop a number of key statements that could be used to guide the management of patients with IBD during this pandemic. Using RAND panel methodology all IOIBD members and selected content experts were asked to participate in a survey before the first webinar that included statements related to risks of SARS-CoV-2 and COVID-19 in patients with IBD, as well as statements related to disease management under a variety of clinical scenarios.
During the first webinar, held on March 20, 2020, IOIBD members shared their direct experiences with COVID-19 in the epicenters of China and Italy (Zhihua Ran and Silvio Danese, respectively), followed by information about the successful population-wide response in Hong Kong (Siew Ng). Also discussed was the organization of international registries.3 , 4 At the time of this first webinar, there were only 15 cases of IBD and COVID-19 reported. Also presented and discussed at length were the possible effects of immunotherapies on infection in patients with IBD (Maria Abreu, Markus Neurath), which is summarize elsewhere in this article. Next, the results from the first round of voting were reviewed and the group focused on statements in which there was disagreement or uncertainty.
Subsequent to this first webinar, as per RAND panel protocol, a second round of voting with modified statements was sent to the participants. These results led to the statements summarized below and presented in their entirety in Table 1 . A second webinar occurred on March 27, 2020, to review results and to continue discussions for ongoing efforts to provide guidance.
Table 1.
Final Assessment of Statements Related to Risk of Infection with SARS-CoV-2 or development of COVID-19 in Patients with IBD by the IOIBD Panel (n = 66 participants)
| 76 Statements | Median | SD | Category | DI |
|---|---|---|---|---|
| Risk of infection/disease | ||||
| The risk of infection with SARS-CoV-2 is the same whether a patient has IBD or does not have IBD. | 8 | 1.7 | Appropriate | –0.71 |
| Independent of treatment, patients with Crohn’s disease have a greater risk of infection with SARS-CoV-2 than the general population. | 2 | 1.7 | Inappropriate | 0.16 |
| Independent of treatment, patients with ulcerative colitis have a greater risk of infection with SARS-CoV-2 than the general population. | 2 | 1.7 | Inappropriate | 0.16 |
| Having active inflammation from IBD increases the risk of getting SARS-CoV-2. | 5.5 | 1.8 | Uncertain | 0.63 |
| Patients with IBD who are exposed to SARS-CoV-2 have a higher risk of developing COVID-19 compared to patients without IBD. | 5 | 1.7 | Uncertain | 0.52 |
| Patients with IBD who have COVID-19 have a higher mortality compared to patients without IBD. | 3.5 | 1.7 | Inappropriate | 0.52 |
| Patients with an ostomy are at increased risk for COVID-19. | 2 | 1.2 | Inappropriate | 0.13 |
| Patients with a J pouch are at increased risk for COVID-19. | 2 | 1.2 | Inappropriate | 0.13 |
| Elective surgeries and endoscopies should be postponed at this time. | 8.5 | 1.6 | Appropriate | –0.34 |
| Healthcare workers with IBD on immune modifying medications working in an environment with known or suspected COVID-19 patients should continue working, assuming they are following standard prevention methods. | 5.5 | 2.0 | Uncertain | 2.02 |
| Patients with IBD on immune-modifying medications should discontinue any nonessential travel. | 9 | 1.2 | Appropriate | –0.17 |
| It is safe to continue infusions in an infusion center assuming the infusion center has a screening protocol in place. | 8 | 1.0 | Appropriate | –0.71 |
| Therapy class: 5-ASA | ||||
| 5-ASA increases the risk of infection with SARS-CoV-2. | 1 | 0.7 | Inappropriate | 0.00 |
| 5-ASA increases the risk of COVID-19. | 1 | 0.7 | Inappropriate | 0.12 |
| Patients taking 5-ASA therapy should reduce the dose of therapy to prevent SARS-CoV-2 infection. | 1 | 0.7 | Inappropriate | 0.00 |
| Patients taking 5-ASA therapy should discontinue therapy to prevent SARS-CoV-2 infection. | 1 | 0.7 | Inappropriate | 0.00 |
| Patients taking 5-ASA therapy should stop therapy if they test positive for SARS-CoV-2 but do not have COVID-19. | 1 | 1.1 | Inappropriate | 0.00 |
| Patients taking 5-ASA therapy should stop therapy if they develop COVID-19. | 1 | 1.5 | Inappropriate | 0.13 |
| Therapy class: oral budesonide | ||||
| Budesonide increases the risk of infection with SARS-CoV-2. | 3 | 1.4 | Inappropriate | 0.16 |
| Budesonide increases the risk of COVID-19. | 3 | 1.5 | Inappropriate | 0.22 |
| Patients taking budesonide therapy should reduce the dose of therapy to prevent SARS-CoV-2 infection. | 3 | 1.8 | Inappropriate | 0.16 |
| Patients taking budesonide therapy should discontinue therapy to prevent SARS-CoV-2 infection. | 2 | 1.6 | Inappropriate | 0.16 |
| Patients taking budesonide therapy should stop therapy if they test positive for SARS-CoV-2 but do not have COVID-19. | 4 | 2.1 | Uncertain | 0.52 |
| Patients taking budesonide therapy should stop therapy if they develop COVID-19. | 5 | 2.2 | Uncertain | 0.85 |
| Therapy class: oral prednisone (≥20 mg/d) | ||||
| Prednisone (≥20 mg/d) increases the risk of infection with SARS-CoV-2. | 7 | 2.1 | Appropriate | 2.35 |
| Prednisone (≥20 mg/d) increases the risk of COVID-19. | 7 | 2.0 | Appropriate | 10.00 |
| Patients taking prednisone therapy (≥20 mg/d) should reduce the dose of therapy to prevent SARS-CoV-2 infection. | 7 | 2.0 | Appropriate | 0.00 |
| Patients taking prednisone therapy (≥20 mg/d) should discontinue therapy (taper as appropriate) to prevent SARS-CoV-2 infection. | 7 | 2.3 | Appropriate | 2.35 |
| Patients taking prednisone therapy (≥20 mg/d) should stop therapy (taper as appropriate) if they test positive for SARS-CoV-2 but do not have COVID-19. | 7 | 1.7 | Appropriate | –0.71 |
| Patients taking prednisone therapy (≥20 mg/d) should stop therapy (taper as appropriate) if they develop COVID-19. | 8 | 1.6 | Appropriate | –0.71 |
| Therapy class: thiopurines | ||||
| Azathioprine/6-MP increases the risk of infection with SARS-CoV-2. | 5 | 2.0 | Uncertain | 0.85 |
| Azathioprine/6-MP increases the risk of COVID-19. | 6 | 1.9 | Uncertain | 0.63 |
| Patients taking azathioprine/6-MP should reduce the dose of therapy to prevent SARS-CoV-2 infection. | 3 | 2.1 | Inappropriate | 0.56 |
| Patients taking azathioprine/6-MP should discontinue therapy to prevent SARS-CoV-2 infection. | 3 | 1.9 | Inappropriate | 0.35 |
| Patients taking azathioprine/6-MP should stop therapy if they test positive for SARS-CoV-2 but do not have COVID-19. | 7 | 2.0 | Appropriate | –2.32 |
| Patients taking azathioprine/6-MP should stop therapy if they develop COVID-19. | 8 | 1.5 | Appropriate | –0.71 |
| Therapy: methotrexate | ||||
| Methotrexate increases the risk of infection with SARS-CoV-2. | 4 | 1.7 | Uncertain | 0.52 |
| Methotrexate increases the risk of COVID-19. | 5 | 1.9 | Uncertain | 0.44 |
| Patients taking methotrexate should reduce the dose of therapy to prevent SARS-CoV-2 infection. | 3 | 1.6 | Inappropriate | 0.16 |
| Patients taking methotrexate should discontinue therapy to prevent SARS-CoV-2 infection. | 3 | 1.5 | Inappropriate | 0.16 |
| Patients taking methotrexate should stop therapy if they test positive for SARS-CoV-2 but do not have COVID-19. | 7 | 2.0 | Appropriate | 10.00 |
| Patients taking methotrexate should stop therapy if they develop COVID-19. | 7 | 1.6 | Appropriate | –0.71 |
| Therapy class: anti-TNF | ||||
| Anti-TNF therapy increases the risk of infection with SARS-CoV-2. | 4 | 1.7 | Uncertain | 0.22 |
| Anti-TNF therapy increases the risk of COVID-19. | 4 | 1.7 | Uncertain | 0.52 |
| Patients taking anti-TNF therapy should reduce the dose of therapy to prevent SARS-CoV-2 infection. | 2 | 1.4 | Inappropriate | 0.16 |
| Patients taking anti-TNF therapy should discontinue therapy to prevent SARS-CoV-2 infection. | 2 | 1.2 | Inappropriate | 0.00 |
| Patients taking anti-TNF therapy should stop therapy if they test positive for SARS-CoV-2 but do not have COVID-19. | 6 | 2.2 | Uncertain | 2.35 |
| Patients taking anti-TNF therapy should stop therapy if they develop COVID-19. | 7 | 2.0 | Appropriate | –0.71 |
| Therapy: vedolizumab | ||||
| Vedolizumab increases the risk of infection with SARS-CoV-2. | 3 | 1.5 | Inappropriate | 0.16 |
| Vedolizumab increases the risk of COVID-19. | 3 | 1.6 | Inappropriate | 0.37 |
| Patients taking vedolizumab should reduce the dose of therapy to prevent SARS-CoV-2 infection. | 2 | 1.3 | Inappropriate | 0.15 |
| Patients taking vedolizumab should discontinue therapy to prevent SARS-CoV-2 infection. | 2 | 1.2 | Inappropriate | 0.00 |
| Patients taking vedolizumab should stop therapy if they test positive for SARS-CoV-2 but do not have COVID-19. | 5 | 2.2 | Uncertain | 0.85 |
| Patients taking vedolizumab should stop therapy if they develop COVID-19. | 6 | 2.1 | Uncertain | 2.35 |
| Therapy: ustekinumab | ||||
| Ustekinumab increases the risk of infection with SARS-CoV-2. | 3 | 1.5 | Inappropriate | 0.16 |
| Ustekinumab increases the risk of COVID-19. | 3 | 1.6 | Inappropriate | 0.16 |
| Patients taking ustekinumab should reduce the dose of therapy to prevent SARS-CoV-2 infection. | 2 | 1.1 | Inappropriate | 0.16 |
| Patients taking ustekinumab should discontinue therapy to prevent SARS-CoV-2 infection. | 2 | 1.1 | Inappropriate | 0.00 |
| Patients taking ustekinumab should stop therapy if they test positive for SARS-CoV-2 but do not have COVID-19. | 6 | 2.1 | Uncertain | 2.35 |
| Patients taking ustekinumab should stop therapy if they develop COVID-19. | 7 | 2.1 | Appropriate | –1.57 |
| Therapy: tofacitinib | ||||
| Tofacitinib increases the risk of infection with SARS-CoV-2. | 5 | 1.9 | Uncertain | 0.52 |
| Tofacitinib increases the risk of COVID-19. | 5 | 1.9 | Uncertain | 0.32 |
| Patients taking tofacitinib should reduce the dose of therapy to prevent SARS-CoV-2 infection. | 3 | 1.9 | Inappropriate | 0.19 |
| Patients taking tofacitinib should discontinue therapy to prevent SARS-CoV-2 infection. | 3 | 1.5 | Inappropriate | 0.16 |
| Patients taking tofacitinib should stop therapy if they test positive for SARS-CoV-2 but do not have COVID-19. | 7 | 1.9 | Appropriate | 10.00 |
| Patients taking tofacitinib should stop therapy if they develop COVID-19. | 8 | 1.6 | Appropriate | –0.71 |
| Combination therapy | ||||
| Patients taking combination therapy with an anti-TNF and thiopurine/methotrexate should reduce the dose of the thiopurine/methotrexate to prevent infection from SARS-CoV-2. | 4 | 2.2 | Uncertain | 0.91 |
| Patients taking combination therapy with an anti-TNF and thiopurine/methotrexate should stop the thiopurine/methotrexate if they test positive for SARS-CoV-2 but do not have COVID-19. | 7 | 2.2 | Appropriate | –3.30 |
| Patients taking combination therapy with an anti-TNF and thiopurine/methotrexate should stop the thiopurine/methotrexate if they develop COVID-19. | 8 | 1.3 | Appropriate | 0.00 |
| Clinical trials | ||||
| Patients taking clinical trial drugs should discontinue therapy to prevent SARS-CoV-2 infection. | 2 | 1.4 | Inappropriate | 0.16 |
| Patients taking clinical trial drugs should stop therapy if they test positive for SARS-CoV-2 but do not have COVID-19. | 7 | 1.9 | Appropriate | 10.00 |
| Patients taking clinical trial drugs should stop therapy if they develop COVID-19. | 8 | 1.6 | Appropriate | –0.32 |
| Approach to active disease | ||||
| A patient with moderately to severely active Crohn’s disease or ulcerative colitis (new diagnosis or relapsing disease) should be treated with the same therapies you would choose in the pre-COVID-19 era. | 7 | 2.1 | Appropriate | 10.00 |
| Treatment of IBD after SARS-coV-2 infection or COVID-19 | ||||
| In an IBD patient who tests positive for SARS-CoV-2 and whose IBD meds have been stopped because of this, IBD meds can be restarted after 14 days (provided they have not developed COVID-19). | 7 | 1.5 | Appropriate | –0.71 |
| In an IBD patient who develops COVID-19 and whose IBD meds have been stopped, IBD meds can be restarted after COVID-19 symptoms resolve. | 7 | 1.9 | Appropriate | 10.00 |
| In an IBD patient who develops COVID-19 and whose IBD meds have been stopped, IBD meds can be restarted after 2 nasopharyngeal PCR tests are negative. | 8 | 1.6 | Appropriate | –0.71 |
5-ASA, 5-aminosalicylic acid; 6-MP, mercaptopurine; COVID-19, coronavirus disease 2019; DI, disagreement index; IBD, inflammatory bowel disease; IOIBD, International Organization for the Study of Inflammatory Bowel Diseases; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2; SD, standard deviation; TNF, tumor necrosis factor.
Methodology for Developing the IOIBD Statements on COVID-19 and IBD
IOIBD used the established RAND/UCLA method, which uses a Delphi panel approach to address the appropriateness of specific medical interventions or medical decisions.5 We used a modified RAND panel to allow for a rapid cycle of 2 rounds of voting by the expert panel. The panel was presented a web-based questionnaire that included clinical scenarios specific to patients with IBD during the COVID-19 pandemic. The questionnaire was created and iteratively improved by 3 of the authors (DTR, MTA, CAS) and then distributed electronically to the respondents. The panelists included the membership of IOIBD in addition to other invited specialists in IBD. Respondents rated each of the patient scenarios on a scale of 1–9, such that statements rated 1–3 are considered inappropriate, 4–6 are uncertain, and 7–9 are appropriate. After the first round of anonymous voting, the first webinar occurred and related content was reviewed as summarized and the results of the first round of voting were reviewed. The subsequent discussion focused on scenarios that had a median in the uncertainty range and those with a high standard deviation. The goal of the discussion was to understand views of the panel in preparation for a second round of voting, not necessarily to achieve consensus. The second round questionnaire was nearly identical to the first, except for clarifying a few of the original scenarios and adding 2 additional sections that were not covered in round 1 (how to manage patients in IBD clinical trials and when to restart medications if they were being held for active COVID-19 infection). Table 1 lists the statements from the second round of voting. The final appropriateness category is based on the second round voting median. The mean, standard deviation, and disagreement index (DI) were also calculated.
The DI expresses the spread of responses and is calculated using a previously described approach5 and the following formula:
Using this formula, agreement is defined as a DI of <1, whereas disagreement is defined as a DI of ≥1.5
Summary of RAND Panel and Development of Guidance Statements
Of the 76 statements in the second-round survey, 26 were rated as appropriate, 19 as uncertain, and 31 as inappropriate. Although agreement is not required, there was agreement (DI of <1) in 64 of 76 scenarios (84%).
-
•
The panel agreed that having IBD (either Crohn’s disease or ulcerative colitis) did not increase the risk of becoming infected with SARS-CoV-2 or developing COVID-19 and having an ostomy or J-pouch did not increase the risk for COVID-19.
-
•
The panel agreed that it is safe to continue to receive infusions in an infusion center, assuming that the infusion center has a SARS-CoV-2 screening protocol in place.
-
•
The group was in agreement that it is appropriate to reduce the dose or discontinue prednisone to prevent infection from SARS-CoV-2, but voted that it was inappropriate to reduce the dose or stop other IBD therapies to prevent infection from SARS-CoV-2.
-
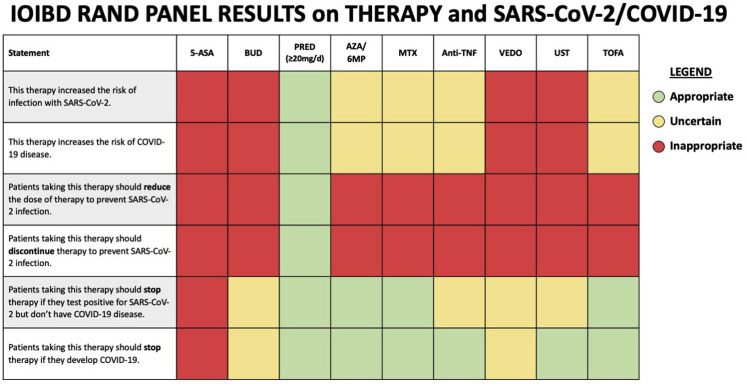
•
There were mixed responses related to the other clinical scenarios and therapies. The key findings regarding the management of medical therapy for IBD in the setting of the COVID-19 pandemic are summarized in Figure 1 .
-
•
In regards to the scenario of a patient receiving combination therapy of an anti-tumor necrosis factor (TNF) and immune modulator, the group was uncertain if the immune modulator should be dose reduced to potentially modify the risk of infection with SARS-CoV-2, but was in agreement and did vote that it is appropriate to discontinue the immune modulator in a patient who is known to be infected with SARS-CoV-2 or when a patient develops COVID-19.
-
•
In the scenario of a patient who stopped IBD medications because either they tested positive for SARS-CoV-2 infection or had COVID-19, the group voted that it is appropriate to restart their medications if they do not develop symptoms after 2 weeks, or when symptoms have completely resolved.
-
•
The group was in agreement and voted it was appropriate to postpone nonessential endoscopic procedures.
-
•
Furthermore, the panel voted that patients in clinical trials should continue those therapies unless they become infected by SARS-CoV-2 or develop COVID-19.
-
•
The group voted that it was appropriate to discontinue the clinical trial drug if a patient tests positive for SARS-CoV-2 or develops COVID-19, but there was some disagreement in the responses.
Figure 1.
Final results of the RAND appropriateness panel for the use of medications to treat IBD in the setting of SARS-CoV-2 or COVID-19. 5-ASA, 5-aminosalicylate; 6MP, 6-mercaptopurine; AZA, azathioprine; anti-TNF, anti-tumor necrosis factor; Bud, budesonide; COVID-19, coronavirus disease; IBD, inflammatory bowel disease; MTX, methotrexate; Pred, prednisone; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2; TOFA, tofacitinib; UST, ustekinumab; VEDO, vedolizumab.
The full results of the first survey (before the webinar) and second survey are available in Supplementary Table 2.
Summary of the Discussion about Immune Activity in COVID-19 Infections and Possible Effects of IBD Therapies
It is now known that similar to the 2002 SARS-CoV, the 2019 SARS-CoV-2 requires the angiotensin-converting enzyme 2 (ACE-2) receptor to enter the cell as well as TMPRSS2, a serine protease that cleaves the viral spike to permit viral entry.6 The ACE-2 receptor is found at high levels in alveolar type-2 cells in the lung. However, important for IBD, the small intestine is also known to express ACE-2; in a patient who was infected with SARS-CoV-2, ACE-2 and the virus were found in stomach, duodenal, and rectal biopsies.7 Moreover, single cell transcriptomics of the gut corroborate that ACE-2 is expressed in the gastrointestinal tract.8 The virus has also been found in the stool of infected patients for longer periods of time than in sputum, although in smaller amounts.9
ACE-2 can be up-regulated after infection with SARS-CoV and Middle Eastern Respiratory Syndrome (MERS)-CoV–related viruses, suggesting there is a positive feed forward loop once patients are infected with virus. In vitro, IFN-γ can induce ACE-2 and the promoter region of ACE-2 contains several immune and cytokine responsive transcription factor binding sites, suggesting that inflammation may increase expression of ACE-2.8 The other important factor for viral entry is endocytosis. Baricitinib is a JAK1, JAK2 and TYK2 inhibitor currently available for rheumatoid arthritis and in clinical development for IBD and it may inhibit endocytosis.10 This effect does not occur with the less selective JAK inhibitor, tofacitinib.
Early in the disease course for SARS-CoV and MERS-CoV, the innate immune response to the virus through interferon and interferon-stimulated genes may limit viral replication, but is also subverted by the virus itself.11 In later stages, the cytokine release syndrome, characterized by high levels of proinflammatory cytokines such as IL-6, has been suggested to trigger acute respiratory distress syndrome in SARS-CoV-2 infections and lead to fatal outcomes. In terms of the therapies we use for IBD and the potential effect on SARS-CoV2, previous experience with high-dose steroids as a treatment for SARS-CoV or MERS-CoV was not effective and delayed viral clearance.12 Therefore, steroids are not recommended as a treatment for SARS-CoV-2, but the doses that were used in these studies are much higher than the doses that are used in IBD.12 With respect to thiopurines, mercaptopurine and 6-thioguanine have potential antiviral activity against MERS and SARS, at least in vitro.13 There are no publications, however, showing that this finding was tested in patients. The other issue that has to be considered in the context of thiopurines is lymphopenia, because patients on thiopurines may develop lymphopenia from the drug. Unfortunately, patients with lymphopenia caused by SARS-CoV-2 have a worse prognosis and have an increased risk of death associated with the virus.14 , 15 At the current time, there are no specific data available on methotrexate and COVID-19, but in theory the pulmonary toxicity of methotrexate may be of interest in the setting of a virus that involves the respiratory tract.
Many patients with IBD are using monotherapy with biologics or the novel small molecule Janus kinase inhibitor, tofacitinib. Anti-TNF therapy may impact viral immunity given that there is a small increased risk of herpes zoster and hepatitis B virus reactivation. Although high IL-2R and IL-6 serum levels have been associated with severe COVID-19 cases, no effect on TNF levels was noted.16 IFN-γ and TNF production by CD4+ T cells have been associated with severe SARS-CoV and, therefore, inhibition of TNF has been proposed as a treatment of the cytokine release syndrome that can occur in some of these patients.17 , 18
Vedolizumab primarily inhibits α-4, β-7 lymphocyte homing of Th17 and Th9 cells as well as regulatory T cells to the intestine. Viral infections are rare with vedolizumab therapy.19 In patients who had concurrent hepatitis B or C virus infection, there was no viral reactivation and there was sustained virologic control in simian immunodeficiency–infected macaques after antiretroviral and vedolizumab therapy.20 Vedolizumab has been used for clinical therapy in patients with IBD and early clinical trials in HIV have not shown effects on viral load.21 With respect to ustekinumab, this agent can prevent Th1+ T-cell priming and IFN-γ production by CD4+ T cells and suppress Th17+ T-cell activation and cytokine production. There has been no increase in viral infections in IBD or psoriasis patients receiving ustekinumab in large postmarketing registries.22 Tocilizumab, an anti–IL-6R antibody, is currently being tested in patients with severe COVID-19 and may decrease the severity of acute respiratory distress syndrome.23 , 24 Finally, tofacitinib has been associated with a clear increase in reactivation of herpes zoster. Thus, tofacitinib might inhibit viral immunity; in contrast, it might help with the severe inflammatory response that ultimately leads to death. In certain studies, tofacitinib can inhibit interferon secretion.
When considering the management of IBD medications, one must also take into account the half-life of the drugs. In general, 5.5 half-lives are required to achieve very low levels of drug. Corticosteroids have a half-life of about 24 hours, but it is dose dependent and the higher the dose, the longer the biological effect. Thiopurines have been studied in patients with IBD and the median 6-thioguanine elimination half-life is 6.8 days with very low levels of 6-thioguanine present by 40 days.25 Studies of vaccine responses in patients on thiopurines demonstrate that patients were able to mount normal immune responses.26 Methotrexate has a 6-hour half-life and takes <3 days to eliminate. Tofacitinib has a half-life of between 3 and 6 hours, depending on whether it is an immediate- or extended-release formulation.27 Studies in normal volunteers treated with tofacitinib, however, found defects in immune function for 1 month after drug withdrawal.28 For the subcutaneous and IV-based biologics, the half-life is much longer. The half-life of adalimumab is between 10 and 20 days and the half-life of infliximab is between is 7 and 12 days. The median half-life for ustekinumab is approximately 19 days in patients with Crohn’s disease, and the serum half-life of vedolizumab is 25 days. Thus, even after cessation of these immune therapies, there may be continued effects on the patient and implications for the infection.
Summary and Next Steps
This meeting was convened by an international group of IBD physician-scientists to develop guidance for the management of patients with IBD during the COVID-19 pandemic. Using a RAND panel methodology, the group developed a series of statements regarding the risk of infection and management of therapies that will assist patients and healthcare providers during this uncertain time. These statements are based on expert opinion in the absence of definitive data or, in some cases, any data. They are meant to help inform clinical decision making, but should not replace individualized management decisions. The IOIBD and the invited experts are participating in weekly updates of this rapidly evolving situation and will update this information for patients and colleagues as appropriate.
Acknowledgments
The authors thank all the participants of the RAND panel.
The authors acknowledge Cindy Traboulsi, Amarachi I. Erondu, and Seth R. Shaffer for their assistance in data management and Raymond Kulig and Marischka Konings for invaluable help in logistical coordination.
Participating IOIBD Members: Vineet Ahuja, Matthieu Allez, Ashwin N. Ananthakrishnan, Charles N. Bernstein, Jonathan G. Braun, Yehuda Chowers, Jean-Frederic Colombel, Silvio Danese, Geert D'Haens, Andre D'Hoore, Axel Dignass, Iris Dotan, Marla C. Dubinsky, Anders Ekbom, Phillip R. Fleshner, Miquel A. Gassull, Richard B. Gearry, Subrata Ghosh, Anne M. Griffiths, Jonas Halfvarson, Stephen B. Hanauer, Noam Harpaz, Ailsa Hart, Michael A. Kamm, Gil G. Kaplan, Ioannis Koutroubakis, Peter L. Lakatos, Arie Levine, James D. Lewis, James O. Lindsay, Edward V. Loftus Jr., Edouard Louis, Milan Lukas, Fernando Magro, Uma Mahadevan, Gerasimos J. Mantzaris, Dermot P. McGovern, Bjørn A. Moum, Pia Munkholm, Markus F. Neurath, Siew C. Ng, Colm O'Morain, Remo Panaccione, Julian Panes, Laurent Peyrin-Biroulet, Cosimo Prantera, Zhihua Ran, Walter Reinisch, Feza H. Remzi, David B. Sachar, William J. Sandborn, R. Balfour Sartor, Jürgen Schölmerich, Stefan Schreiber, Britta Siegmund, Mark S. Silverberg, Johan D. Söderholm, Eduard F. Stange, Flavio Steinwurz, Dan Turner, Morten H. Vatn, and Severine Vermeire. Additional Invited Participants: Erica J. Brenner, Britt Christensen, Ferdinando D'Amico, Chris M. Griffiths, Peter D. Higgins, Michael D. Kappelman, Charlie Lees, Miguel D. Regueiro, Joel R. Rosh, Ryan Ungaro
Footnotes
Conflicts of interest The authors have made the following disclosures: David Rubin, Maria Abreu, and Corey Siegel are members of IOIBD. In addition, they have the following disclosures. David T. Rubin has received grant support from Takeda; has served as a consultant for Abbvie, Abgenomics, Allergan Inc., Boehringer Ingelheim Ltd., Bristol-Myers Squibb, Celgene Corp/Syneos, Dizal Pharmaceuticals, GalenPharma/Atlantica, Genentech/Roche, Gilead Sciences, Ichnos Sciences S.A., GlaxoSmithKline Services, Janssen Pharmaceuticals, Eli Lilly, Pfizer, Prometheus Laboratories, Reistone, Shire, Takeda, and Techlab Inc. Maria T. Abreu has received grant support from Prometheus Laboratories, Pfizer, and Takeda; and has served as a consultant for Abbvie, Eli Lilly, Janssen, Takeda, Focus Medical Communications, Boehringer Ingelheim, Gilead, Imedex, Cornerstones Health, Landos Biopharma, and UCB Biopharma SRL. Victoria Rai has nothing to disclose. Corey A. Siegel has received grant support from the Crohn’s and Colitis Foundation, Broad Medical Research Program, Abbvie, Janssen, Pfizer, and Takeda; and has served as consultant for Abbvie, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Pfizer, Prometheus, Sebela, and Takeda.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2020.04.002.
Contributor Information
International Organization for the Study of Inflammatory Bowel Disease:
Vineet Ahuja, Matthieu Allez, Ashwin N. Ananthakrishnan, Charles N. Bernstein, Jonathan G. Braun, Yehuda Chowers, Jean-Frederic Colombel, Silvio Danese, Geert D'Haens, Andre D'Hoore, Axel Dignass, Iris Dotan, Marla C. Dubinsky, Anders Ekbom, Phillip R. Fleshner, Miquel A. Gassull, Richard B. Gearry, Subrata Ghosh, Anne M. Griffiths, Jonas Halfvarson, Stephen B. Hanauer, Noam Harpaz, Ailsa Hart, Michael A. Kamm, Gil G. Kaplan, Ioannis Koutroubakis, Peter L. Lakatos, Arie Levine, James D. Lewis, James O. Lindsay, Edward V. Loftus, Jr., Edouard Louis, Milan Lukas, Fernando Magro, Uma Mahadevan, Gerasimos J. Mantzaris, Dermot P. McGovern, Bjørn A. Moum, Pia Munkholm, Markus F. Neurath, Siew C. Ng, Colm O'Morain, Remo Panaccione, Julian Panes, Laurent Peyrin-Biroulet, Cosimo Prantera, Zhihua Ran, Walter Reinisch, Feza H. Remzi, David B. Sachar, William J. Sandborn, R. Balfour Sartor, Jürgen Schölmerich, Stefan Schreiber, Britta Siegmund, Mark S. Silverberg, Johan D. Söderholm, Eduard F. Stange, Flavio Steinwurz, Dan Turner, Morten H. Vatn, Severine Vermeire, Erica J. Brenner, Britt Christensen, Ferdinando D'Amico, Chris M. Griffiths, Peter D. Higgins, Michael D. Kappelman, Charlie Lees, Miguel D. Regueiro, Joel R. Rosh, and Ryan Ungaro
Supplementary Material
Supplementary Table 1.
Types of IBD Therapies Assessed by the IOIBD Panel
| Therapy type | Also known as |
|---|---|
| 5-Aminosalicylic acid (5-ASA) | Asacol, Apriso, balsalazide, Dezicol, Lialda, mesalamine, mesalazine, Pentasa |
| Oral budesonide | Entocort, Uceris |
| Steroids (the dose discussed is oral prednisone and ≥20 mg/d) | Prednisone, Medrol, Hydrocortisone |
| Thiopurines | 6-Mercaptopurine, azathioprine, Azasan, Purinethol |
| Methotrexate | Trexal, Rheumatrex |
| JAK inhibitor | Tofacitinib (Xeljanz) |
| Anti-TNF | Adalimumab (Humira, Abrilada, Ajevita, Cyltezo, Hyrimoz, Hadlima), certolizumab pegol (Cimzia), golimumab (Simponi), infliximab (Remicade, Avsola, Inflectra, Ixifi Remsima, Renflexis) |
| Anti-IL12/23 | Ustekinumab (Stelara) |
| Anti-integrin | Vedolizumab (Entyvio), (the panel did not discuss natalizumab (Tysabri) |
IBD, inflammatory bowel disease; IOIBD, International Organization for the Study of Inflammatory Bowel Diseases; TNF, tumor necrosis factor.
Supplementary Table 2.
Full Results of Assessment of Statements Related to Risk of Infection with SARS-CoV-2 or Development of COVID-19 in Patients with IBD by the IOIBD Panel
| Statements | First RAND Panel Voting |
Second RAND Panel Voting After 20 March 2020 Webinar |
||||||
|---|---|---|---|---|---|---|---|---|
| (n = 64 participants, 69 statements) |
(n = 66 participants, 76 statements) |
|||||||
| Median | SD | Category | DI | Median | SD | Category | DI | |
| Risk of infection/disease | ||||||||
| The risk of infection with SARS-CoV-2 is the same whether a patient has IBD or does not have IBD. | 7 | 1.9 | Appropriate | 2.35 | 8 | 1.7 | Appropriate | –0.71 |
| Independent of treatment, patients with Crohn’s disease have a greater risk of infection with SARS-CoV-2 than the general population. | 3 | 1.8 | Inappropriate | 0.55 | 2 | 1.7 | Inappropriate | 0.16 |
| Independent of treatment, patients with ulcerative colitis have a greater risk of infection with SARS-CoV-2 than the general population. | 3 | 1.8 | Inappropriate | 0.65 | 2 | 1.7 | Inappropriate | 0.16 |
| Having active inflammation from IBD increases the risk of getting SARS-CoV-2. | 5 | 1.9 | Uncertain | 0.69 | 5.5 | 1.8 | Uncertain | 0.63 |
| Patients with IBD who are exposed to SARS-CoV-2 have a higher risk of developing COVID-19 compared to patients without IBD. | 5 | 1.9 | Uncertain | 0.37 | 5 | 1.7 | Uncertain | 0.52 |
| Patients with IBD who have COVID-19 have a higher mortality compared to patients without IBD. | 4 | 1.7 | Uncertain | 0.52 | 3.5 | 1.7 | Inappropriate | 0.52 |
| Patients with an ostomy are at increased risk for COVID-19. | 3 | 1.8 | Inappropriate | 0.16 | 2 | 1.2 | Inappropriate | 0.13 |
| Patients with a J pouch are at increased risk for COVID-19. | 3 | 1.8 | Inappropriate | 0.16 | 2 | 1.2 | Inappropriate | 0.13 |
| Elective surgeries and endoscopies should be postponed at this time. | 8 | 2.0 | Appropriate | -0.44 | 8.5 | 1.6 | Appropriate | –0.34 |
| Healthcare workers with IBD on immune modifying medications working in an environment with known or suspected COVID-19 patients should continue working, assuming they are following standard prevention methods. | 6 | 2.2 | Uncertain | 2.35 | 5.5 | 2.0 | Uncertain | 2.02 |
| Patients with IBD on immune-modifying medications should discontinue any nonessential travel. | 9 | 1.5 | Appropriate | 0.00 | 9 | 1.2 | Appropriate | –0.17 |
| It is safe to continue infusions in an infusion center assuming the infusion center has a screening protocol in place. | 8 | 1.3 | Appropriate | -0.71 | 8 | 1.0 | Appropriate | –0.71 |
| Therapy class: 5-ASA | ||||||||
| 5-ASA increases the risk of infection with SARS-CoV-2. | 1 | 1.2 | Inappropriate | 0.13 | 1 | 0.7 | Inappropriate | 0.00 |
| 5-ASA increases the risk of COVID-19. | 1 | 1.2 | Inappropriate | 0.13 | 1 | 0.7 | Inappropriate | 0.12 |
| Patients taking 5-ASA therapy should reduce the dose of therapy to prevent SARS-CoV-2 infection. | 1 | 0.8 | Inappropriate | 0.13 | 1 | 0.7 | Inappropriate | 0.00 |
| Patients taking 5-ASA therapy should discontinue therapy to prevent SARS-CoV-2 infection. | 1 | 0.9 | Inappropriate | 0.00 | 1 | 0.7 | Inappropriate | 0.00 |
| Patients taking 5-ASA therapy should stop therapy if they test positive for SARS-CoV-2 but do not have COVID-19. | 1 | 1.0 | Inappropriate | 0.13 | 1 | 1.1 | Inappropriate | 0.00 |
| Patients taking 5-ASA therapy should stop therapy if they develop COVID-19. | 1 | 1.4 | Inappropriate | 0.13 | 1 | 1.5 | Inappropriate | 0.13 |
| Therapy class: oral budesonide | ||||||||
| Budesonide increases the risk of infection with SARS-CoV-2. | 3 | 1.8 | Inappropriate | 0.63 | 3 | 1.4 | Inappropriate | 0.16 |
| Budesonide increases the risk of COVID-19. | 4 | 1.7 | Uncertain | 0.52 | 3 | 1.5 | Inappropriate | 0.22 |
| Patients taking budesonide therapy should reduce the dose of therapy to prevent SARS-CoV-2 infection. | 3 | 2.0 | Inappropriate | 0.52 | 3 | 1.8 | Inappropriate | 0.16 |
| Patients taking budesonide therapy should discontinue therapy to prevent SARS-CoV-2 infection. | 3 | 1.7 | Inappropriate | 0.31 | 2 | 1.6 | Inappropriate | 0.16 |
| Patients taking budesonide therapy should stop therapy if they test positive for SARS-CoV-2 but do not have COVID-19. | 4 | 2.1 | Uncertain | 0.52 | 4 | 2.1 | Uncertain | 0.52 |
| Patients taking budesonide therapy should stop therapy if they develop COVID-19. | 5 | 2.4 | Uncertain | 1.76 | 5 | 2.2 | Uncertain | 0.85 |
| Therapy class: oral prednisone (≥20 mg/d) | ||||||||
| Prednisone (≥20 mg/d) increases the risk of infection with SARS-CoV-2. | 7 | 1.9 | Appropriate | 10.00 | 7 | 2.1 | Appropriate | 2.35 |
| Prednisone (≥20 mg/d) increases the risk of COVID-19. | 7 | 1.4 | Appropriate | 0.00 | 7 | 2.0 | Appropriate | 10.00 |
| Patients taking prednisone therapy (≥20 mg/d) should reduce the dose of therapy to prevent SARS-CoV-2 infection. | 7 | 2.0 | Appropriate | 10.00 | 7 | 2.0 | Appropriate | 0.00 |
| Patients taking prednisone therapy (≥20 mg/d) should discontinue therapy (taper as appropriate) to prevent SARS-CoV-2 infection. | 5 | 2.0 | Uncertain | 0.85 | 7 | 2.3 | Appropriate | 2.35 |
| Patients taking prednisone therapy (≥20 mg/d) should stop therapy (taper as appropriate) if they test positive for SARS-CoV-2 but do not have COVID-19. | 6 | 2.1 | Uncertain | 2.35 | 7 | 1.7 | Appropriate | –0.71 |
| Patients taking prednisone therapy (≥20 mg/d) should stop therapy (taper as appropriate) if they develop COVID-19. | 7 | 2.3 | Appropriate | -4.49 | 8 | 1.6 | Appropriate | –0.71 |
| Therapy class: thiopurines | ||||||||
| Azathioprine/6-MP increases the risk of infection with SARS-CoV-2. | 6 | 1.9 | Uncertain | 0.63 | 5 | 2.0 | Uncertain | 0.85 |
| Azathioprine/6-MP increases the risk of COVID-19. | 6 | 1.7 | Uncertain | 2.35 | 6 | 1.9 | Uncertain | 0.63 |
| Patients taking azathioprine/6-MP should reduce the dose of therapy to prevent SARS-CoV-2 infection. | 3 | 1.9 | Inappropriate | 0.52 | 3 | 2.1 | Inappropriate | 0.56 |
| Patients taking azathioprine/6-MP should discontinue therapy to prevent SARS-CoV-2 infection. | 3 | 2.1 | Inappropriate | 0.60 | 3 | 1.9 | Inappropriate | 0.35 |
| Patients taking azathioprine/6-MP should stop therapy if they test positive for SARS-CoV-2 but do not have COVID-19. | 7 | 2.3 | Appropriate | 2.35 | 7 | 2.0 | Appropriate | –2.32 |
| Patients taking azathioprine/6-MP should stop therapy if they develop COVID-19. | 8 | 2.0 | Appropriate | -0.71 | 8 | 1.5 | Appropriate | –0.71 |
| Therapy: methotrexate | ||||||||
| Methotrexate increases the risk of infection with SARS-CoV-2. | 5 | 1.9 | Uncertain | 0.92 | 4 | 1.7 | Uncertain | 0.52 |
| Methotrexate increases the risk of COVID-19. | 5 | 1.7 | Uncertain | 0.85 | 5 | 1.9 | Uncertain | 0.44 |
| Patients taking methotrexate should reduce the dose of therapy to prevent SARS-CoV-2 infection. | 3 | 1.9 | Inappropriate | 0.52 | 3 | 1.6 | Inappropriate | 0.16 |
| Patients taking methotrexate should discontinue therapy to prevent SARS-CoV-2 infection. | 3 | 2.1 | Inappropriate | 0.57 | 3 | 1.5 | Inappropriate | 0.16 |
| Patients taking methotrexate should stop therapy if they test positive for SARS-CoV-2 but do not have COVID-19. | 6.5 | 2.2 | Appropriate | 2.35 | 7 | 2.0 | Appropriate | 10.00 |
| Patients taking methotrexate should stop therapy if they develop COVID-19. | 7 | 2.2 | Appropriate | -0.71 | 7 | 1.6 | Appropriate | –0.71 |
| Therapy class: anti-TNFs | ||||||||
| Anti-TNF therapy increases the risk of infection with SARS-CoV-2. | 3 | 1.9 | Inappropriate | 0.52 | 4 | 1.7 | Uncertain | 0.22 |
| Anti-TNF therapy increases the risk of COVID-19. | 5 | 2.1 | Uncertain | 0.52 | 4 | 1.7 | Uncertain | 0.52 |
| Patients taking anti-TNF therapy should reduce the dose of therapy to prevent SARS-CoV-2 infection. | 3 | 1.6 | Inappropriate | 0.16 | 2 | 1.4 | Inappropriate | 0.16 |
| Patients taking anti-TNF therapy should discontinue therapy to prevent SARS-CoV-2 infection. | 2 | 1.8 | Inappropriate | 0.16 | 2 | 1.2 | Inappropriate | 0.00 |
| Patients taking anti-TNF therapy should stop therapy if they test positive for SARS-CoV-2 but do not have COVID-19. | 5 | 2.4 | Uncertain | 0.85 | 6 | 2.2 | Uncertain | 2.35 |
| Patients taking anti-TNF therapy should stop therapy if they develop COVID-19. | 7 | 2.5 | Appropriate | 30.00 | 7 | 2.0 | Appropriate | –0.71 |
| Therapy: vedolizumab | ||||||||
| Vedolizumab increases the risk of infection with SARS-CoV-2. | 3 | 2.0 | Inappropriate | 0.65 | 3 | 1.5 | Inappropriate | 0.16 |
| Vedolizumab increases the risk of COVID-19. | 4 | 2.0 | Uncertain | 0.65 | 3 | 1.6 | Inappropriate | 0.37 |
| Patients taking vedolizumab should reduce the dose of therapy to prevent SARS-CoV-2 infection. | 2 | 1.7 | Inappropriate | 0.22 | 2 | 1.3 | Inappropriate | 0.15 |
| Patients taking vedolizumab should discontinue therapy to prevent SARS-CoV-2 infection. | 2 | 1.6 | Inappropriate | 0.13 | 2 | 1.2 | Inappropriate | 0.00 |
| Patients taking vedolizumab should stop therapy if they test positive for SARS-CoV-2 but do not have COVID-19. | 4 | 2.4 | Uncertain | 0.92 | 5 | 2.2 | Uncertain | 0.85 |
| Patients taking vedolizumab should stop therapy if they develop COVID-19. | 5 | 2.6 | Uncertain | 1.77 | 6 | 2.1 | Uncertain | 2.35 |
| Therapy: ustekinumab | ||||||||
| Ustekinumab increases the risk of infection with SARS-CoV-2. | 3 | 1.7 | Inappropriate | 0.22 | 3 | 1.5 | Inappropriate | 0.16 |
| Ustekinumab increases the risk of COVID-19. | 3 | 1.9 | Inappropriate | 0.52 | 3 | 1.6 | Inappropriate | 0.16 |
| Patients taking ustekinumab should reduce the dose of therapy to prevent SARS-CoV-2 infection. | 2 | 1.6 | Inappropriate | 0.16 | 2 | 1.1 | Inappropriate | 0.16 |
| Patients taking ustekinumab should discontinue therapy to prevent SARS-CoV-2 infection. | 2 | 1.6 | Inappropriate | 0.16 | 2 | 1.1 | Inappropriate | 0.00 |
| Patients taking ustekinumab should stop therapy if they test positive for SARS-CoV-2 but do not have COVID-19. | 5 | 2.2 | Uncertain | 0.92 | 6 | 2.1 | Uncertain | 2.35 |
| Patients taking ustekinumab should stop therapy if they develop COVID-19. | 6 | 2.4 | Uncertain | 18.25 | 7 | 2.1 | Appropriate | –1.57 |
| Therapy: tofacitinib | ||||||||
| Tofacitinib increases the risk of infection with SARS-CoV-2. | 6 | 2.2 | Uncertain | 0.63 | 5 | 1.9 | Uncertain | 0.52 |
| Tofacitinib increases the risk of COVID-19. | 6 | 2.1 | Uncertain | 2.35 | 5 | 1.9 | Uncertain | 0.32 |
| Patients taking tofacitinib should reduce the dose of therapy to prevent SARS-CoV-2 infection. | 3 | 2.4 | Inappropriate | 0.52 | 3 | 1.9 | Inappropriate | 0.19 |
| Patients taking tofacitinib should discontinue therapy to prevent SARS-CoV-2 infection. | 3 | 2.1 | Inappropriate | 0.60 | 3 | 1.5 | Inappropriate | 0.16 |
| Patients taking tofacitinib should stop therapy if they test positive for SARS-CoV-2 but do not have COVID-19. | 6 | 2.4 | Uncertain | 2.35 | 7 | 1.9 | Appropriate | 10.00 |
| Patients taking tofacitinib should stop therapy if they develop COVID-19. | 7 | 2.3 | Appropriate | -3.08 | 8 | 1.6 | Appropriate | –0.71 |
| Combination therapy of anti-TNF and immunomodulator | ||||||||
| Patients taking combination therapy with an anti-TNF and thiopurine/methotrexate should reduce the dose of the thiopurine/methotrexate to prevent infection from SARS-CoV-2. | 5 | 2.4 | Uncertain | 0.97 | 4 | 2.2 | Uncertain | 0.91 |
| Patients taking combination therapy with an anti-TNF and thiopurine/methotrexate should stop the thiopurine/methotrexate if they test positive for SARS-CoV-2 but do not have COVID-19. | 7 | 2.6 | Appropriate | -3.08 | 7 | 2.2 | Appropriate | –3.30 |
| Patients taking combination therapy with an anti-TNF and thiopurine/methotrexate should stop the thiopurine/methotrexate if they develop COVID-19. | 8 | 2.2 | Appropriate | -0.93 | 8 | 1.3 | Appropriate | 0.00 |
| Clinical trials | ||||||||
| Patients taking clinical trial drugs should discontinue therapy to prevent SARS-CoV-2 infection. | 2 | 1.4 | Inappropriate | 0.16 | ||||
| Patients taking clinical trial drugs should stop therapy if they test positive for SARS-CoV-2 but do not have COVID-19. | 7 | 1.9 | Appropriate | 10.00 | ||||
| Patients taking clinical trial drugs should stop therapy if they develop COVID-19. | 8 | 1.6 | Appropriate | –0.32 | ||||
| Approach to active disease | ||||||||
| A patient with moderately to severely active Crohn’s disease or ulcerative colitis (new diagnosis or relapsing disease) should be treated with the same therapies you would choose in the pre-COVID-19 era. | 7 | 2.1 | Appropriate | 10.00 | ||||
| Treatment of IBD after SARS-CoV-2 infection or COVID-19 | ||||||||
| In an IBD patient who tests positive for SARS-CoV-2 and whose IBD meds have been stopped because of this, IBD meds can be restarted after 14 days (provided they have not developed COVID-19). | 7 | 1.5 | Appropriate | –0.71 | ||||
| In an IBD patient who develops COVID-19 and whose IBD meds have been stopped, IBD meds can be restarted after COVID-19 symptoms resolve. | 7 | 1.9 | Appropriate | 10.00 | ||||
| In an IBD patient who develops COVID-19 and whose IBD meds have been stopped, IBD meds can be restarted after 2 nasopharyngeal PCR tests are negative. | 8 | 1.6 | Appropriate | –0.71 | ||||
5-ASA, 5-aminosalicylic acid; 6-MP, mercaptopurine; COVID-19, coronavirus disease 2019; DI, disagreement index; IBD, inflammatory bowel disease; IOIBD, International Organization for the Study of Inflammatory Bowel Diseases; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2; SD, standard deviation; TNF, tumor necrosis factor.
Supplementary Data
Google form survey instrument (second round voting).
References
- 1.Ghebreyesus T. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 Available: Accessed June, 2020.
- 2.Johns Hopkins Center for Systems Science and Engineering Coronavirus COVID-19 Global Cases by Johns Hopkins CSSE. https://coronavirus.jhu.edu/ Available:
- 3.Secure-IBD Database. https://covidibd.org/ Available:
- 4.ESPGHAN: Porto and IBD Interest Group ESPGHAN. www.espghan.org/about-espghan/committees/gastroenterology/working-groups/porto-and-ibd-interest-group/ Available:
- 5.Fitch K., editor. The Rand/UCLA appropriateness method user’s manual. RAND; Santa Monica: 2001. [Google Scholar]
- 6.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ace2 and tmprss2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao F., Tang M., Zheng X. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang P.-H., Cheng Y. Increasing host cellular receptor—angiotensin-converting enzyme 2 (ACE2) expression by coronavirus may facilitate 2019-nCoV infection. bioRxiv. 2020 doi: 10.1002/jmv.26139. 2020.02.24.963348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ianiro G., Mullish B.H., Kelly C.R. Screening of faecal microbiota transplant donors during the COVID-19 outbreak: suggestions for urgent updates from an international expert panel. Lancet Gastroenterol Hepatol. 2020;5:430–432. doi: 10.1016/S2468-1253(20)30082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson P., Griffin I., Tucker C. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng K.-W., Cheng S.-C., Chen W.-Y. Thiopurine analogs and mycophenolic acid synergistically inhibit the papain-like protease of Middle East respiratory syndrome coronavirus. Antiviral Res. 2015;115:9–16. doi: 10.1016/j.antiviral.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 Mar 13 doi: 10.1001/jamainternmed.2020.0994. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L., Liu H.G., Liu W. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E005. doi: 10.3760/cma.j.issn.1001-0939.2020.0005. [DOI] [PubMed] [Google Scholar]
- 17.Li C.K., Wu H., Yan H. T cell responses to whole SARS coronavirus in humans. J Immunol. 2008;181:5490–5500. doi: 10.4049/jimmunol.181.8.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A clinical study for the efficacy and safety of Adalimumab Injection in the treatment of patients with severe novel coronavirus pneumonia (COVID-19) Chinese Clinical Trial Register (ChiCTR) www.chictr.org.cn/showprojen.aspx?proj=49889 Available:
- 19.Ng S.C., Hilmi I.N., Blake A. Low frequency of opportunistic infections in patients receiving vedolizumab in clinical trials and post-marketing setting. Inflamm Bowel Dis. 2018;24:2431–2441. doi: 10.1093/ibd/izy153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrareddy S.N., Arthos J., Cicala C. Sustained virologic control in SIV+ macaques after antiretroviral and α4β7 antibody therapy. Science. 2016;354:197–202. doi: 10.1126/science.aag1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uzzan M., Tokuyama M., Rosenstein A.K. Anti-α4β7 therapy targets lymphoid aggregates in the gastrointestinal tract of HIV-1-infected individuals. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aau4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghosh S., Gensler L.S., Yang Z. Ustekinumab safety in psoriasis, psoriatic arthritis, and Crohn’s disease: an integrated analysis of phase II/III clinical development programs. Drug Saf. 2019;42:751–768. doi: 10.1007/s40264-019-00797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu X., Han M., Li T. Effective treatment of severe COVID-19 patients with tocilizumab. ChinaXiv. 2020:12. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roche to start phase III trial of Actemra in Covid-19 patients Clinical Trials Arena. www.clinicaltrialsarena.com/news/roche-actemra-covid-19-trial/ Available:
- 25.Ben-Horin S., Van Assche G., Chowers Y. Pharmacokinetics and immune reconstitution following discontinuation of thiopurine analogues: implications for drug withdrawal strategies. J Crohns Colitis. 2018;12:1410–1417. doi: 10.1093/ecco-jcc/jjy122. [DOI] [PubMed] [Google Scholar]
- 26.Dotan I., Werner L., Vigodman S. Normal response to vaccines in inflammatory bowel disease patients treated with thiopurines. Inflamm Bowel Dis. 2012;18:261–268. doi: 10.1002/ibd.21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamba M., Wang R., Fletcher T. Extended-release once-daily formulation of tofacitinib: evaluation of pharmacokinetics compared with immediate-release tofacitinib and impact of food. J Clin Pharmacol. 2016;56:1362–1371. doi: 10.1002/jcph.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinhold K.J., Bukowski J.F., Brennan T.V. Reversibility of peripheral blood leukocyte phenotypic and functional changes after exposure to and withdrawal from tofacitinib, a Janus kinase inhibitor, in healthy volunteers. Clin Immunol. 2018;191:10–20. doi: 10.1016/j.clim.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Google form survey instrument (second round voting).