Abstract
Due to the government's early intervention such as mass lockdown and curtailment strategies towards mass gatherings, amid the COVID-19 outbreak, the organization of the voluntary blood donation camps have been suspended. It's most significant impact on the blood community has been a dramatic decrease in the number of blood donors. Therefore, our blood stock has almost dried up and put our inventory in a state of jeopardy. Additionally, all the elective surgeries and non-urgent clinical interventions have also been deferred during this time. This has led to a drop in the blood collection, demand as well as the issue at our blood center. With this backdrop, we intended to assess the effect of this mass lockdown on our blood supply management, particularly in two phases [phase-I prior to the outbreak] and phase-II [during the outbreak]. Transitioning back to the normal conditions would most likely depend on the extent and the time duration of this pandemic and associated behavioural change, which is foreseen to remain in effect well beyond the original estimates.
Keywords: Coronavirus Infection, Pandemic, Voluntary Blood Donation, Blood Management, Blood Transfusion Services
1. Introduction
The corona-virus disease-2019 [COVID-19] caused by the novel coronavirus [nCoV] also known as “SARS-CoV-2” is a declared pandemic. This is primarily transmitted by the respiratory route [1]. Although WHO noted in 2003 that no cases of SARS-CoV had been reported due to transfusion of blood products, the transfusion transmission risk of this novel virus despite being theoretical, cannot be ignored at this juncture [2]. The outbreak of this nCoV has had a hugely negative impact on the blood community by decreasing the number of donations, thereby, affecting the blood transfusion services [BTS] worldwide [3], [4]. Therefore, all the BTS must assess, plan, and respond in a timely yet proportionate manner towards the upsurge of this devastating nCoV that has killed thousands globally till date. Blood community in these harrowing times must balance the procurement and the issuing of blood in a rather pro-active manner. The global recommendations of “social distancing” are being seen as the only effective measure to mitigate the community spread of this deadly pathogen. In the context of BTS, today's mandate to “flatten the curve of this pandemic” raises many questions that may transform its basic functioning. Few questions it raises are namely, how can we “ensure sufficient blood donations” within the existing limitations of social distancing? How should we protect our BTS staffs who are obligated to work in close proximity to the apparently healthy blood donors? How do we plan the duty-hours of our BTS staff in the wake of this pandemic when fear and anxiety are genuine among the healthcare workers? What could be the short and long-term implications of the reduced collections on our services? The answers to these questions will depend on the extent and time-duration of this pandemic and associated behavioural change, which is anticipated to remain in effect well beyond the original estimates. Additionally, according to the recent advisory, by the government, all the voluntary blood donation camps [VBDCs] have been put on a hold as a part of the “curtailment strategy” to avoid mass gatherings [5]. With this background, we aimed to study the pattern of blood collection, its demand and issue both before and during this outbreak. We also intended to derive crucial lessons towards the blood-supply management both for now as well as in the future.
2. Study methodology
This was a retrospective descriptive study of six months [Oct’19 to Mar’20] data of a blood centre supporting a 1200-bedded multi-specialty tertiary care academic hospital in the Dehradun district of North India. The study was divided into two phases namely:
-
•
Phase-I: the pre-pandemic phase in India [Oct’19 to Initial half-Feb’20];
-
•
Phase-II: the full-blown pandemic phase in India [Later half-Feb’20 to Mar’20].
Details of the blood units collected both in-house as well as in the VBDCs were retrieved from the dedicated hospital management software [Akhil Systems Pvt. Ltd. New Delhi]. The date of collection, date of expiry and the date of issue for each packed red blood cell [PRBC] units were noted. The difference between the date of collection and the date of issue was taken as the storage life for that particular blood unit. Blood usage [on a weekly basis] was also tabulated on a spreadsheet. At our centre, we follow the rule of ‘first in, first out’ policy for issuing of red cell units towards the majority of transfusions. The numbers of red blood cell units which were not utilized and were outdated beyond the date of expiry [OBDE] were noted as well. We used the following formula to calculate the percentage of OBDE units:
The data was captured and analyzed using SPSS software version 20 [IBM, USA].
3. Results
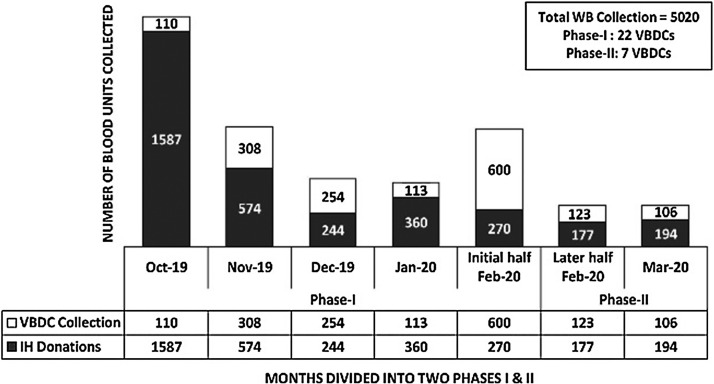
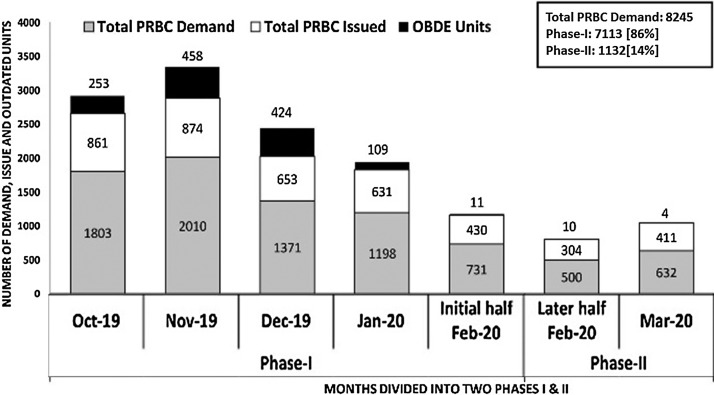
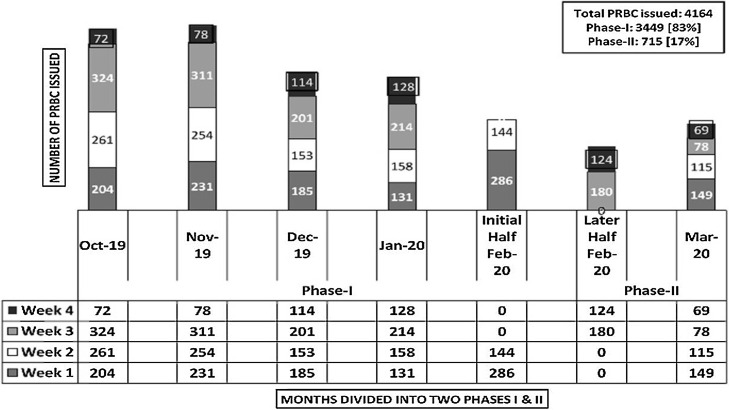
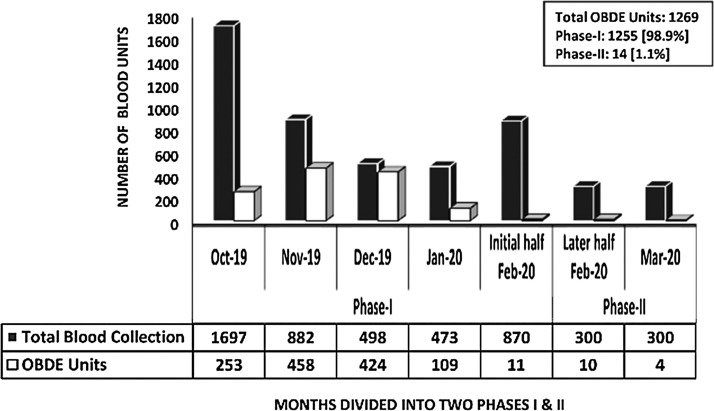
The total whole blood [WB] collections in phase-I and phase-II were 88% [n = 4420/5020] and 12% [n = 600/5020] respectively. In phase-I, we conducted twenty-two VBDCs as against seven in phase-II (Fig. 1 ). In phase-I, the average in-house [IH] WB collections were 22/d [n = 3035 in 137 days] which dropped to 8/d [n = 371 in 45 days] during the phase-II respectively. Percentage-wise, this reduction in the number of donations amounted to a significant 64%. Of the total 5020 WB collections, 4,772 PRBC units were prepared in the last six-month period [Oct’19 to Mar’20]. The total demand raised for PRBC during these six-months were, n = 8,245. This was scaled down to 16% [n = 1132/8245] in phase-II, when compared to 84% [n = 7113/8245] in phase-I respectively (Fig. 2 ). For the estimation of blood requirement, we assessed the number of PRBC units issued on a weekly basis over a period of six months [n = 4,164]. The mean number of PRBC units issued on a weekly basis was 148.7 units (Range: 40 to 324) (Fig. 3 ). For the estimation of blood requirement, we evaluated the number of PRBC units issued on a weekly basis over a period of six months [n = 4,164]. The average number of PRBC units issued on a weekly basis was 148.7 units [(Range: 40 to 324), fig. 3]. As depicted in fig.2, the issue was high in the month of Oct’19 [n = 861 units] and Nov’19 [n = 874 units] respectively, and least in the month of Mar’20 [n = 411 units]. There was a generally a decreasing trend in the number of OBDE units starting from the month of Nov’19 [n = 458 units] to only four units in Mar’20. This reduction in the trend of OBDE units followed the lesser collection of blood in phase II as against phase-I [Fig. 4 , n = 0.0001]. Majority of the PRBC of blood group ‘B’ and ‘AB’ were issued towards the end of their shelf life. The majority of the PRBC of blood group ‘B’ and ‘AB’ were issued towards the end of their shelf life. The mean storage period of the PRBC units of blood group ‘B’, AB’, ‘O’, ‘A’ were 29, 27, 15 and 14 days respectively.
Fig. 1.
WB collections at voluntary blood donation camps [VBDCs] and in-house [IH] donations.
Fig. 2.
Significant reduction in the demand, issue & OBDE of PRBC units during Phase-II.
Fig. 3.
Comparison of the weekly issue of PRBC units during Phase-I and Phase-II.
Fig. 4.
Lesser WB collection in the Phase-II led to a reduction in the number of OBDE units.
4. Discussion
It is a well-known fact that apart from donations, there is no other way to maintain sufficient bloodstock in the inventory. The emergence of COVID-19 has led to an enormous anxiety among blood donors and therefore, the blood community has been affected adversely. Having disrupted so many processes world-wide, the BTS are no exception. Hence, it merited a compelling and justifiable review of the blood collection, demand, issue as well as the OBDE pattern at our facility. The monthly average collection of the whole blood was 805 units [SD: 485, Range: 285–1607] and the average issue of the PRBC units was 694 [SD: 172, Range: 411–874] showing the maintenance of the overall balance between the two. Despite strict vigilance and adherence to the first-in, first-out principle, the number of OBDE units was high during certain months of the study period, particularly in phase-I. The increased number of outdated units was due to both a higher number of collections as well as the decreased requirement of blood. The phase-II had lesser number of OBDE units due to lesser collection. The study by Bashwari et al at eastern Saudi Arabia has shown a rate of 3.6% of discarding of blood units due to expiry and the study concludes that performing regular internal audits and reviewing statistics are critical tools of any BTS [6]. The overall discard rate of the South Korean blood services was 3.2% and according to them, the shortened blood reserve in the inventory resulted in the drop in the OBDE rate of the unused blood components [7]. The overall rate of OBDE units during the study period at our blood centre was 30% in phase-I and it was mainly due to the large number of units outdated (458 units in Nov’19 and 424 units in Dec’19) due to lesser requirement of blood bed-side, despite high demand raised during the two occasions. Although our technicians cross-matched and reserved the PRBC units however, the reserved blood was hardly utilized bed-side according to our records. This led to a higher percentage of OBDE on those two occasions. To further analyze the cause for a higher rate of blood wastage due to OBDE, we compared its trend with the pattern of blood collection. Almost 30% [n = 1255/4187] of the PRBC units were outdated in phase-I following the higher WB collection. This is strikingly different from phase-II where lesser number of donations during the COVID-19 outbreak resulted in only 2.39% [n = 14/585] of OBDE. Another issue raised in recent literature is about the safety of the stored blood transfusion, and these studies concluded that transfusion of older blood units adversely affects the recipients’ health. Based on the meta-analysis, Dong Wang et al concluded that the use of older stored blood is associated with a significantly increased risk of mortality [8]. In the present study, the mean storage period of the red cell units was 21.2 days. Similar to the trend in the OBDE units, the issuing of older red cell units [‘B’ and AB’ at 29 and 27 days respectively] increased following VBDCs collections [642 units in Nov’19 and 396 units in Dec’19]. Although the use of older blood units quantitatively does not amount to wastage, qualitatively this is generally not considered as ‘good blood transfusion practices.’
The government's restrictions on conducting VBDCs as a part of the curtailment strategy has directly influenced the blood supply chain. To avoid shortage in these difficult times, in accordance to the national blood transfusion council, we are issuing an appointment letter for the voluntary blood donors, encouraging them to come to our blood centre individually during the lock-down period. Although prepared, till date we have not had any COVID-19 positive patients admitted in our hospital. Therefore, an increase in blood to address specific needs due to either inflammation or DIC [which is shown to happen in such COVID-19 positive cases] could not be evaluated at our facility. Furthermore, we have routinely been involved in the blood donor screening program that is able to prevent any individuals with active respiratory symptoms from donating blood. Additionally, the safety of both the donor as well as the BTS staff remains our priority during this time. An experience during the SARS epidemic [2002 to 2003] has shown that the most significant impact on the blood supply during a pandemic is likely to be a dramatic reduction in blood donors [9]. Similarly, a significant 64% drop in the frequency of IH blood donations from pre-pandemic to the full-blown pandemic phase has been observed at our facility.
Literature suggests that influenza and its complications are generally not associated with the need for blood transfusions, and an increased need for blood components during a pandemic is highly unlikely. Conversely, there may be a decrease in the blood demand as healthcare capacity is shifted towards providing the basic healthcare support, and non-urgent clinical interventions are usually deferred. It is estimated that demand for red cells will decrease by 5 to 25%, and frozen and fractionated products by 0–10%, with little or no change in platelet demands [10]. In our study, we noted that due to the deferral of elective surgeries and routine procedures during phase-II, there was a reduction in both the demand as well as the issue of blood units. A blood component wise reduction in the demand was 14%, 11% and 1.6% for PRBC, fresh frozen plasma and platelets respectively. Likewise, we noticed a reduction in the PRBC issued during the phase-II as 17% as against 83% in phase-I
5. Conclusion
Knowledge of the COVID-19 is evolving every day. An efficient communication strategy with the voluntary blood donors to motivate them for donations and networking with the nearby blood establishments will help go a long way into the blood supply management during this pandemic.
Research involving human participants and/or animals
Human participant.
Informed consent
As per the department policy an informed consent is obtained from all donors prior to blood donation in accordance to our department protocol
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional ethics committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding and resources
None.
Each author's contributions are detailed below
| S. No | Categories of Contribution | Author 1: Manish Raturi | Author 2: Anuradha Kusum |
|---|---|---|---|
| 1 | Conceptual design | Yes | No |
| 2 | Literature search | Yes | No |
| 3 | Data Compilation | Yes | No |
| 4 | Manuscript preparation and editing | Yes | Yes |
| 5 | Manuscript review | Yes | Yes |
| 6 | Guarantor [taking responsibility of the integrity of the work as a whole from inception to published article] | Yes | No |
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.World Health Organization. Interim Guidance on COVID-19. https://www.who.int/publications-detail/maintaining-a-safe-and-adequate-blood-supply-during-the-pandemic-outbreak-of-coronavirus-disease-(covid-19).[Last accessed 2020-3-28].
- 2.World Health Organization . 2003. WHO recommendations on SARS and blood safety.https://www.who.int/csr/sars/guidelines/bloodsafety/en/ [Last accessed 2020-3-28] [Google Scholar]
- 3.Shan H., Zhang P. Viral attacks on the blood supply: the impact of severe acute respiratory syndrome in Beijing. Transfusion. 2004;44:467–469. doi: 10.1111/j.0041-1132.2004.04401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teo D. Blood supply management during an influenza pandemic. ISBT Science Series. 2009;4:293–298. [Google Scholar]
- 5.Ministry of Health and family Welfare. https://www.mohfw.gov.in/pdf/advisoryformassgathering.pdf.[Last accessed 2020-3-28].
- 6.Bashwari L.A. Pattern of blood procurement, ordering and utilization in a University Hospital in Eastern Saudi Arabia. Saudi Medical Journal. 2002;23:555–561. [PubMed] [Google Scholar]
- 7.Oh D.J. The progress of South Korean blood transfusion services (2004-2006) Asian J Transfus Sci. 2008;2:87–89. doi: 10.4103/0973-6247.42697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D., Sun J., Solomon S.B., Klein H.G., Natanson C. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52:1184–1195. doi: 10.1111/j.1537-2995.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Blood Alliance . European Blood Alliance; 2009. Pandemic Influenza–Planning for Blood Organisations.http://isbt-web.org/uploads/documents/PandemicInfluenza.pdf [Last accessed202 -3-30] [Google Scholar]
- 10.W.H.O. World Health Organization; Geneva: 2006. Guidelines for National Blood Transfusion Services: Maintaining a Safe and Adequate Blood Supply in the Event of Pandemic Influenza.http://www.who.int/bloodsafety/publications/WHO-Guidelines on Pandemic Influenza and Blood Supply.pdf [Last accessed 2020-3-30] [Google Scholar]