Highlights
-
•
COVID -19 cases are now confirmed in multiple countries.
-
•
Assessed the prevalence of comorbidities in infected patients.
-
•
Comorbidities are risk factors for severe compared with non-severe patients.
-
•
Help the health sector guide vulnerable populations and assess the risk of deterioration.
Keywords: SARS-CoV-2, COVID-19, Comorbidities, Clinical characteristics, Epidemiology, Meta-analysis
Abstract
Background
An outbreak of coronavirus disease 2019 (COVID-19) occurred in Wuhan, China; the epidemic is more widespread than initially estimated, with cases now confirmed in multiple countries.
Aims
The aim of this meta-analysis was to assess the prevalence of comorbidities in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infected patients and the risk of underlying diseases in severe patients compared to non-severe patients.
Methods
A literature search was conducted using the databases PubMed, EMBASE, and Web of Science through February 25, 2020. Odds ratios (ORs) and 95% confidence intervals (CIs) were pooled using random-effects models.
Results
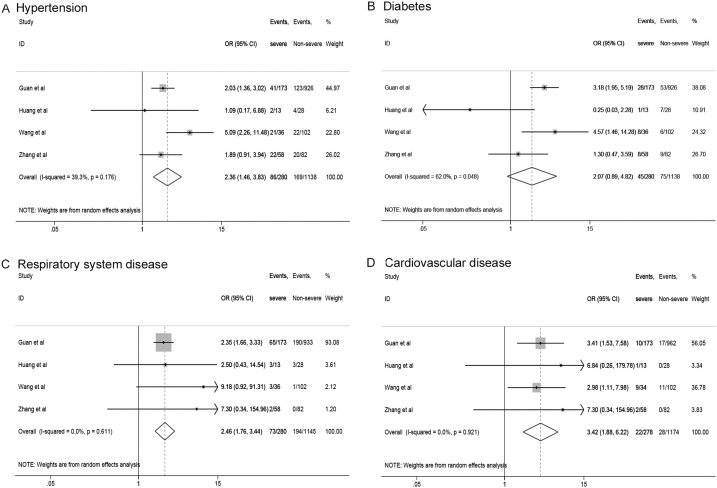
Seven studies were included in the meta-analysis, including 1 576 infected patients. The results showed the most prevalent clinical symptom was fever (91.3%, 95% CI: 86–97%), followed by cough (67.7%, 95% CI: 59–76%), fatigue (51.0%, 95% CI: 34–68%) and dyspnea (30.4%, 95% CI: 21–40%). The most prevalent comorbidities were hypertension (21.1%, 95% CI: 13.0–27.2%) and diabetes (9.7%, 95% CI: 7.2–12.2%), followed by cardiovascular disease (8.4%, 95% CI: 3.8–13.8%) and respiratory system disease (1.5%, 95% CI: 0.9–2.1%). When compared between severe and non-severe patients, the pooled OR of hypertension, respiratory system disease, and cardiovascular disease were 2.36 (95% CI: 1.46–3.83), 2.46 (95% CI: 1.76–3.44) and 3.42 (95% CI: 1.88–6.22) respectively.
Conclusion
We assessed the prevalence of comorbidities in the COVID-19 patients and found that underlying disease, including hypertension, respiratory system disease and cardiovascular disease, may be risk factors for severe patients compared with non-severe patients.
Introduction
In December, 2019, a cluster of cases of “pneumonia of unknown origin” has been reported in Wuhan, China. Only a few days later, Chinese health authorities confirmed that this cluster was associated with coronavirus (Hui et al., 2020) and the disease caused by it was named coronavirus disease 2019 (COVID-19) by WHO. Confirmed by comparative homology analysis, COVID-19 is closely associated with bat-derived severe acute respiratory syndrome (SARS)-like coronavirus (bat-SL-covzc45 and bat-SL-covzxc21, with 88% identity), but is far away from severe acute respiratory syndrome coronavirus (SARS-CoV) (about 79%) and Middle East respiratory syndrome coronavirus (MERS-CoV) (about 50%) (Lu et al., 2020). A total of 77 658 confirmed cases, including 9 162 with severe illnesses, and 2 663 deaths had been reported as of February 25, 2020, by the National Health Commission of the People's Republic of China.
Huang et al. firstly reported the clinical features of 41 confirmed patients, and indicated 13 (32%) of them had underlying diseases (Huang et al., 2020), including cardiovascular disease, diabetes, hypertension, and chronic obstructive pulmonary disease. Subsequently, Wang et al. reported findings from 138 cases of COVID-19; the results suggested that 64 (46.4%) of them had comorbidities. Importantly, the patients who were admitted to the intensive care unit (ICU) had a higher number of comorbidities (72.2%) than those not admitted to the ICU (37.3%). This suggested that comorbidities maybe risk factors for adverse outcomes (Wang et al., 2020). Assessing the prevalence of these chronic diseases are the basis for mitigating complications in patients infected with SARS-CoV-2. However, the effort was hampered by the limited number of cases.
To get more convincing results, we will provide a systematic evaluation and detail, which will not only estimate the prevalence of comorbidities in all patients, but also assess the risk of underlying diseases in severe patients compared to non-severe patients. The results may aid in patient management while helping to develop policies for prevention and response to COVID-19 and its critical outcomes.
Methods
Search strategy and selection criteria
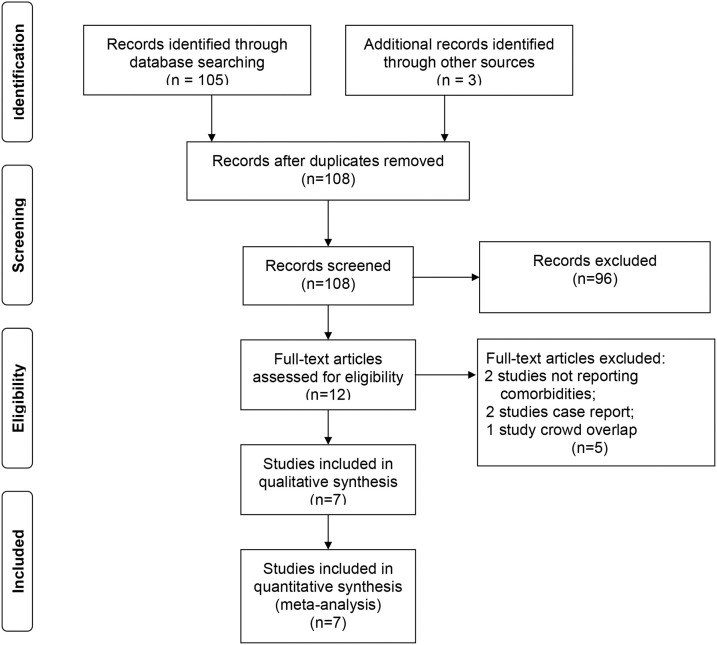
A systematic search was conducted on studies published from January 1, 2019 to February 25, 2020 in PubMed, EMBASE, and Web of Science databases. Records were managed by EndNote X9.0 software to exclude duplicates. According to the indices of the various databases, we use the search term “2019 novel coronavirus and COVID-19” AND “comorbidities, clinical characteristics, epidemiological” without any language restriction. To identify missing studies, we checked the reference list for each selected paper. Eligible were those that described the epidemiological, clinical features of COVID-19, and the prevalence of chronic diseases in infected patients. Studies that were (a) duplicate publications, (b) reviews, editorials, case reports, letters, and family-based studies, or (c) only have children’s cases were excluded. The steps of the literature search are shown in Figure 1 .
Figure 1.
Flow diagram of the number of studies screened and included in the meta-analysis.
Data extraction and analysis
The two investigators (J Yang and YP Wang) who performed the literature search also independently extracted the data from included studies. Disagreements were resolved with a third investigator (YN Zhou) or by consensus. We extracted the following variables: author, date, age, gender, number of participants in severe and non-severe, and the prevalence of clinical symptoms such as fever, cough, fatigue, and dyspnea, together with comorbidities including hypertension, diabetes, respiratory system disease, and cardiovascular disease. All calculations were performed by Stata MP version 13.0 (Stata corporation, College Station, TX, USA). The odds ratios (ORs) was used to describe the risk of different comorbidities in severe patients compared with non-severe patients. Owing to heterogeneity within and between studies, random-effect models were used to estimate the average effect and its precision, which would give a more conservative estimate of the 95% confidence intervals (CIs). The I2 statistic and Cochran's Q test were used to assess statistical heterogeneity.
Results
Search terms initially found a total of 108 articles. After we removed duplicates, checked the title and abstract, and reviewed full-text, eight studies remained for analysis. But we found that the population in one of the eight studies overlapped with populations in others, and it was not entirely in line with the purpose of our research, so we excluded this article (The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team, 2020). Seven studies (Guan et al., 2020; Huang et al., 2020; K. Liu et al., 2020; Y. Liu et al., 2020; Wang et al., 2020; J. Zhang et al., 2020; M. Zhang et al., 2020) eventually met the predetermined inclusion and exclusion criteria. As of February 25, 2020, a total of 1 576 participants were included in our meta-analysis. As presented in Table 1 , the median age was 49.6 years and 890 (56.5%) were men.
Table 1.
Main Characteristics of included studies in the meta-analysis.
| Study | Date (month, day) |
Patients (No.) |
Age (year) |
Symptoms (%) |
Comorbidities (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Male | Fever | Cough | Fatigue | Dyspnea | Hypertension | Diabetes | Respiratory system disease | Cardiovascular diseases | |||
| Huang et al. (2020) | 12.16-01.02 | 41 | 30 | 49.0 | 98.0 | 76.0 | 44.0 | 55.0 | 15.0 | 20.0 | 2.0 | 15.0 |
| Liu et al. (2020) | 12.30-01.24 | 137 | 61 | 57.0 | 81.8 | 48.2 | 32.1 | 19.0 | 9.5 | 10.2 | 1.5 | 7.3 |
| Liu et al. (2019) | 01.10-01.21 | 12 | 8 | 53.7 | 83.3 | 91.7 | 25.0 | 16.7 | 8.3 | 33.3 | ||
| Wang et al. (2020) | 01.01-01.28 | 138 | 75 | 56.0 | 98.6 | 59.4 | 69.6 | 31.2 | 31.2 | 10.1 | 2.9 | 14.5 |
| Zhang et al. (2020a) | 01.06-02.03 | 140 | 71 | 57.0 | 91.7 | 75.0 | 75 | 36.7 | 30.0 | 12.1 | 1.4 | 5.0 |
| Zhang et al. (2020b) | 01.18-02.03 | 9 | 5 | 35.2 | 88.9 | 55.6 | 44.4 | 0 | 11.1 | 0 | 0 | |
| Guan et al. (2020) | −01.29 | 1 099 | 640 | 47.0 | 87.9 | 67.7 | 38.1 | 18.6 | 14.9 | 7.4 | 1.4 | 2.5 |
| Total | 12.16-02.03 | 1 576 | 890 | 49.6 a | ||||||||
| NCPERETb | 12.31-02.11 | 44 672 | 22 981 | 45.9 | 12.8 | 5.3 | 2.4 | 4.2 | ||||
| Prevalence(%)c | 91.3 | 67.7 | 51.0 | 30.4 | 21.1 | 9.7 | 1.5 | 8.4 | ||||
| 95% CI | 86–97 | 59–76 | 34–68 | 21–40 | 13.0–27.2 | 7.2–12.2 | 0.9–2.1 | 3.8–13.8 | ||||
| I2 (%) | 92.1 | 84.9 | 96.4 | 91.1 | 86.2 | 28.7 | 0 | 83.4 | ||||
| P for heterogeneity | 0 | 0 | 0 | 0 | 0 | 0.209 | 0.872 | 0 | ||||
aaverage age.
bThe Novel Coronavirus Pneumonia Emergency Response Epidemiology Team.
cMeta-analysis for the prevalence was calculated from binary random-effects model analysis.
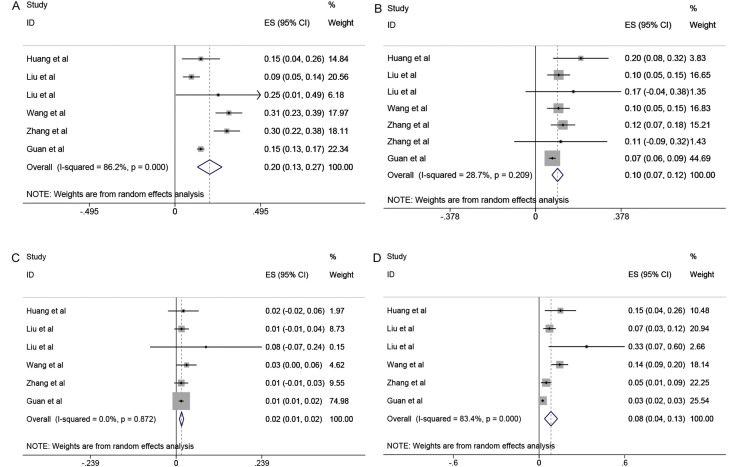
The results of this meta-analysis showed the most prevalent clinical symptom was fever (91.3%, 95% CI: 86–97%), followed by cough (67.7%, 95% CI: 59–76%), fatigue (51.0%, 95% CI: 34–68%) and dyspnea (30.4%, 95% CI: 21–40%). However, the I2 varying from 84.9% to 96.4% in the evaluates of the clinical features showed significant statistical heterogeneity (p = 0.000). The comorbidities included hypertension, diabetes, respiratory system disease, and cardiovascular disease. As shown in Figure 2 (inserts A, B, C, D), the most prevalent comorbidities were hypertension (21.1%, 95% CI: 13.0–27.2%) and diabetes (9.7%, 95% CI: 7.2–12.2%), followed by cardiovascular disease (8.4%, 95% CI: 3.8–13.8%) and respiratory system disease (1.5%, 95% CI: 0.9–2.1%). In analysis by the proportion of comorbidities, significant heterogeneities were observed for estimates of hypertension and cardiovascular disease (p = 0.000), but not for diabetes (p = 0.209) and respiratory system disease (p = 0.872) with I2 indexes ranging from 0 to 86.2%.
Figure 2.
Meta-analysis of the comorbidities in COVID-19 cases. A, B, C, D represent proportions of hypertension, diabetes, respiratory system disease, and cardiovascular disease.
In Figure 3 , we analyzed comorbidities between severe group and non-severe group. Higher risk of hypertension (OR 2.36, 95% CI: 1.46–3.83), respiratory system disease (OR 2.46, 95% CI: 1.76–3.44), and cardiovascular disease (OR 3.42, 95% CI: 1.88–6.22) were observed in the severe group. They showed low heterogeneity, with I2 from 0 to 39.3 %. However, there was not statistically significant difference in diabetes (OR 2.07, 95% CI: 0.89-4.82).
Figure 3.
The risk of comorbidities in severe patients compared to non-severe patients. (A) hypertension, (B) diabetes, (C) respiratory system disease, (D) cardiovascular disease.
Discussion
The meta-analysis was based on data from 7 studies with laboratory-confirmed COVID-19. All cases were from hospitals in China. The results observed males took a larger amount than females; statistics showed about 890:686 in the COVID-19 patients. The results consisted with that the Chinese Center for Disease Control and Prevention (China CDC) reported (The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team, 2020). MERS-CoV and SARS-CoV have also been found that males are more likely to be infected than females (Badawi and Ryoo, 2016, Channappanavar et al., 2017). It is customary to think women are less likely to be affected by many bacteria and viruses than are men, partly because of their more robust innate and adaptive immune responses (Jaillon et al., 2019). Elderly people and severe patients are more susceptible to SARS-CoV-2, which may be associated with a higher frequency of comorbidities (Zhang et al., 2020a).
A meta-analysis of the comorbidities suggested that hypertension was prevalent in approximately 21.1% of the patients; diabetes, cardiovascular disease, and respiratory system disease were present in 9.7%, 8.4%, and 1.5% of the cases, respectively. Hypertension and diabetes mellitus consistent with the prevalence of hypertension and diabetes in China were 23.2% (Hu et al., 2019) and 10.9% (Liu et al., 2019) in adults. A recent study about influenza illness suggested that compared to patients with no comorbidities, the risk of death for severe patients was higher in those who had chronic obstructive pulmonary disease (OR 1.49, 95% CI: 1.10–2.01), and in those who had cardiovascular disease (OR 2.92, 95% CI: 1.76–4.86) or hypertension (OR 1.49, 95% CI: 1.10–2.10) (Mertz et al., 2013). The comorbidities had also been noted to have similar effects in other respiratory illnesses, such as MERS (Badawi and Ryoo, 2016). In our study, the associations were also observed in hypertension, cardiovascular disease, and respiratory system disease groups. Overall, the severe patients were older (Wang et al., 2020) and had more significant number of comorbid conditions than those who were non-severe. These results may suggest that age and comorbidities are risk factors for critical patients.
Diseases such as hypertension, diabetes, respiratory system disease, cardiovascular disease, and their susceptibility conditions may be linked to the pathogenesis of COVID-19. Chronic diseases share several standard features with infectious disorders, such as the proinflammatory state, and the attenuation of the innate immune response. For instance, diabetes occurs in part because the accumulation of activated innate immune cells in metabolic tissues leads to the release of inflammatory mediators, especially IL-1β and TNFα, which promote systemic insulin resistance and β-cell damage (Odegaard and Chawla, 2012). Additionally, metabolic disorders may lead to low immune function by impairing macrophage and lymphocyte function (Dooley and Chaisson, 2009), which may make individuals more susceptible to disease complications (Badawi and Ryoo, 2016). Recently, Guo et al. (2019) retrospectively analyzed the clinical data of patients with viral pneumonia and found that the absolute count levels of CD3+T cells, CD3+CD8+ T cells and CD3+CD4+ T cells in the deceased group were significantly lower than those in the survival group, suggesting that the levels of various inflammatory factors in the deceased group were higher than those in the survival group. A prospective case control study about seasonal influenza was conducted by Hong et al. (2014). Their results showed that diabetes and chronic cardiovascular disease were significantly related to development of complications, and diabetes was an independent risk factor for severe seasonal influenza (OR 3.63, 95% CI: 1.15–11.51, p = 0.02). Furthermore, a study analyzed the risk factors for patients with MERS-CoV infection, finding diabetes, smoking, and heart disease were also significantly associated with MERS-CoV illness (Alraddadi et al., 2016).
Limitations of this meta-analysis should be addressed. First, high heterogeneity statistics could be found. This may relate to the study designs and large variation among studies in the sample size (9 to 1 099 patients). Second, different lengths of follow-up may miss the events leading to heterogeneity; some patients in the included studies are still in hospital. Third, because only a few studies compared the comorbidities of severe and non-severe patients, we did not conduct sensitivity analysis and subgroup analysis.
If causality exists between chronic diseases and severe COVID-19 patients, it will help the health sector guide vulnerable populations and assess the risk of deterioration. Lau et al. (2013) followed up the fiu vaccinations of 91 605 people with diabetes. The results showed that the flu vaccine could decrease the incidence of influenza and pneumonia in people with diabetes aged <65 years by 43%, and diabetes in elderly people (≥65 years) by 55%. The symptoms of COVID-19 are similar to those of influenza (e. g, fever, cough or fatigue), and the COVID-19 outbreaks occured during a year of a high prevalence of respiratory diseases caused by influenza, respiratory syncytial virus, and other respiratory viruses. Vaccines can still be useful in preventing flu and will reduce possible confusion with the SARS-CoV-2 infection (Patel and Jernigan, 2020).
The people with hypertension, diabetes, respiratory system disease, and cardiovascular disease should be included in influenza and future SARS-CoV-2 vaccination recommendations. Given the limited level of evidence, more adequately powered studies should be conducted to prove the association. The prevalence of chronic diseases is increasing year by year, and targeted public health vaccination interventions must be adopted to better protect people with chronic diseases from infection with SARS-CoV-2 and other respiratory viruses.
Conflict of interest
The authors declared that they have no conflicts of interest to this work.
Ethics
The study does not require ethical approval because the meta-analysis is based on published research and the original data are anonymous.
Contributor Information
Yuping Wang, Email: wangyuping@lzu.edu.cn.
Yongning Zhou, Email: zhouyn@lzu.edu.cn.
References
- Alraddadi B.M., Watson J.T., Almarashi A., Abedi G.R., Turkistani A., Sadran M. Risk factors for primary middle east respiratory syndrome coronavirus illness in humans, Saudi Arabia, 2014. Emerging Infect Dis. 2016;22:49–55. doi: 10.3201/eid2201.151340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fett C., Mack M., Ten Eyck P.P., Meyerholz D.K., Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017;198:4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley K.E., Chaisson R.E. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9:737–746. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Wei D., Zhang X., Wu Y., Li Q., Zhou M. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA Score. Front Microbiol. 2019;10:2752. doi: 10.3389/fmicb.2019.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K.W., Cheong H.J., Choi W.S., Lee J., Wie S.H., Baek J.H. Clinical courses and outcomes of hospitalized adult patients with seasonal influenza in Korea, 2011-2012: Hospital-based Influenza Morbidity & Mortality (HIMM) surveillance. J Infect Chemothe. 2014;20:9–14. doi: 10.1016/j.jiac.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Hu S., Gao R., Liu L., Zhu M., Wang W., Wang Y. Summary of the 2018 report on cardiovascular diseases in China. Chin Circ J. 2019;34:209. [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., IA E., Madani T.A., Ntoumi F., Kock R., Dar O. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health – the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillon S., Berthenet K., Garlanda C. Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol. 2019;56:308–321. doi: 10.1007/s12016-017-8648-x. [DOI] [PubMed] [Google Scholar]
- Lau D., Eurich D.T., Majumdar S.R., Katz A., Johnson J.A. Effectiveness of influenza vaccination in working-age adults with diabetes: a population-based cohort study. Thorax. 2013;68:658–663. doi: 10.1136/thoraxjnl-2012-203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020 doi: 10.1097/cm9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Liu S.W., Wang L.J., Bai Y.M., Zeng X.Y., Guo H.B. Burden of diabetes, hyperglycaemia in China from to 2016: findings from the 1990 to 2016, global burden of disease study. Diabetes Metab. 2019;45:286–293. doi: 10.1016/j.diabet.2018.08.008. [DOI] [PubMed] [Google Scholar]
- Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz D., Kim T.H., Johnstone J., Lam P.P., Science M., Kuster S.P. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013;347:f5061. doi: 10.1136/bmj.f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard J.I., Chawla A. Connecting type 1 and type 2 diabetes through innate immunity. Cold Spring Harbor Perspect Med. 2012;2 doi: 10.1101/cshperspect.a007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A., Jernigan D.B. Initial public health response and interim clinical guidance for the 2019 novel coronavirus outbreak – United States, December 31, 2019-February 4, 2020. Am J Transpl. 2020;20:889–895. doi: 10.1111/ajt.15805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)-China, 2020. China CDC Wkly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q. Clinical characteristics of 140 patients infected by SARS-CoV-2 in Wuhan, China. Allergy. 2020;0:1–12. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- Zhang M.Q., Wang X.H., Chen Y.L., Zhao K.L., Cai Y.Q., An C.L. Clinical features of 2019 novel coronavirus pneumonia in the early stage from a fever clinic in Beijing. Zhonghua jie he he hu xi za zhi. 2020;43:E013. doi: 10.3760/cma.j.issn.1001-0939.2020.0013. [DOI] [PubMed] [Google Scholar]