Abstract
This study was performed to clarify the acute phase response following infectious bronchitis virus inoculation. Ninety clinically healthy 1-d-old Ross chicks were randomly assigned into 2 groups: control (n = 20) and infected group (n = 70). At the age of 20 d, all birds in the infected group were challenged intranasally with allantoic fluid containing 105 embryo lethal dose (ELD50)/0.1 mL of the infectious bronchitis virus. Blood samples were collected from 20 clinically healthy and 70 infected chicks at prior and 1, 2, 3, 5, 7, 11, 13, 15, and 20 d postinoculation. On d 1, 7, and 11 postinoculation 4 chickens from the experimental group and 2 chickens from the control group were randomly selected. Their trachea, lungs, and cecal tonsil were collected for virus detection and quantitation by real-time reverse-transcription PCR assay. In the serum the acute phase proteins (haptoglobin and serum amyloid A), pro-inflammatory cytokines (interferon-γ and tumor necrosis factor-α), and serum sialic acid (total, TSA; lipid-bound, LBSA; and protein-bound, PBSA) concentrations were measured using validated standard procedures. All variables were significantly higher in the infected birds after virus inoculation compared with the healthy group (P < 0.05). There were positive correlations between all variables in the infected group. Correlation coefficients were significantly positive between haptoglobin and interferon-γ, LBSA and TSA, and TSA and LBSA (P < 0.05). There were positive correlations among viral RNA and all studied variables; however, these correlations were not statistically significant (P > 0.05).
Key words: avian infectious bronchitis virus, real-time reverse-transcription PCR, acute phase protein, inflammatory cytokine
INTRODUCTION
Infectious bronchitis virus (IBV) primarily causes an acute respiratory disease in domestic chickens. The disease is characterized by high morbidity, low mortality, and substantial loss of production (Cavanagh and Gelb, 2008). The etiologic agent of infectious bronchitis (IB) is an enveloped, positive-sense, and single-stranded RNA virus that belongs to the family Coronaviridae, genus Coronavirus (Cavanagh, 2007). Chickens of all ages may be infected, and infected chickens show signs of depression, coughing, sneezing, nasal discharge, polyuria, and death. The IBV replicates in many organs such as the upper respiratory tract, reproductive, renal, and digestive organs, and lead to inflammatory responses (Cavanagh and Gelb, 2008). Live attenuated and inactivated vaccines have been developed to control IB (Cavanagh, 2007). Despite the wide use of various vaccines to protect commercial chickens in Iran, IB still remains responsible for serious financial losses to the poultry industry of the country. A new isolate of IBV (IRFIBV32; GenBank: HQ123359.1) from broiler chicken in Fars province, Southern Iran, was isolated and identified by Boroomand et al. (2011). Furthermore, the pathogenesis and clinical signs of experimentally infected chicks by this isolate were also studied (Boroomand et al., 2012).
The inflammatory reactions consist of a series of complex physiological events occurring in the host against infection or tissue injury. The purposes of these events are to eliminate the infecting agent, prevent further tissue damage, and restore the homeostasis of the host organism. The early sets of reactions that occur immediately after tissue damage are known as the acute phase response (APR). Acute phase response includes pro-inflammatory cytokines and acute phase protein (APP) production (Petersen et al., 2004). The APP are blood proteins primarily synthesized by the liver, in which the concentration of APP change to infection, inflammation, surgical trauma, or stress (Gabay and Kushner, 1999; Eckersall, 2004; Murata et al., 2004; Gruys et al., 2005). Many advances in monitoring the APP response in animals for clinical and experimental purposes have been achieved in the last 2 decades (Eckersall, 2000).
On exposure to various inflammatory conditions, white blood cells release pro-inflammatory cytokines, which are the major mediators of APP synthesis in the liver and are essential for the recruitment of neutrophils to the site of inflammation (Yoshioka et al., 2002; Ananian et al., 2005). Two major pro-inflammatory cytokines are tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), which activate macrophages and promote cell-mediated immune responses against invasive intracellular pathogens (Rich et al., 2008).
Chickens, similar to other animals, produce APP during inflammation and diseases. Several research studies have been published about APP and their changes due to different inflammatory and noninflammatory conditions in birds (Nielsen et al., 1999; Henry et al., 2000; Barnes et al., 2001; Koutsos and Klasing, 2001; Juul-Madsen et al., 2003; Kokosharov, 2006). However, the importance of and sensitivity toward the evaluation of APP and inflammatory cytokines in the diagnosis of infection and disease are not clearly known. Few research studies have been undertaken on changes of APP and sialic acid (SA) in IB with regard to finding the relationship between APP and sialic acid in IB. Based on this evidence, the present study was undertaken to evaluate alteration in concentrations of the acute phase proteins [haptoglobin (Hp) and serum amyloid A (SAA)], pro-inflammatory cytokines (TNF-α and IFN-γ), and the serum level of total sialic acid (TSA), lipid-bound sialic acid (LBSA), and protein-bound sialic acid (PBSA) in experimentally infected chicks by IBV isolate IRFIBV32 compared with healthy chicks.
MATERIALS AND METHODS
Birds
Ninety apparently healthy 1-d-old Ross broiler chicks were divided randomly into 2 experimental groups consisting of challenged (n = 70) and control (n = 20) groups. Chicks were reared separately in the Animal Research Unit of the Veterinary School of Shiraz University and received feed and water ad libitum during the experiment. All experiments were conducted after institutional approval of the animal use committee of Shiraz University.
Experimental Design
The IBV isolate IRFIBV32 (GenBank: HQ123359.1) was used in the present study. This isolate was well characterized previously (Boroomand et al., 2011). It was propagated in 10-d-old embryonating chicken eggs. The embryo lethal dose (ELD50) was calculated according to the formula of Reed and Muench (1938). Allantoic fluid containing 105 ELD50/0.1 mL of the virus was used to induce disease. The infected group was challenged intranasally at 20 d old. The remaining 20 birds were left as unchallenged control. Blood sampling was performed on all chicks via wing vein. All samples were collected prior to and 1, 2, 3, 5, 7, 11, 13, 15, and 20 d postinoculation (PI) into plane tubes. Sera were separated by centrifugation (for 15 min at 750 × g and 25°C) and stored at −20°C until assayed. A TaqMan real-time reverse-transcription PCR was used for early and accurate detection and quantify the viral RNA load of IBV isolate IRFIBV32 from infected chicken clinical samples at 1, 7, and 11 d PI. The cDNA synthesis from normalized RNA of positive samples was carried out using AccuPowder RT PreMix kit (BioNeer Corporation, Daejeon, South Korea) according to the manufacturer's suggestions. The reaction was performed with a mixture of 20 pmol of random hexamer and 20 pmol of reverse primer. The primer was specific to a highly conserved region of nucleocapsid protein gene of IBV that was described previously by Adzhar et al. (1996; Table 1 ). The reaction mixture was incubated at 70°C for 5 min, then incubated at 42°C for 60 min, heated to 95°C for 5 min, cooled to 4°C, then stored at −70°C until assayed. The quantitative real-time PCR primers and TaqMan probe used in this study were described previously by Chousalkar et al. (2009; Table 1). The primers amplified a 76-bp fragment in the N gene of IBV. The probe annealed to the part of the sequence amplified by 2 primers (Chousalkar et al., 2009). The assays were performed on a 48-well microtiter plate of Bio-Rad MiniOpticon System. The reaction mixture contained 5 µL of target cDNA, 1 µL of each primer at a concentration of 10 pmol/µL, 1 µL of the TaqMan probe at a concentration of 10 pmol/µL, 10 µL of master mix 5x, 0.2 µL of uracil-N-glycosylase (UNG), and 1.8 µL of distilled water in a final volume of 20 µL. At first UNG treatment at 50°C for 2 min and UNG inactivation at 95°C for 10 min was performed and then cDNA was amplified by 44 two-step cycles: denaturation (95°C, 15 s), primer annealing (55°C, 30 s). Viral cDNA copy numbers (expressed as copies per 1 µg of total RNA) were quantified by comparison with a 10-fold serially diluted plasmid standard of known concentration. The data and standard curves were obtained during target cDNA and recombinant plasmid amplification (unpublished data).
Table 1.
The cDNA synthesis and real-time PCR primer and probe sequences
| Specificity | Primer/probe | Sequence |
|---|---|---|
| cDNA synthesis | Forward (UTR1: Adzhar et al., 1996) | 5′-GCTCTAACTCTATACTAGCCTAT-3′ |
| Real-time PCR | Forward | 5′-CCA TTA GTT CGA TGT ACG GAT A-3′ |
| Reverse | 5′-CAG ATG AAT CAT CGG ACC TTT G-3′ | |
| Probe | 5′-FAM-GCG GTC CCT TTA CAG A BHQ-2-3′ |
UTR = untranslated region.
Biochemical Assays
APP (Hp and SAA) Determination.
The Hp was measured according to prevention of the peroxidase activity of hemoglobin, which is directly proportional to the amount of Hp (Tridelta PHASE Haptoglobin Assay kit). The analytical sensitivity of this test in serum has been determined as 0.0156 mg/mL for Hp by the manufacturer (Tridelta Development Plc, Wicklow, Ireland).
The SAA was measured by a solid phase sandwich ELISA (Tridelta phase range SAA kit). The analytical sensitivity of this test in serum has been determined as 0.3 μg/mL for SAA by the manufacturer (Tridelta Development Plc).
Inflammatory Mediator (IFN-γ and TNF-α) Determination.
The TNF-α and IFN-γ were measured by a solid phase sandwich ELISA (AbC 606 and AbC 607, respectively; Votre Fournisseur AbCys S.A., Paris, France).
TSA, LBSA, and PBSA Determination.
The TSA concentration was determined by the TBA method previously described by Warren (1959). The amount of TSA was determined by the use of a standard curve developed from a standard sample of N-acetylneuraminic acid. The LBSA concentration was determined by the method described by Katopodis et al. (1982). The amount of LBSA was determined by use of a standard curve developed from a standard sample of N-acetylneuraminic acid. The PBSA concentration was measured by subtracting serum TSA from LBSA.
Statistical Analysis
Descriptive statistics including mean, SD, median, minimum, and maximum were calculated for all variables. Statistical analysis was performed using a paired samples t-test to determine differences between 2 different hours in each experimental group. Two independent samples t-test was used to detect differences between control and infected groups in similar hours of each serological parameter. One-way ANOVA with LSD post-hoc test was used to compare mean serum concentrations of PBSA, LBSA, and TSA in similar hours of infected groups. Pearson's rank correlation coefficients were calculated to determine relationships between variables on d 7 PI (the time of highest variable concentration). The relationship among the studied variables and viral RNA in various organs was investigated using Pearson correlation coefficient. Data were analyzed using SPSS software (SPSS for Windows, version 11.5, SPSS Inc., Chicago, IL). A P-value less than 0.05 was considered as statistically significant.
RESULTS
The results of the 2 independent samples t-test showed significant differences between diseased birds and the control group for Hp, SAA, TNF-α, IFN-γ, TSA, LBSA, and PBSA. All of these variables were significantly higher in diseased birds compared with the control group (P < 0.05). The serum concentrations of IFN-γ, TNF-α, Hp, SAA, TSA, LBSA, and PBSA were increased significantly 2.50, 1.98, 1.82, 3.33, 2.25, 2.44, and 1.77 times, respectively, during experimental infection with IBV in chickens. This increase reached maximum levels at d 7 after inoculation and then serum levels decreased; however, their concentrations at d 20 PI were higher than the baseline at prior virus inoculation (P < 0.05, Figures 1 , 2 , and 3 ).
Figure 1.
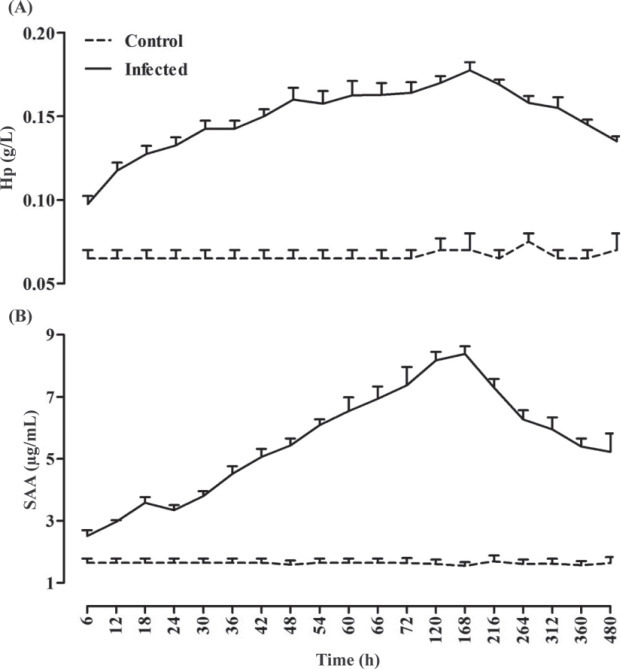
Haptoglobin (Hp, A) and serum amyloid A (SAA, B) alterations due to experimentally induced infectious bronchitis in broiler chicks (n = 70).
Figure 2.
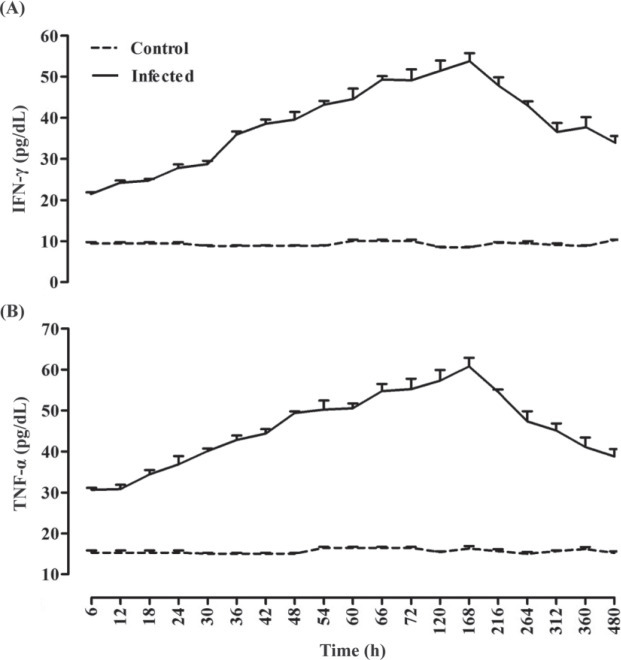
Interferon-gamma (IFN-γ, A) and tumor necrosis factor-α (TNF-α, B) alterations due to experimentally induced infectious bronchitis in broiler chicks (n = 70).
Figure 3.
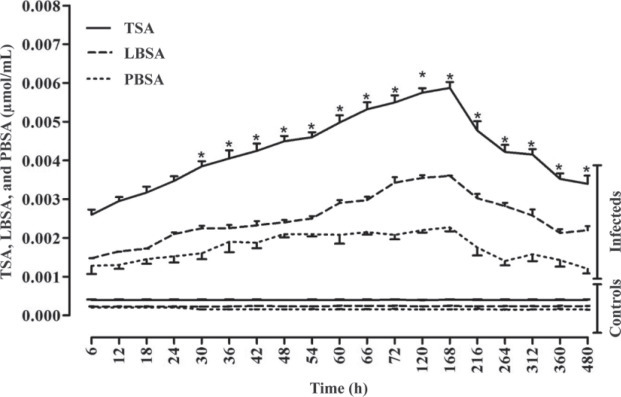
Total sialic acid (TSA), lipid-bound sialic acid (LBSA), and protein-bound sialic acid (PBSA) alterations due to experimentally induced infectious bronchitis in broiler chicks (n = 70). *Significant differences between different types of sialic acids in infected groups (P < 0.05).
The results of the paired samples t-test revealed that elevation of each parameters from the baseline level was significantly different, and the serum concentrations of each parameter at d 20 postinfection were significantly higher than its concentrations at prior infection (P < 0.05).
The results of the one-way ANOVA showed that there were significant differences between various types of SA in the infected groups after the second day postinfection (P < 0.05, Figure 3).
Results of the correlation coefficient showed that there were positive correlations between all variables in the infected group. Correlation coefficients were significantly positive between Hp and IFN-γ, LBSA and TSA, and TSA and LBSA (P < 0.05, Table 2 ). There were positive correlations among viral RNA and all studied variables; however, these correlations were not statistically significant (P > 0.05).
Table 2.
Pearson correlation coefficient between study variables in diseased birds at d 7 postvirus inoculation1
| Item | IFN-γ | Hp | TNF-α | SAA | LBSA | TSA |
|---|---|---|---|---|---|---|
| Hp | 0.949* | |||||
| TNF-α | 0.400 | 0.311 | ||||
| SAA | 0.800 | 0.632 | 0.300 | |||
| LBSA | 0.800 | 0.949* | 0.200 | 0.400 | ||
| TSA | 0.810 | 0.948* | 0.200 | 0.400 | 1.000* | |
| PBSA | 0.400 | 0.316 | 0.400 | 0.800 | 0.200 | 0.200 |
Hp: haptoglobin; TNF-α: tumor necrosis factor-α; SAA: serum amyloid A; LBSA: lipid-bound sialic acid; TSA: total sialic acid; PBSA: protein-bound sialic acid.
Significant correlation (P < 0.05).
The IBV RNA levels were calculated over time in various tissues of infected chickens. The mean of viral copies per 1 µg of total RNA in trachea at 1 d PI was 221,740.1. The viral load decreased in trachea at 7 (811,372.2) and 11 d PI (in sampled bird). The mean IBV RNA levels in the lung were 10,155.3 at 1 d PI and increased to 1,274,234.1 copies at 7 d PI. The viral load decreased in the lung at 11 d PI. The IBV RNA level in cecal tonsils at 1 d PI was 2,707,071 copies per 1 µg of total RNA. The mean of viral copies per 1 µg of total RNA in cecal tonsils at 7 d PI was 2,043,642.4. The viral mRNA load in cecal tonsil was high (6,973,352.9) at 11 d PI (Table 3 ).
Table 3.
Copy number of viral genome per microgram of total RNA of different infected tissues at different days postinoculation (PI)
| Days PI | Tissue |
||
|---|---|---|---|
| Trachea | Lung | Cecal tonsil | |
| 1 | 221,740.1385 | 110,155.2665 | 2,707,070.919 |
| 7 | 811,372.2199 | 1,274,234.159 | 2,043,642.467 |
| 11 | 18,894.25094 | 63,984.47335 | 6,973,352.916 |
There were positive correlations among viral RNA and all studied variables; however, these correlations were not statistically significant (P > 0.05).
DISCUSSION
Evaluating the serum concentrations of APP is generally regarded as being a sensitive, although nonspecific, marker of inflammation and disease in veterinary and medical science (Eckersall, 2000). Several studies have shown that inflammatory conditions and common diseases induce in poultry a state of inflammation associated with an elevation in plasma APP concentration (Chamanza et al., 1999; Holt and Gast, 2002; Rath et al., 2003, Rath et al., 2007; Rath, 2005; Nazifi et al., 2010, 2011).
In the present study, change in serum Hp, SAA, TNF-α, IFN-γ, TSA, LBSA, and PBSA concentrations were evaluated as biomarkers for the assessment of inflammatory processes associated with IBV. As demonstrated, the serum concentrations of Hp, SAA, TNF-α, IFN-γ, TSA, LBSA, and PBSA were significantly higher in infected group after IBV inoculation compared with the healthy group (Figures 1, 2, and 3; P < 0.05). The results of the present study showed that serum concentrations of these factors were elevated after virus inoculation up to d 7 and decreased at d 20 (Figures 1, 2, and 3). It may be stated that serum concentrations of these inflammatory biomarkers decreased along with the depletion of viral mRNA load in different tissues.
Haptoglobin is an α2-globulin and one of the acute phase proteins that serum levels increase in infections, inflammations or tissue damages (Murata et al., 2004). Serum amyloid A is an apolipoprotein of high-density lipoprotein. Serum amyloid A is one of the major acute phase proteins in which, following inflammation and physical stress or at parturition, the serum SAA levels elevate (Murata et al., 2004). Serum amyloid A and Hp have the most important change to inflammation among APP in many animal species (Piñeiro et al., 2007). In chickens, SAA is likely to be a reliable APP for diagnosing inflammatory lesions (Chamanza et al., 1999). As already mentioned, the concentrations of SAA and Hp were significantly higher in challenged chicks after IBV inoculation (P < 0.05, Figure 1). These results are consistent with the previous studies. Kovács et al. (2007) showed an increase in SAA concentration in goose following administration of a fowl cholera vaccine containing inactivated Pasteurella multocida. Nazifi et al. (2010) assessed a significant increase in SAA and Hp levels in infected chicks with gumboro. Nazifi et al. (2011) reported a significant increase of SAA and Hp levels in chicks that had been infected naturally with IBV. In the study of Mosleh et al. (2012), Japanese quail with retained yolk sac showed a significant increase in Hp and SAA concentration. It is documented that SAA and transferrin concentrations increase following administration of terpentin to pullet and Staphylococcus aureus infection in chicks (Chamanza et al., 1999).
Avian cytokines, like their mammalian counterparts, are influential in host immune response to pathogenic infection (Kaiser and Stabeli, 2008).
Interferon-γ is a type II interferon produced by white blood cells and viral-infected somatic cells in response to viral infections, immune activation, inflammatory stimulation, and chemical stimulants (Opal and DePalo, 2000). The TNF-α is a cytokine involved in systemic inflammation and is a member of a group of cytokines that stimulate the APR. The TNF-α and IFN-γ are responsible for a broad spectrum of synergistic or antagonistic effects that influence the specific immune response of the stressed organism against foreign antigens and invading microorganisms (van Miert, 1995; Pinelli, 1996). In the present experiment, the results of the IFN-γ and TNF-α assay showed that the serum concentrations of these inflammatory cytokines in the infected group were significantly higher than in the control group after virus inoculation (P < 0.05; Figure 2). Inflammatory reaction in infected chicks following IBV inoculation causes the release and elevation of pro-inflammatory cytokine concentrations. These pro-inflammatory cytokines then induce the synthesis of APP by the liver. Therefore, the elevation in the IFN-γ, TNF-α, SAA, and Hp concentration in the present experiment could be explained. Arnold and Holt (1996) reported that the level of the tumor necrosis factor in the intestinal tract of infected birds with Salmonella enteritis was higher than healthy ones. Nang et al. (2011) determined infection of chickens with H9N2 influenza virus may induce the production of TNF-α, IFN-γ in trachea, lung, and intestinal cells. Liu et al. (2010) showed that infection with infectious bursal disease virus induces changes in the level of cytokine gene expression in the chicken bursa.
Sialic acid, an acetylated derivative of neuroaminic acid, is widely distributed in mammals’ tissue. Sialic acid is a terminal component of the nonreducing end of carbohydrate chains of glycoproteins and glycolipids (Seyrek et al., 2008). The concentration of SA increases rapidly following the inflammatory and injury process. The measurement of serum SA concentration is of importance in the diagnosis and prognosis of inflammation and cancer (Citil et al., 2004). Based on our findings, concentrations of TSA, LBSA, and PBSA in infected birds significantly increased compared with healthy ones (P < 0.05; Figure 3). Increasing serum TSA, LBSA, and PBSA concentrations revealed apparent tissue damage and inflammatory disorders. This is supported by increases of other measured parameters (Hp, SAA, TNF-α, and IFN-γ) in the present research. This result is in good agreement with the study of Nazifi et al. (2011), which reported that the concentrations of TSA, LBSA, and PBSA in chicks infected naturally with IBV were higher than control group. The TSA, LBSA, and PBSA concentrations in cattle with traumatic reticulo-peritonitis were higher than those of healthy cattle (Citil et al., 2004). Mosleh et al. (2012) demonstrated a significant increase in TNF-α, IFN-γ, TSA, LBSA, and PBSA concentrations in Japanese quail with retained yolk sac.
In the current experimental study, the mean of viral copies per 1 µg of total RNA in trachea, lung, and cecal tonsils at d 7 PI were significantly higher than d 1. The viral RNA load decreased after d 7 PI in trachea and lung, and these depletions occurred along with decreasing the serum concentrations of APR biomarkers. There were positive correlations among viral RNA and all studied variables; however, these correlations were not statistically significant (P > 0.05).
In conclusion, IRFIBV32 isolate can cause inflammatory processes and responses that stimulate the synthesis of pro-inflammatory cytokine and proteins. There are direct relationships between viral RNA loads and inflammatory responses during experimentally infected IB.
ACKNOWLEDGMENTS
The authors thank the members of the Research Committee of Shiraz University (Shiraz, Iran) for providing financial support.
REFERENCES
- Adzhar A., Shaw K., Britton P., Cavanagh D. Universal oligonucleotides for the detection of infectious bronchitis virus by the polymerase chain reaction. Avian Pathol. 1996;25:817–836. doi: 10.1080/03079459608419184. [DOI] [PubMed] [Google Scholar]
- Ananian P., Hardwigsen J., Bernard D., Le Treut Y.P. Serum acute-phase protein level as indicator for liver failure after liver resection. Hepatogastroenterology. 2005;52:857–861. [PubMed] [Google Scholar]
- Arnold J.W., Holt P.S. Cytotoxicity in chicken alimentary secretions as measured by a derivative of the tumor necrosis factor assay. Poult. Sci. 1996;75:329–334. doi: 10.3382/ps.0750329. [DOI] [PubMed] [Google Scholar]
- Barnes D.M., Song Z., Klasing K.C., Bottje W. Protein metabolism during an acute phase response in chickens. Amino Acid. 2001;22:15–26. doi: 10.1007/s726-002-8198-6. [DOI] [PubMed] [Google Scholar]
- Boroomand Z., Asasi K., Mohammadi A. Pathogenesis and tissue distribution of avian infectious bronchitis virus isolate IRFIBV32 (793/B serotype) in experimentally infected broiler chickens. Sci. World. J. 2012;2012:402537. doi: 10.1100/2012/402537. 10.1100/2012/402537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroomand Z., Razeghian I., Asasi K., Mohammadi A., Hoseini A. Isolation and identification of a new isolate of avian infectious bronchitis virus IRFIBV32 and a study of its pathogenicity. Online J. Vet. Res. 2011;15:366–380. [Google Scholar]
- Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007;38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Gelb J. Infectious bronchitis. In: Saif L.J., editor. Diseases of Poultry. Blackwell Publishing; Ames, IA: 2008. pp. 117–135. [Google Scholar]
- Chamanza R., Toussaint M.J., van Ederen A.M., van Veen L., Hulskamp-Koch C., Fabri T.H. Serum amyloid A and transferrin in chicken. A preliminary investigation of using acute-phase variables to assess diseases in chickens. Vet. Q. 1999;21:158–162. doi: 10.1080/01652176.1999.9695012. [DOI] [PubMed] [Google Scholar]
- Chousalkar K.K., Cheetham B.F., Roberts J.R. LNA probe-based real-time RT-PCR for the detection of infectious bronchitis virus from the oviduct of unvaccinated and vaccinated laying hens. J. Virol. Methods. 2009;155:67–71. doi: 10.1016/j.jviromet.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Citil M., Gunes V., Karapehlivan M., Atalan G., Marasli S. Evaluation of serum sialic acid as an inflammation marker in cattle with traumatic reticulo peritonitis. Rev. Méd. Vét. 2004;155:389–392. [Google Scholar]
- Eckersall P.D. Recent advances and future prospects for the use of acute phase proteins as markers of disease in animals. Rev. Méd. Vét. 2000;151:577–584. [Google Scholar]
- Eckersall P.D. The time is right for acute phase protein assays. Vet. J. 2004;168:3–5. doi: 10.1016/j.tvjl.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Gabay C., Kushner I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Gruys E., Toussaint M.J.M., Niewold T.A., Koopmans S.J. Acute phase reaction and acute phase proteins. J. Zhejiang Univ. Sci. B. 2005;6:1045–1056. doi: 10.1631/jzus.2005.B1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M.H., Wyatt R.D., Fletchert O.J. The toxicity of purified fumonisin B1 in broiler chicks. Poult. Sci. 2000;79:1378–1384. doi: 10.1093/ps/79.10.1378. [DOI] [PubMed] [Google Scholar]
- Holt P.S., Gast R.K. Comparison of the effects of infection with Salmonella enteritidis, in combination with an induced molt, on serum levels of the acute phase protein α1-acid glycoprotein in hens. Poult. Sci. 2002;8:1295–1300. doi: 10.1093/ps/81.9.1295. [DOI] [PubMed] [Google Scholar]
- Juul-Madsen H.R., Munch M., Handberg K.J., Sørensen P., Johnson A.A., Norup L.R., Jørgensen P.H. Serum levels of mannan-binding lectin (MBL) in chickens prior to and during experimental infection with avian infectious bronchitis virus (IBV) Poult. Sci. 2003;82:235–241. doi: 10.1093/ps/82.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser P., Stabeli P. In: Davison F., Kaspers B., Schat K.A., Kaiser P., editors. Vol. 10. Elsevier Ltd.; London, UK: 2008. pp. 203–222. (Avian Cytokines and Chemokines: Avian Immunology). Chapter 1. [Google Scholar]
- Katopodis N., Hirshaut Y., Geller N.L., Stock C.C. Lipid associated sialic acid test for the detection of human cancer. Cancer Res. 1982;42:5270–5275. [PubMed] [Google Scholar]
- Kokosharov T. Changes in the protein profile in birds with experimental acute fowl typhoid. Bulg. J. Vet. Med. 2006;9:189–192. [Google Scholar]
- Koutsos E.A., Klasing K.C. The acute phase response in Japanese quail (Coturnix coturnix japonica) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001;128:255–263. doi: 10.1016/s1532-0456(00)00199-x. [DOI] [PubMed] [Google Scholar]
- Kovács B.M., Toussaint M.J., Gruys E., Fabian I.B., Szilagyil J.J., Rudas P. Evaluation of goose serum amyloid A acute phase response by enzyme-linked immunosorbent assay. Acta Vet. Hung. 2007;55:349–357. doi: 10.1556/AVet.55.2007.3.9. [DOI] [PubMed] [Google Scholar]
- Liu H., Zhang M., Han H., Yuan J., Li Z. Comparison of the expression of cytokine genes in the bursal tissues of the chickens following challenge with infectious bursal disease viruses of varying virulence. Virol. J. 2010;7:364. doi: 10.1186/1743-422X-7-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosleh N., Nazifi S., Alaeddini A. Changes in serum acute phase reactants, inflammatory mediators and gangliosides in Japanese quail (Coturnix japonica) with retained yolk sac. Pakistan. Vet. J. 2012;32:251–254. [Google Scholar]
- Murata H., Shimada N., Yoshioka M. Current research on acute phase proteins in veterinary diagnosis: An overview. Vet. J. 2004;168:28–40. doi: 10.1016/S1090-0233(03)00119-9. [DOI] [PubMed] [Google Scholar]
- Nang N.T., Lee J.S., Song B.M., Kang Y.M., Kim H.S., Seo S.H. Induction of inflammatory cytokines and toll-like receptors in chickens infected with avian H9N2 influenza virus. Vet. Res. 2011;42:64. doi: 10.1186/1297-9716-42-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazifi S., Dadras H., Hoseinian S.A., Ansari-Lari M., Masoudian M. Measuring acute phase proteins (haptoglobin, ceruloplasmin, serum amyloid A and fibrinogen) in healthy and infectious bursal disease virus-infected chicks. Comp. Clin. Pathol. 2010;19:283–286. [Google Scholar]
- Nazifi S., Tabande M.R., Hoseinian S.A., Ansari-Lari M., Safari H. Evaluation of sialic acid and acute-phase proteins (haptoglobin and serum amyloids A) in healthy and avian infection bronchitis virus infected chicks. Comp. Clin. Pathol. 2011;20:69–73. doi: 10.1007/s00580-009-0939-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen O.L., Jensenius J., Jørgensen P.H., Laursen S.B. Serum levels of chicken mannan-binding lectin (MBL) during virus infections: Indication that chicken MBL is an acute phase reactant. Vet. Immunol. Immunopathol. 1999;70:309–316. doi: 10.1016/s0165-2427(99)00090-2. [DOI] [PubMed] [Google Scholar]
- Opal S.M., DePalo V.A. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- Petersen H.H., Nielsen J.P., Heegaard P.M. Application of acute phase protein measurements in veterinary clinical chemistry. Vet. Res. 2004;35:163–187. doi: 10.1051/vetres:2004002. [DOI] [PubMed] [Google Scholar]
- Piñeiro M., Pineiro C., Carpintero R., Morales J., Campbell F.M., Eckersall P.D., Toussaint M.J., Lampreave F. Characterization of the pig acute phase protein response to road transport. Vet. J. 2007;173:669–674. doi: 10.1016/j.tvjl.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Pinelli, E. 1996. Protective immune responses against Leishmania in dogs. PhD Thesis. Utrecht University, Utrecht, the Netherlands.
- Rath, N. C. 2005. Ovotransferrin as an avian acute phase protein and its immunomodulatory function. Page 18 in Proceedings of the 5th International Colloquium on Animal Acute Phase Proteins, Dublin, Ireland.
- Rath N.C., Xie H., Huff W.E., Huff G.R. Avian acute phase protein ovotransferrin modulates phagocyte function. Poult. Dis. 2007;19:22–28. [Google Scholar]
- Rath, N. C., H. Xie, W. E. Huff, G. R. Huff, and J. M. Balog. 2003. Modulation of phagocyte function by ovotransferrine, a chicken acute phase protein. West Poultry Disease Conference 332.
- Reed L.J., Muench H. A simple method of estimating fifty percent endpoint. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Rich R.R., Fleisher T.A., Shearer W.T., Schroeder H.W., Frew A.J., Weyand C.M. Clinical Immunology. 3rd ed. Elsevier Ltd.; London, UK: 2008. [Google Scholar]
- Seyrek K., Yaylak E., Akşit H. Serum sialic acid, malondialdehyde, retinol, zinc, and copper concentrations in dairy cows with lameness. Bull. Vet. I. Pulawy. 2008;52:281–284. [Google Scholar]
- van Miert A.S. Pro-inflammatory cytokines in a ruminant model: Pathophysiological, pharmacological, and therapeutic aspects. Vet. Q. 1995;17:41–50. doi: 10.1080/01652176.1995.9694530. [DOI] [PubMed] [Google Scholar]
- Warren L. The thiobarbituric acid assay of sialic acids. J. Biol. Chem. 1959;234:1971–1975. [PubMed] [Google Scholar]
- Yoshioka M., Watanabe A., Shimada N., Murata H., Yokomizo Y., Nakajima Y. Regulation of haptoglobin secretion by recombinant bovine cytokines in primary cultured bovine hepatocytes. Domest. Anim. Endocrinol. 2002;23:425–433. doi: 10.1016/s0739-7240(02)00174-1. [DOI] [PubMed] [Google Scholar]


