Abstract
Since December 2019, increasing attention has been paid to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic in Wuhan, China. SARS-CoV-2 primarily invades the respiratory tract and lungs, leading to pneumonia and other systemic disorders. The effect of SARS-CoV-2 in transplant recipients has raised significant concerns, especially because there is a large population of transplant recipients in China. Based on the current epidemic situation, this study reviewed publications on this virus and coronavirus disease 2019 (COVID-19), analyzed common features of respiratory viral pneumonias, and presented the currently reported clinical characteristics of COVID-19 in transplant recipients to improve strategies regarding the diagnosis and treatment of COVID-19 in this special population.
Keywords: Novel coronavirus, Corona virus disease 2019, Transplantation, Pneumonia, Recipients
In December 2019, a cluster of pneumonia cases of unknown cause were detected in some hospitals in Wuhan, China. These patients seemed to be epidemiologically linked to a fresh seafood market. Analysis of the whole genome sequence of respiratory tract specimens of patients revealed that the pathogen was a novel coronavirus that was different from respiratory coronavirus found previously and completely different from common respiratory viruses like influenza and adenovirus.1,2 This novel coronavirus has been officially named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses (ICTV). SARS-CoV-2 primarily invades the respiratory tract and lungs, leading to coronavirus pneumonia. Moreover, SARS-CoV-2 infection also causes disorders of other systems, such as the digestive or nervous system. The disease caused by SARS-CoV-2 was named corona virus disease 2019 (COVID-19) by the World Health Organization (WHO) on February 12, 2020. COVID-19 has become rapidly more prevalent in China over the past few weeks. According to data released by the National Health Commission (NHC) of the People's Republic of China, as of 0:00 on February 22, there were a total of 76,936 confirmed infected cases, 22,888 cured cases, and 2442 deaths in China.3
In China, a large population of transplant recipients have received hematopoietic stem cell transplantation (HSCT)4 and/or solid organ transplantation (SOT).5 The primary diseases in HSCT recipients leading to their need for a transplant were all hematologic malignancies.6 For the SOT recipients, solid organ failure was the main reason for organ transplantation. Both HSCT and SOT have undergone rapid development in China in recent years. In 2017, China ranked second in the world regarding the number of the solid organ donations and transplantations. To date, the total number of SOT recipients in China is more than 50,000.5 Both HSCT and SOT recipients have attracted much attention and concern with respect to COVID-19. The transplanted recipients are referred to as immunosuppressive hosts because almost all of them have to take multiple immunosuppressants (including glucocorticoid methylprednisolone, azathioprine, tacrolimus, cyclosporine A, mycophenolate mofetil, and other medicines) for life to prevent rejection after transplantation. The main role of the above immunosuppressants is to suppress the body's immune system, significantly reducing their capabilities to prevent infections with various pathogens. Based on the current epidemic situation, this study reviewed the publications regarding SARS-CoV-2 and COVID-19, analyzed the common features of the respiratory viral pneumonias, and presented the currently reported clinical characteristics of COVID-19 in transplant recipients, in order to improve strategies regarding the diagnosis and treatment of COVID-19 in this special population.
Characteristics of SARS-CoV-2
SARS-CoV-2, a single-stranded and positive-sense RNA virus, belongs to the Coronaviridae family, which is part of the Nidovirales order. The novel coronavirus isolated in 2019 is a new strain that had never been found in humans before.7 Currently, the source, characteristics, intermediate hosts, transmission routes, and pathogenic virulence of SARS-CoV-2 are unclear. Current investigations have shown that the geographical distribution of the initially infected population is closely related to Wuhan South China Seafood Market, and the following characteristics have been reported.8, 9, 10, 11
-
1.
Unknown host: at present, the natural host (probably Rhinolophus sinicus) of SARS-CoV-2 has not been completely identified; however, it is considered that it is more likely to be transmitted to the human body via an intermediate host (certain wild animals, “game meat”). However, currently, there is no definitive evidence.
-
2.
Multiple means of transmission: respiratory droplet transmission is the most important route of transmission for SARS-CoV-2. However, it can also be transmitted via direct contact with secretions or via the fecal–oral route.
-
3.
Strong pathogenic virulence: many common respiratory viruses, such as adenovirus, influenza virus, and respiratory syncytial virus, can lead to pneumonia, but the likelihood of developing pneumonia is relatively low and the virulence of these pathogens is lower. However, there is a much higher risk of causing pneumonia after the human body is infected by the SARS-CoV-2, which has stronger virulence.
-
4.
Inactivation characteristics: SARS-CoV-2 is sensitive to ultraviolet rays and heat. The virus can be effectively inactivated under conditions of 56 °C for 30 min, using ether, 75% alcohol, chlorine-containing disinfectant, and chloroform.
-
5.
Invasion of organs: after SARS-CoV-2 invades the human body, its effects are mainly manifested as respiratory infections, including upper respiratory symptoms, tracheitis-bronchitis, and pneumonia. In addition, there is also involvement of the digestive tract and/or nervous system.
-
6.
Infected population: currently available studies have shown that SARS-CoV-2 is easily transmissible among humans and people can be easily infected. The cases of COVID-19 are mainly in middle-aged and elderly patients with underlying diseases, such as cancer, coronary heart disease, or diabetes. To date, this study has collected 10 cases of SOT recipients infected with the SARS-CoV-2, and most are 30–60 years old. Regarding HSCT recipients, no case has been reported up to February 12, 2020.
-
7.
Detection methods: there are several methods, including virus nucleic acid tests using real-time polymerase chain reaction (RT-PCR), virus culture, and serologic specific antibody detection, for detecting SARS-CoV-2. Testing for viral nucleic acid is the best approach and is thus the current standard method for confirming infection. RT-PCR is the most commonly performed detection method because of its specificity and sensitivity. This method can quickly distinguish virus types and subtypes and generally provides results within 4–6 h; thus, it can meet the needs of clinical practice. In comparison, it takes 2–3 weeks to obtain virus culture results; thus, this method cannot meet the needs of clinical diagnosis. SARS-CoV-2-specific IgM and IgG antibody levels can also help in diagnosis. The dynamic detection of the IgG antibody titer in double serum specimens is needed, and if the results in the recovery phase are four or more times higher than those in the acute phase, the results have positive significance. However, this method is only useful in retrospective diagnosis. Another method is the detection of virus-specific inclusion bodies in the cells of lung tissues. This method is relatively difficult for patients and clinicians because lung biopsy is an invasive method. In addition, it is currently unavailable for the detection of SARS-CoV-2-specific antigens and infection-specific memory T cells. Nevertheless, the current detection methods used for transplant recipients are the same as those for the general population. SARS-CoV-2 infection in transplant recipients is also confirmed based on positive PCR test results from throat swab specimens or other respiratory specimens such as bronchoalveolar lavage fluid (BALF).
Common characteristics of viral pneumonia in transplant recipients
Both HSCT and SOT recipients should be the most susceptible population to COVID-19 as they are immunosuppressed hosts. Moreover, the prognosis for these patients is poor, based on previous studies of respiratory viral infections in this population.12,13 Infection is the most common complication in transplant recipients and the respiratory tract is the most common site of infection, especially in lung transplant recipients or combined heart-lung transplant recipients. In fact, there are many viruses in the respiratory tract. The immune system of healthy people can resist pathogens without causing pneumonia or other infectious disease in most cases. However, for transplant recipients, respiratory viral pneumonia is prevalent and its common characteristics are described below 14, 15, 16
-
1.
Relatively weak seasonality: respiratory tract infections in the general population occur frequently in the epidemic seasons of viral infections such as winter and spring; however, for SOT and HSCT recipients, respiratory tract infections occur throughout the whole year and are more frequent in winter and spring.
-
2.
Regularity of postoperative onset time: the postoperative incidence of respiratory infections, including pneumonia, in transplant recipients is higher than that in the general population over a lifetime. However, respiratory infections mainly occur at 1 month to 1 year after transplantation. In general, viral infections occur more frequently early in the postoperative period, especially within 2–6 months post-transplantation. The main reason for the higher risk of infection during the early stage is that recipients are on high-dose immunosuppressive treatment or are receiving immunosuppressive induction therapy.
-
3.
Atypical clinical symptoms: clinical symptoms after respiratory tract infections in transplant recipients are always atypical and sometimes do not match the severity of the disease. For example, these transplant recipients may not develop fever in the early stage; only some subjective symptoms are present, such as palpitations and chest tightness, without any typical symptoms; these symptoms can range from mild nasal congestion to severe tracheobronchitis, pneumonia, and even respiratory failure.
-
4.
Rapid disease progression: many patients initially feel slightly unwell without paying much attention to it when they are initially infected with a respiratory virus; however, it can progress to respiratory failure within a short period of time and require tracheal intubation for assisted breathing. Therefore, presentation to healthcare professionals may be delayed and the best opportunity for treatment may be lost; thus, the disease may be aggravated to a very serious and even life-threatening level.
-
5.
Poor prognosis and slow recovery: the viral virulence is stronger, detoxification time is more prolonged, and damage to the body lasts longer when transplant recipients are infected by a virus than when the general population is infected. Even if targeted medications are employed, long-term invasion will occur, which may increase the risk of variation in drug resistance. Moreover, the recovery time for patients will be significantly prolonged in contrast to that for the general population.
-
6.
Prone to complications: compared with the general population, transplant recipients have a higher risk of bacterial or/and fungal infections secondary to viral pneumonia. The airway mucosal barrier function is impaired after the viral infection and airway adhesion molecules are upregulated, which is likely to cause secondary bacterial, fungal, and other pathogen infections, resulting in respiratory failure and other organ dysfunctions.
-
7.
Difficulty in differential diagnosis: the incidence of viral pneumonia, especially cytomegalovirus (CMV) pneumonia and adenoviral pneumonia, is significantly higher in transplant recipients than that in the general population. Therefore, once the recipients have symptoms such as fever, muscular soreness, shortness of breath, and decreased white blood cell count, thoracic imaging may show some non-specific findings, without providing any clues for the specific pathogen. All of these contribute to difficulties in the clinical differential diagnosis. In addition, for patients after lung transplantation and patients with HSCT, any pathology needs to be distinguished from non-infectious disease, such as acute rejection and graft-versus-host disease (GVHD).
-
8.
Induced rejection: molecular cross-reactivity after respiratory viral infection can induce acute and/or chronic rejection in SOT recipients, and lung injury due to GVHD in HSCT recipients, which always occurs within the first year after transplantation.16,17
-
9.
Population characteristics: common viral pneumonia in transplant recipients has different risks of infection at different ages. In general, pediatric recipients have a significantly higher risk and frequency of respiratory viral infection than adults, which is different from COVID-19. The incidence of COVID-19 is relatively lower in children in contrast to that in adults in the general population, based on recent observations in China.3
Characteristics of COVID-19 in the general population and manifestations in immunosuppressed hosts
-
1.
Different incubation periods18: generally, the incubation period is on average 3–7 days in most patients. However, it is reported that the shortest incubation period was 1 day and the longer was 24 days. It shows that the incubation period for COVID-19 varies greatly among the general population. Thus, a close observation period of at least 14 days is recommended by the NHC for the following populations3: first, those who have stayed or lived in Wuhan and other related epidemic areas, or contacted diagnosed patients during the past 14 days; and second, patients with fever accompanied by some respiratory symptoms in other urban areas where a significantly increasing number of cases have been confirmed to be COVID-19. In summary, based on the scant data available at present, the incubation period ranges 2–8 days among SOT recipients.
-
2.
Diversified clinical symptoms19, 20, 21: observational studies have shown that COVID-19 shares some similarities to common viral pneumonia. Typical symptoms include fever, dry cough, fatigue, and muscular soreness during the early stages and shortness of breath, increasing dyspnea, and hypoxemia during the late stages or in severe cases. However, there are some differences between this new type of pneumonia and the other types of viral pneumonias, such as the severe acute respiratory syndrome (SARS) epidemic. The biggest difference between COVID-19 and SARS, which is caused by another type of coronavirus viral, regards the presence of fever. It is well known that SARS had a pandemic in China in 2003.7 Fever was not only the major manifestation but also the most prominent one among most patients with SARS in 2003. In comparison, there is no fever or a low/slight fever in some patients with COVID-19. Moreover, symptoms related to the digestive systems, such as nausea and diarrhea, and ophthalmic symptoms, including photophobia, xenoma, and blepharitis, are also part of the clinical manifestations of COVID-19. However, some patients without any symptoms are important sources of transmission during the latent period or the early stages of infection. Slight fever, headache, dry cough, and widespread skeletal muscles ache with a flu-like illness are the most common manifestations within the early days of COVID-19. In addition, fatigue, diarrhea, and streaming eyes, among other symptoms, are part of the atypical manifestation of early-stage COVID-19. According to current data, the clinical manifestations of immunosuppressed hosts with COVID-19 are slightly different from those of the general population; dry cough, chest tightness, fatigue, and progressive shortness of breathing are the main clinical manifestations in immunosuppressed patients, which may be hints that the disease progresses more rapidly than that in the general population.
-
3.
High pathogenicity22: there are many different common respiratory viruses in the respiratory tract in human bodies, such as adenovirus, influenza virus, parainfluenza virus, respiratory syncytial virus, and rhinovirus. However, the likelihood of these viruses causing pneumonia is relatively low and the number of such cases is relatively small. However, the incidence of pneumonia is much higher when the patient is infected with SARS-CoV-2 than with other viruses. Furthermore, SARS-CoV-2 is more virulent, which means a poorer prognosis among the patients with COVID-19, especially among patients on high dose immunosuppressive drugs. Up to February 18, 2020, two SOT recipients died among the 10 who contracted COVID-19 and there were no reported deaths in HSCT recipients.
-
4.
Imaging manifestations23,24: the imaging manifestations of COVID-19 share common features with those of other viral pneumonias, but there are also some differences between them. The majority of the general population have multiple small patchy shadows and interstitial changes at an early stage, which gradually progresses to multiple ground-glass and infiltration opacities in both lungs during the progressive stage. Severe cases may have pulmonary consolidation, which is particularly obvious in the outer zone of the lung, and may also be complicated by pleural effusion. Regarding immunosuppressed hosts, the prominent characteristics of COVID-19 are the varied radiologic manifestations, including diffuse ground-glass, multiple infiltration opacities, and big patchy consolidation in bilateral lungs. Rapidly progressing radiologic manifestations are a major characteristic in most patients, especially in severe cases. In addition, the complications of pleural effusion and pericardial effusion may be present in some patients on computed tomography (CT). Based on currently reported cases, it seems that pleural effusion is more likely to be a complication in patients with liver transplantation and pericardial effusion is more likely in patients with heart transplantation.
-
5.
No effective drug25: there is no effective antiviral medicine for COVID-19 to date. Once a patient develops COVID-19, the main strategy is to prevent and treat complications secondary to the infection according to the clinical condition of the patient. Although some old drugs, such as anti-human immunodeficiency virus drugs have shown some level of effectiveness in some patients, large-scale clinical validation has not been conducted yet, let alone safety and efficacy evaluations in immunosuppressed populations. Scientists have urgently tried to develop new antiviral drugs targeting COVID-19 and some have shown promise (phase III clinical trials for remdesivir in China have been conducted since February 1, 2020), while vaccines for COVID-19 are still under development.
-
6.
Susceptible population: the following patients have been noted to be particularly susceptible: adults aged 30–79 years, especially those who are elderly with some comorbidity, such as cancer, diabetes, or coronary heart disease.26,27 The latest study showed that incidence of COVID-19 is much higher in patients with cancer than in the general population. Among patients with cancer, more than 25% had received chemotherapy or surgery. The above information obtained from the general population indicates that the HSCT and SOT recipients are a susceptible population. At the moment, 10 SOT recipients have been reported to have developed confirmed COVID-19 and no case in the HSCT population have been reported yet.
-
7.
Laboratory tests28: patients with COVID-19 are prone to have a decrease in white blood cell and lymphocyte counts, which is also observed in other viral pneumonia patients. However, a similar decrease in HSCT or SOT recipients might be attributed to the side effects of immunosuppressive medication; thus, these medicine-related factors should be excluded. Levels of inflammatory markers, such as C-reactive protein (CRP), were elevated in some patients to a varying degree, and the procalcitonin level was marginally elevated in some patients. Myocardial enzyme levels, including troponin I and myoglobulin, were found to be slightly higher in some patients. Serum lactate dehydrogenase (LDH) and alanine aminotransferase (ALT) levels were also increased in some patients.
-
8.
Prognosis26,27: the all-cause mortality is less than 3%, on average, in the general population, based on the latest national report. It seems that the prognosis of COVID-19 is better than that of SARS, which was prevalent in China in 2003. An observational study showed that symptoms in children are mild, a small portion of patients are critically ill, and most patients who die were those with chronic diseases. Moreover, patients with underlying comorbidities have a higher mortality than other after they develop COVID-19. Among immunosuppressed hosts, the number of reported cases is too limited to jump to a conclusion regarding mortality; however, rapid progression into a severe condition in some SOT recipients within a short period after they were confirmed to have COVID-19 has been observed. Further studies will be needed to see if this population has a higher mortality rate than the general population.
Differential diagnosis of the COVID-19 from cytomegalovirus pneumonia28, 29, 30, 31
CMV pneumonia is the most common viral infection in both HSCT and SOT recipients. It is not only one of the common infectious complications, but also an important cause of death in this population. It is important to differentiate COVID-19 from CMV pneumonia in the transplant recipients. The main points for differentiation include the following aspects.
-
1.
Significant risk factors: serum CMV-IgG in the recipient and donor before transplantation is the main determinant. Donor+/recipient− is indicative of an extremely high risk of the occurrence of CMV pneumonia in SOT recipients after transplantation. In contrast, there are no data on significant risk factors for developing COVID-19 in the transplant population currently. However, elderly recipients with some underlying diseases, such as cardiovascular disease and diabetes, are more susceptible based on the study results in the general population.
-
2.
Clinical manifestations: there are no significant differences in the clinical manifestations of the two types of pneumonia. Fever, dry cough, chest tightness, progressively aggravated shortness of breath, and decreased oxygen saturation are common manifestations in the early stage, and yellow purulent sputum may develop if the secondary bacterial infection occurred at a later stage.
-
3.
Imaging manifestations: typical CMV pneumonia mainly manifests as diffuse ground-glass opacities in bilateral lungs, and bilateral pulmonary interstitial fibrosis appears in the late or chronic stage; big areas of consolidation are rare. For COVID-19, the imaging manifestations are diverse, with scattered ground-glass and consolidation opacities in the unilateral or bilateral lung, and these consolidation opacities are predominant in the middle and outer zones of the lung fields. According to the cases recorded so far, imaging manifestations in SOT recipients with COVID-19 are similar to those in the general population. However, the prominent characteristic of COVID-19 is that the manifestations are varied, with multiple consolidation shadows being localized to the outer zone of the lung in SOT recipients.
-
4.
Laboratory tests: a decreased white blood cell count is more common and more prominent in CMV pneumonia in SOT recipients, while lymphocyte counts seem to be decreased to a larger extent in SOT recipients with COVID-19. Myocardial enzymes are generally elevated in patients with COVID-19 while this is not common in CMV pneumonia except if it is accompanied by concomitant CMV myocarditis.
-
5.
Diagnostic criteria: viral detection using RT-PCR is the common diagnostic criteria for both COVID-19 and CMV. In addition, the detection of the specific antigen for CMV (PP65) in the blood is another criterion for diagnosing CMV infection. In contrast, a specific antigen for SARS-CoV-2 has not yet been detected. Nevertheless, positive results for different viruses using RT-PCR of a respiratory specimen or the blood are gold standards for differential diagnosis.
-
6.
Specific anti-viral medicine: for CMV pneumonia, most patients improve clinically after treatment with ganciclovir; however, for COVID-19, no specific effective medications are currently available. Therefore, the main therapeutic strategy is to prevent complications and provide adjuvant treatment.
Fig. 1, Fig. 2 show CT images of COVID-19 and CMV pneumonia in transplant recipients, respectively. Table 1 lists the key points for the differentiation of COVID-19 from CMV pneumonia.
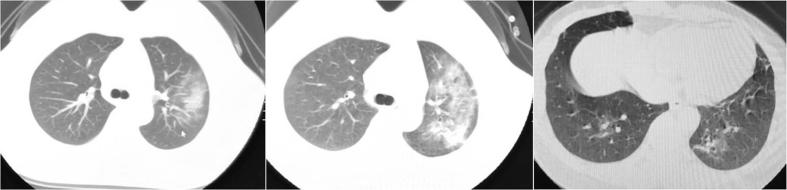
Fig. 1.
Imaging manifestation of a kidney transplant recipient infected with the COVID-19; female, 39 years old, 19 months after kidney transplant; moderate fever, dry cough and fatigue are the main symptoms of the patient.
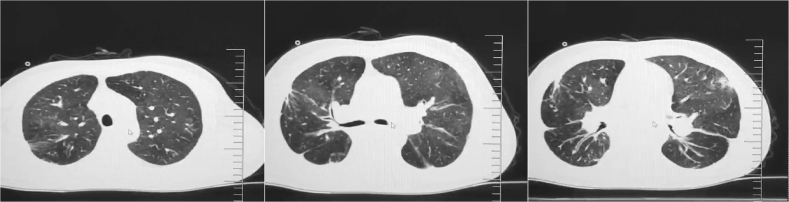
Fig. 2.
Imaging manifestation of a HSCT recipient infected with the CMV pneumonia; male, 40 years old, 3 years after HSCT; fever, cough, fatigue and progressive shortness of breath are the main symptoms of the patient.
Table 1.
Key points for differentiating COVID-19 from CMV pneumonia or Pneumocystis jirovecii pneumonia.
| Diseases | Susceptible patients | Manifestations | Laboratory tests | CT findings | Pharmaceutical treatment |
|---|---|---|---|---|---|
| COVID-19 | All recipients are susceptible, but the elderly with underlying disease, such as cancer, diabetes, or cardiovascular disease, may be more susceptible | No or slight fever, dry cough, fatigue, and widespread skeletal muscle aching | A slight decrease in white blood cell and moderate decrease in lymphocyte counts; increased levels of serum myocardial and liver enzymes, such as LDH and ALT | The manifestations are varied; scattered ground-glass and multiple consolidation opacities in single or bilateral lungs, which are prominent in the middle and outer zones of the lung | No specific effective drugs at the moment |
| CMV pneumonia | Serum CMV-IgG is the main determinant, with donor+ and recipient- being a highly significant risk factor for the recipient | Fever, mild to high fever, dry cough, fatigue, chest tightness and shortness of breath | A moderate decrease in white blood cells; in most cases, no significant increase in serum levels of myocardial or liver enzymes | Diffused ground-glass opacities in the double lungs in the early stage, and interstitial fibrosis appear in the late or chronic stage, rare to have a big consolidation | Ganciclovir, Val-ganciclovir, foscarnet sodium, cidofovir |
| Pneumocystis jirovecii pneumonia | Patients with severe immunosuppression, especially with CD4+ lymphocytes decrease or deficiency | Fever, dry cough, dyspnea, chest tightness, severe hypoxemia | Severe decrease in lymphocyte counts; CD4+ lymphocytes are always less than 200/L, serum G test (+), LDH always increased; trophozoites and cysts can be found in BALF | Diffused ground-glass opacities in the upper lungs with slight interstitial fibrosis in the lower lungs, rare to have consolidation | Compound sulfamethoxazole, pentamidine, clindamycin, caspofungin |
CMV: cytomegalovirus; COVID-19: novel coronavirus disease; LDH: lactate dehydrogenase; ALT: alanine-aminotransferase; BALF: bronchoalveolar lavage fluid.
Diagnosis, prophylaxis, and treatment strategies for COVID-19
-
1.
Diagnosis: for new-onset COVID-19 infection in both HSCT and SOT recipients, the criteria for the diagnosis are consistent with the guidelines published by the NHC.3
-
2.
Prophylaxis: because HSCT and SOT recipients are immunosuppressed hosts, prophylaxis is one of the most important strategies. There are no prophylactic drugs for COVID-19; therefore, protection against coming into contact with the pathogen should be enhanced for this population.32
-
3.
General treatments: the principles of treatment strategies are consistent with the guidelines published by the NHC,3 which include rest, supportive treatment, maintenance of water-electrolyte balance and homeostasis, oxygen therapy, respiratory support, and supportive treatment of corresponding vital organs for critically ill patients.
Special precaution strategies for transplant recipients33, 34, 35, 36, 37
For both HSCT and SOT recipients, most some form of immunosuppressants to prevent rejection; thus, much attention should be paid to the adjustment of medications. In terms of special medications, they should be adjusted according to the patient's age, overall clinical condition, severity of respiratory failure, speed of progression of the disease, immune status, type of transplanted organs, and number of postoperative days. It is recommended that the diagnosis and treatment scheme should be performed after multidisciplinary team (MDT) consultation. The MDT should include at a minimum a respiratory specialist, pharmacist, transplantation specialist, and infectious disease physician.
-
1.
Antiviral therapy-related precautions: oral lopinavir/ritonavir (200/50 mg) (Kaletra) has been observed to be effective in some COVID-19 patients. For both HSCT and SOT recipients, the following three points should be noted if the medications are prescribed. First, adverse reactions should be considered; the most common adverse reactions include diarrhea, nausea, vomiting, hypertriglyceridemia, and hypercholesteremia. Transplant recipients always have impaired digestive function and poor gastrointestinal tolerance; therefore, this should be carefully monitored. Second, precautions to avoid pancreatitis induced by severe hypertriglyceridemia or/and hypercholesteremia should be administered because some transplant recipients have already had hypertriglyceridemia or/and hypercholesteremia before the onset of COVID-19. Third, the whole-blood concentration of tacrolimus or cyclosporine-A should be closely monitored as lopinavir/ritonavir can increase their levels significantly. Large-sample data are currently unavailable regarding the safety and effectiveness of the drugs in these transplant recipients. As for the aerosol inhalation of α interferon, there are no known interactions or interactivities between the medications.
-
2.
Antibiotics: preventive antibiotics can be administrated as appropriate, but the combination of multiple antibiotics is not recommended to use routinely.
-
3.
Methylprednisolone: the initial maintenance dose should be appropriately increased. It can be administered before the oxygenation index is <300. The specific dose should be individualized. The generally recommended dose is 1–2 mg/kg per day, with a 5–7-day course of treatment. The dose should then be tapered down to the baseline dosage within 2–3 weeks.
-
4.
Cell cycle inhibitors: based on previous diagnosis and treatment experience of viral pneumonia and current small sample studies of COVID-19 cases in SOT recipients, it is recommended to discontinue this type of drug during the period of infection, especially for the patients in severe condition.
-
5.
Calcineurin inhibitors: calcineurin inhibitors (CNIs) mainly comprise cyclosporine A and tacrolimus in current clinical practice. Generally, the dose should be reduced, while the specific dosage should be individualized, depending on multiple factors such as the patient's age, severity of respiratory failure, and immune status, among other factors. Complete discontinuation is not recommended if patients are at the early stage post-lung transplantation, but it can be reduced to the lowest acceptable concentration range, with the concentration being closely monitored.
-
6.
Immunoadsorption: if there are no contraindications and it can be tolerated by the patients at the same time, immunoadsorption therapy can be considered for transplant recipients if necessary.
-
7.
Traditional Chinese medicines: for transplant recipients, given the complexity of drug combinations, impaired digestive capabilities, and poor gastrointestinal tolerance, it is not recommended to use traditional Chinese medicine treatment as an ordinary strategy.
Summary
In response to this epidemic situation, it is important to follow an effective strategy regarding the prophylaxis, diagnosis, and treatment of COVID-19 in transplant recipients. As immunosuppressed hosts are susceptible to all kinds of infective pathogens, the pathogenesis should be detected once the patients develop the infectious disease and the differential diagnosis should be accurately performed. Based on the cases that are currently available, diverse symptoms, varied CT manifestations, and rapid progress are the major characteristics of COVID-19 in this population when compared to that in the general population. Combined with the review on the common features of viral pneumonias and the specific features of COVID-19, this paper provides important and practical information for clinicians to deal with the emergence of COVID-19 in this population.
Funding
The study was supported by the grants form the Foundation from The State Key Laboratory of Respiratory Disease (No. SKLRD-QN-201710), the Natural Science Foundation of Guangdong Province (No. 2018A030313107) and Guangzhou Institute of Respiratory Health (No. 2019GIRHZ04).
Conflicts of interest
None.
Acknowledgment
The data of the patients were provided by experts in the following hospitals: Affiliated Renmin Hospital of Wuhan University, Affiliated Tongji Hospital of Huazhong University of Science and Technology, Affiliated Union Hospital of Huazhong University of Science and Technology, Affiliated Zhongnan Hospital affiliated to Wuhan University, Jinyintan Hospital of Wuhan, and Peking University Third Hospital. We would like to express sincere thanks to the experts for sharing the details of COVID-19 cases in transplant recipients.
Edited by Yi Cui
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Bajema K.L., Oster A.M., McGovern O.L., 2019-CoV Persons under Investigation Team Persons evaluated for 2019 novel coronavirus – United States, January 2020. MMWR Morb Mortal Wkly Rep. 2020 Feb 14;69(6):166–170. doi: 10.15585/mmwr.mm6906e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Statement Regarding Cluster of Pneumonia Cases in Wuhan, China. 2020 Jan 9. https://www.who.int/china/news/detail/09-01-2020-who-statement-regarding-cluster-of-pneumonia-cases-in-wuhan-china [Google Scholar]
- 3.Diagnosis and Treatment Scheme for Novel Coronavirus-infected Pneumonia (6th Trial Edition) National Health Commission of the PRC; 2020 Feb 18. http://yzs.satcm.gov.cn/zhengcewenjian/2020-02-19/13221.html [Google Scholar]
- 4.Xu L.P., Wu D.P., Han M.Z. A review of hematopoietic cell transplantation in China: data and trends during 2008–2016. Bone Marrow Transplant. 2017;52:1512–1518. doi: 10.1038/bmt.2017.59. [DOI] [PubMed] [Google Scholar]
- 5.Shi B.Y. The development status of organ transplantation in China – report on the annual meeting of the Chinese medical association on organ transplantation in 2018 (in Chinese) Organ Transpl. 2019;10:32–35. [Google Scholar]
- 6.Xu L., Chen H., Chen J. The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China – recommendations from the Chinese Society of Hematology. J Hematol Oncol. 2018;11:33. doi: 10.1186/s13045-018-0564-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song Z., Xu Y., Bao L. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11 doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J. Pathogenicity and transmissibility of 2019-nCoV – a quick overview and comparison with other emerging viruses. Microbes Infect. 2020 Feb 4 doi: 10.1016/j.micinf.2020.01.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao S., Musa S.S., Lin Q. Estimating the unreported number of novel coronavirus (2019-nCoV) cases in China in the first half of January 2020: a data-driven modelling analysis of the early outbreak. J Clin Med. 2020;9 doi: 10.3390/jcm9020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angeletti S., Benvenuto D., Bianchi M., Giovanetti M., Pascarella S., Ciccozzi M. COVID-2019: the role of the nsp2 and nsp3 in its pathogenesis. J Med Virol. 2020 Feb 21 doi: 10.1002/jmv.25719. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marinelli T., Wee L., Rowe E. Respiratory viruses cause late morbidity in recipients of hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2019 Dec 20 doi: 10.1016/j.bbmt.2019.12.724. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Abbas S., Raybould J.E., Sastry S., de la Cruz O. Respiratory viruses in transplant recipients: more than just a cold. Clinical syndromes and infection prevention principles. Int J Infect Dis. 2017;62:86–93. doi: 10.1016/j.ijid.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Khawaja F., Chemaly R.F. Respiratory syncytial virus in hematopoietic cell transplant recipients and patients with hematologic malignancies. Haematologica. 2019;104:1322–1331. doi: 10.3324/haematol.2018.215152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vakil E., Sheshadri A., Faiz S.A. Risk factors for mortality after respiratory syncytial virus lower respiratory tract infection in adults with hematologic malignancies. Transpl Infect Dis. 2018;20 doi: 10.1111/tid.12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peghin M., Los-Arcos I., Hirsch H.H. Community-acquired respiratory viruses are a risk factor for chronic lung allograft dysfunction. Clin Infect Dis. 2019;69:1192–1197. doi: 10.1093/cid/ciy1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magnusson J., Westin J., Andersson L.M. Viral respiratory tract infection during the first postoperative year is a risk factor for chronic rejection after lung transplantation. Transpl Direct. 2018;4 doi: 10.1097/TXD.0000000000000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020 Feb 7 doi: 10.1001/jama.2020.1585. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu H., Tong Z., Ma P. Intensive care during the coronavirus epidemic. Intensive Care Med. 2020 Feb 20 doi: 10.1007/s00134-020-05966-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manuel O., Estabrook M., American Society of Transplantation Infectious Diseases Community of Practice RNA respiratory viral infections in solid organ transplant recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33 doi: 10.1111/ctr.13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z., Li X., Zhang W., Shi Z.L., Zheng Z., Wang T. Clinical features and treatment of 2019-nCov pneumonia patients in Wuhan: report of a couple cases. Virol Sin. 2020 Feb 7 doi: 10.1007/s12250-020-00203-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu D., Pan Y., Cheng S. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin Chem. 2020 Jan 31 doi: 10.1093/clinchem/hvaa029. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang Y., Zhang H., Xu Y., Xie J., Pang P., Ji W. CT manifestations of two cases of 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020:200280. doi: 10.1148/radiol.2020200280. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernheim A., Mei X., Huang M. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020:200463. doi: 10.1148/radiol.2020200463. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 26.China's center for disease prevention and control in novel coronavirus pneumonia emergency response mechanism group. Analysis on characteristics of Novel Coronavirus pneumonia (in Chinese) Chin J Epidemiol. 2020;41:145–151. [Google Scholar]
- 27.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotton C.N., Kumar D., Caliendo A.M. The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2018;102:900–931. doi: 10.1097/TP.0000000000002191. [DOI] [PubMed] [Google Scholar]
- 29.Lodding I.P., Schultz H.H., Jensen J.U. Cytomegalovirus viral load in bronchoalveolar lavage to diagnose lung transplant associated CMV pneumonia. Transplantation. 2018;102:326–332. doi: 10.1097/TP.0000000000001927. [DOI] [PubMed] [Google Scholar]
- 30.Camargo J.F., Komanduri K.V. Emerging concepts in cytomegalovirus infection following hematopoietic stem cell transplantation. Hematol Oncol Stem Cell Ther. 2017;10:233–238. doi: 10.1016/j.hemonc.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Ju C.R., Wei B., Lian Q.Y. Management strategies of cytomegalovirus pneumonia after SOT-interpretation of ATS guidelines for diagnosis and treatment of cytomegalovirus pneumonia (in Chinese) Organ Transpl. 2019;10:88–90. [Google Scholar]
- 32.Ju C.R., Lian Q.Y., Xu Xin. Prophylaxis strategies on the coronavirus-infected pneumonia for the solid organ transplantation recipients during the period of the epidemic. Organ Transpl. 2020 Feb 1 [Google Scholar]
- 33.Update and Guidance on 2019 Novel Coronavirus (2019-nCov) for Transplant ID Clinicians. The Transplantation Society and Transplant Infectious Diseases; 2020 Jan 27. https://tts.org/tid-about/tid-presidents-message/23-tid/tid-news/657-tid-update-and-guidance-on-2019-novel-coronavirus-2019-ncov-for-trans plant-id-clinicians [Google Scholar]
- 34.HIV Drug Interactions. 2020. https://www.hiv-druginteractions.org Accessed 17.01.2020. [Google Scholar]
- 35.Christians U., Schmidt G., Bader A. Identification of drugs inhibiting the in vitro metabolism of tacrolimus by human liver microsomes. Br J Clin Pharmacol. 1996;41:187–190. doi: 10.1111/j.1365-2125.1996.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 36.Ju C.R., Li L., Shi B.Y. Chinese solid organ transplantation group. Management strategies on the coronavirus-infected pneumonia after post solid organ transplantation during the period of the epidemic (in Chinese) Organ Transpl. 2020 Feb 6 [Epub ahead of print] [Google Scholar]
- 37.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]