Highlights
-
•
Glycosylation of therapeutic proteins affects their bioactivity and immunogenicity.
-
•
Need of reliable method for rapid glycoprofiling of therapeutic proteins.
-
•
Glycan analysis of recombinant IgA by lectin-based microarray and MALDI-MS.
-
•
IgAs were produced at different conditions changing their glycan composition.
-
•
Lectin-based microarray enabled effective high-throughput glycoprofiling of IgAs.
Keywords: IgA, Monoclonal antibody, Therapeutic proteins, Glycosylation, Lectin-based microarray, MALDI-MS
Abstract
Glycosylation of therapeutic glycoproteins significantly affects their physico-chemical properties, bioactivity and immunogenicity. The determination of glycan composition is highly important regarding their development and production. Therefore, there is a demand for analytical techniques enabling rapid and reliable glycoprofiling of therapeutic proteins. For the investigation of changes in glycan structures, we have employed two platforms: lectin-based protein microarray, and MALDI-MS. In lectin-based microarray analysis, the samples of IgA were printed on the microarray slide, incubated with the set of lectins with various specificity and evaluation of changes in glycosylation was based on differences in reactivity of samples with lectins. MALDI-MS was used for N-glycan analysis of IgA1 samples. IgAs are effective as therapeutic agents in defense against viruses that use sialic acid as a receptor. Dimeric IgA1 antibodies were produced by stable cell line IgA1/2G9 on the basal medium at different conditions (different supplementation and feeding) and we also evaluated the effect of different conditions on lactate production, which correlates with IgA productivity. Decrease of lactate levels was observed during supplementation with succinic acid, asparagine, or with mannose feeding. We found by lectin-based microarray analysis that the metabolic shift from glutamine to asparagine or feeding with glucose caused increase of high mannose type glycans what was confirmed also by MALDI-MS. Among other changes in IgA glycosylation determined by lectin-based protein microarray were, for example, reduced galactosylation after supplementation with succinic acid and increase of both sialylation and galactosylation after supplementation with glutamine and feeding with mannose. The elucidation of mechanism of determined changes requires further investigation, but the described analytical approach represent effective platform for determination, screening and evaluation of glycosylation of therapeutic proteins.
1. Introduction
Mammalian cells are widely used for production of therapeutic glycoproteins containing human-like glycans. Most of these are IgGs, but also we have seen an increasing interest in recombinant IgAs since this class of immunoglobulins plays a critical role in providing immunologic protection at mucosal surfaces. Dimeric Immunoglobulin A (IgA) is the most important part of the first mucosal defense against the airborne infectious diseases (Lohse et al., 2011) and can bind, neutralize and eliminate viruses including sendai, influenza, rota, measles, and human immunodeficiency virus (HIV), as well as bacterial LPS (Breedveld et van Egmond, 2019).
Protein glycosylation is a complex post-translational modification (PTM) involving attachment of glycans at specific sites on a protein, most commonly at Asn (N-linked) or Ser/Thr (O-linked) residues (Schachter, 2000). Glycosylation of therapeutic antibodies influences their stability and solubility, bioactivity and immunogenicity. Protein glycosylation also affects pharmacokinetics/pharmacodynamics properties of therapeutic glycoproteins (Ghaderi et al., 2012). For example, partially glycosylated proteins, which usually contain terminal galactose, have much shorter circulatory lifetimes compared to fully glycosylated proteins with terminal sialic acid. Protein glycosylation also depends on the type of host cells and culturing conditions (media, pH, temperature, agitation) (Hossler et al., 2009).
IgA exists as monomeric molecules in serum or as dimeric secretory IgA on mucosal surfaces. Humans and great apes have two IgA subclasses: IgA1 and IgA2, which differ in their glycosylation. IgA1 comprises approximately 85 % of total IgA concentration in serum. IgA1 contains heavily O-glycosylated 23 amino acids long hinge region, whereas IgA2 subclass contains only N-linked oligosaccharides because its shorter hinge region is devoid of O-linked glycosylation. IgA’s N-glycosylation of CH2 at position 263 is as in all Ig isotypes predominantly of biantennary complex-type with α2–6-linked sialic acids. Both IgAs contains also unique C-terminal tail interacting with the J-chain and secretory component to mediate dimerization and that contains N-linked glycans at position 459 of the triantennary type, with α2–6 and α2–3 linked sialic acids Thanks to sialic acid presented in the complex N-linked glycans at position 459 of IgA’s C-terminal tail, over 90 % of the N-linked glycans in IgA1 are sialylated while only about 10 % of the N-linked glycans in IgG1 contain sialic acid (Mattu et al., 1998; Royle et al., 2003; Correa et al., 2013; Wuhrer et al., 2007; Maurer et al., 2018). Since sialic acid competes with viral receptor, IgAs antibodies neutralize virus much more potently than IgG1 (Maurer et al., 2018). Moreover, dimeric IgA containing J-chain was more effective in inhibition of ligand binding and receptor downmodulation that monomeric form (Lohse et al., 2011).
Many studies underscore the importance of the choice of expression system for production of glycoproteins (Goh et Kong, 2018). For example, glycoproteins produced by mammalian cell systems usually contain macro- and micro-heterogeneity in terms of glycosylation patterns (Solá et Griebenow, 2010). They may also contain minor non-human glycans such as N-glycolylneuraminic acid (Neu5Gc) and terminal a1−3-Galactose (a-Gal) modification (Zhang et al., 2016a). CHO cell lines usually have some limitations for culture longevity and the quality of the protein of interest: low specific growth rate and cell concentration (Gupta et al., 2017). Also high consumption of glucose and glutamine results in such undesirable main metabolites as lactate and ammonium. These limits cause low productivity of the production process (Altamirano et al., 2013). Galactose metabolism is slower than that for glucose, allowing a reduction in lactate generation and an increase in cell viability (Altamirano et al., 2000). Reduction of the tricarboxylic acid (TCA) cycle flux without the impairment of glycolytic activity would be another possibility to increase mitochondrial NAD+. Glutamine is directly fed into the TCA cycle and significantly contributes to cell metabolism. Reduction of its specific uptake rate could induce lactate consumption. Therefore, glutamine depletion has been shown to have the potential to induce the lactate metabolic shift (Zagari et al., 2013). Moreover, the effect of the different nucleotide sugar precursors (mannose, galactose, fucose, glucosamine) have been reported to have an effect on the glycosylation patterns of glycoproteins (Blondeel et al., 2015; Lin et al., 2015; Slade et al., 2016; Wong et al., 2010).
With respect to the above mentioned facts, the analysis, characterization and control of glycan composition of therapeutic proteins during their development and production is task of high importance and appropriate glycomics analytical tools have to be employed. Typically used techniques involve high-performance liquid chromatography (HPLC), high-performance anion-exchange chromatography (HPAEC), capillary electrophoresis (CE), mass spectrometry (MS), isoelectric focusing (IEF), and lectin-based microarray (Katrlík et al., 2010; Hirabayashi et al., 2013; Yu et al., 2020). Lectins are glycan binding proteins (GBPs) that selectively recognize glycan epitopes of free carbohydrates or glycoproteins and are used as glycoanalytical tools to probe biological targets (Geisler et Jarvis, 2011; Kobayashi et al., 2014). Lectin microarrays have been used to analyze glycan profiles of purified glycoproteins or cell surface proteins. Glycan patterns of therapeutic glycoproteins were determined by a lectin microarray using commercial lectin chip (Zhang et al., 2016b; Roucka et al., 2017).
In this study, we used lectin-based protein microarray in reverse-phase format developed and prepared in our lab (Zámorová et al., 2017; Robajac et al., 2020). The samples of recombinant monoclonal IgA1 therapeutic proteins were spotted onto microarray chip and glycoprofiled by incubation with the set of biotinylated lectins. The presence of N-glycan moieties was confirmed by MALDI-MS analysis.
2. Material and methods
2.1. Cell culture
We used stable monoclonal cell line producing recombinant dimeric IgA1 antibodies against hemagglutinin of influenza A virus (Argentova et al., 2017). Cell lines cultured in Iscove's Modified Dulbecco's Medium, IMDM (PanEko, Russia) supplemented with l-glutamine (PanEko, Russia) or succinic acid (Komponent-Reaktiv, Russia)/asparagine (Sigma, USA) (in different experiments), 0.1 % Pluronic F68 (Gibco, USA), and antibiotic-antimycotic solution (Gibco, USA) in a CO2 incubator at 37 °C with 96 % humidity in an atmosphere of 8 % CO2. Cell culture were maintained in 125 mL polycarbonate Erlenmeyer flasks (Corning, NY, USA) containing 25 mL of medium. Cells were passaged at 3 × 105 cells/mL and cultured on orbital shakers agitated at 130 rpm in a humidified 37 °C incubator with 8 % CO2.
2.1.1. Experiment in batch and fed-batch mode
Samples of stable cell line IgA1/2G9 were grown in batch mode in a basal medium (IMDM) supplemented with 8 mM glutamine (Sample 1), 8 mM asparagine (Sample 2) and 8 mM succinic acid (Sample 3). The cultures were terminated on Day 14. The cell cultures were seeded also in basal medium supplemented with 8 mM l-glutamine and 10 mM d-(+)-glucose for fed-batch experiments. On Day 5, when glucose was probably depleted in the cultures, alternative sources of sugars were added to shaker flasks in 10 mM final concentration: d-(+)-glucose (Sample 4), d-(+)-galactose (Sample 5) and d-(+)-mannose (Sample 6), all from Sigma-Aldrich, USA. The feeding was started on Day 5 and continued on Days 7 and 10. Samples of cell cultures (both batch and fed-batch modes) were taken: on Days 10 and 14, centrifuged and the supernatant was collected for further productivity analysis (ELISA) and LDH assay; on Days 7, 10 and 14 for viable cells density measurement (cell concentration); and on Day 14 for glycan analysis (lectin-based microarray and MS) measurements.
2.2. Determination of cell concentration
Cell concentration was performed using the Trypan-blue dye exclusion method with a hemocytometer counting chamber and inverted microscope. Total cell density was calculated using the following Eq. (1) (Asher, 1973),
| TCD = N × 1.1 × 105 | (1) |
where, TCD is the total cell density (cell/mL), N is the total number of cells counted in 15 squares of Goryaev chamber.
2.3. ELISA assay
The concentration of secreted mAbs in the samples of culture supernatant after centrifugation was measured using ELISA. The level of IgA1 expression in the cell culture was determined using sandwich ELISA. Mouse mAbs against light kappa-type chains of human Igs (Bialexa, Russia) were added to wells of a 96-well plate at a concentration of 0.5 μg/well. In the analysis, we used α-chain-specific anti-human IgA antibodies conjugated to horseradish peroxidase (Sigma). IgA from human serum (Sigma) was used as the standard. The absorbance of samples at 450 nm was measured in each well using the ELISA microplate reader BioRad-680.
2.4. Determination of LDH enzyme activity
Samples were centrifuged, and the supernatant was collected for further LDH assay. Lactate as the metabolite of anaerobic glycolysis in the samples of culture supernatant after centrifugation was measured indirectly via activity of lactate dehydrogenase (LDH). The release of LDH was measured using a commercially available LDH assay kit (Lactate Dehydrogenase Activity Assay Kit, Sigma). NADH used as control. The intensity of color produced was then measured colorimetrically at a wavelength of 450 nm using a spectrophotometer. The LDH activity of samples was determined using the following Eq. (2) (Vanderline, 1985),
| LDH activity = B × Sample dilution factor/(reaction time) × V | (2) |
where, B is the amount (nmol) of NADH generated (Tinitial - Tfinal).
2.5. Purification and lyophilization of samples
IgA1 was purified from the supernatant of cell culture with HiTrap KappaSelect (GE Healthcare, USA). The purified samples were analysed in gel-electrophoresis by 4–11 % SDS-PAGE under reducing and non-reducing conditions. The concentration of purified IgA1 was measured by Implen NanoPhotometer™ (Implen GmbH, Germany). Purified samples were dialysed in MQ-H2O (MQ Millipore, USA) and concentrated by Pierce Protein Concentrator, PES, 30 K MWCO (Thermo Sci., USA). Concentrated samples of mAbs were lyophilized using vacuum freeze dryer VirTis (Labconco, USA) and used for further glycan analyses.
2.6. Lectin-based microarray analysis
Lyophilized samples of IgA1 were dissolved in PBS (0.1 mg/mL), transferred into source microtiter plate and spotted to the epoxy microarray slides (NEXTERION Slide E, Schott, Germany) in pentaplicates (1.2 nL per spot) using a non-contact piezoelectric sciFLEXARRAYER S1 microarray spotter and piezo dispense capillary PDC 80 (Scienion AG, Berlin, Germany) at the temperature of source plate of 11 °C and humidity of 50 %. The printing was performed into 16 identical subarrays and the slides was incubated at 4 °C for 2 h. Unreacted epoxy groups were blocked with a solution of 3 % BSA in PBS for 1 h at room temperature. After washing the slides with PBS containing 0.1 % Tween-20 (PBST), 16 biotinylated lectins (Table 1 ) from Vector, Burlingame, USA (except PhoSL which was a kind gift from Dr. Yuka Kobayashi, J-Oil Mills, Inc., Japan) at concentrations of 25 μg/mL in PBST were loaded into 16 subarrays for 1 h at room temperature. The slides were washed again with PBST and streptavidin conjugated with a fluorescent dye CF647 (Biotium, Hayward, USA, 0,5 μg/mL in PBST) was loaded into 16 subarrays for 15 min at room temperature. The slides were thoroughly washed with PBST and distilled water and the residual water was removed by centrifugation. Fluorescent signals were detected using InnoScan®710 fluorescent microarray scanner (Innopsys, Carbonne, France) at the wavelength of 635 nm. The signals were analysed by Mapix® 5.5.0 software (Innopsys). The fluorescence of each spot was measured and corrected for the background signal and the intensity of the specific interaction was expressed in arbitrary fluorescence units (AU).
Table 1.
Lectins used in lectin-based microarray analysis and their key sugar specificity.
| Lectin (source) |
Key sugar specificity | |
|---|---|---|
| SNA (Sambucus nigra) |
 |
Siaα2−6 Gal/GalNAc |
| ConA (Canavalia ensiformis) |
 |
Manα1−6Man, Manα1−3Man, Manα1−2Man, high mannose |
| MAL-I (Maackia amurensis) |
Siaα2−3Galβ1−4GlcNAc | |
| MAL-II (Maackia amurensis) |
 |
Siaα2−3Galβ1−3(±Siaα2−6)GalNAc |
| PHA-E (Phaseolus vulgaris) |
 |
di-/triantennary complex type N-glycans with bisecting GlcNAc |
| PHA-L (Phaseolus vulgaris) |
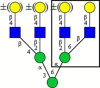 |
tri-/tetraantennary complex type N-glycans with GlcNAcβ1−6Man |
| WGA (Triticum vulgaris) |
 |
GlcNAcβ1−4GlcNAc, Sia |
| RCA (Ricinus communis) |
 |
Galβ1−4GlcNAc, GalNAc, Gal |
| AAL (Aleuria aurantia) |
 |
Fucα1−6GlcNAc, Fucα1−3(Galβ1−4)GlcNAc |
| PhoSL (Pholiota squarrosa) |
Fucα1−6GlcNAc | |
| GNL (Galanthus nivalis) |
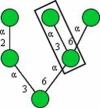 |
Manα1−3Man, high mannose type N-glycans |
| GSL-I (Griffonia simplicifolia) |
Gal, GalNAc | |
| PNA (Arachis hypogaea) |
Galβ1−3GalNAc | |
| HHL (Hippeastrum hybrid) |
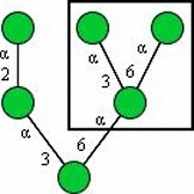 |
Manα1−3Man, Manα1−6Man, high mannose type N-glycans |
| NPL (Narcissus pseudonarcissus) |
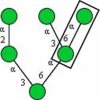 |
Manα1−6Man, high mannose type N-glycans |
| LCA (Lens culinaris) |
 |
αMan in N-glycans with core fucose, αMan in N-glycans |
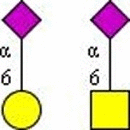
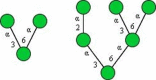
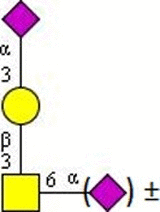
Symbolic representation of monosaccharides - Mannose (Man); - Mannose (Man);  - Galactose (Gal); - N-Acetylglucosamine (GlcNAc); - Galactose (Gal); - N-Acetylglucosamine (GlcNAc);   - N-Acetylgalactosamine (GalNAc); - N-Acetylgalactosamine (GalNAc);  - Fucose (Fuc); - Fucose (Fuc);  - N-Acetylneuraminic acid (Neu5Ac, Sia). | ||
2.7. MALDI-MS analysis of N-glycans
10 μL of lyophilized IgA1 samples was premixed with 40 μL of 10 mM Tris pH 7.5 + 0.1 % SDS buffer. The proteins were alkylated and reduced with 10 mM dithiothreitol (DTT) and 25 mM iodoacetamide (IAA) prior to the addition of 1 U of peptide-N-glycosidase (PNG-ase F; Roche Diagnostics GmbH, DE). After the overnight incubation at 37 °C, the released N-glycans were isolated by Supelclean ENVI-Carb SPE (Supelco/Sigma Aldrich, PA, USA), lyophilized and further subjected to permethylation to increase the signal intensities and to avoid the typical adduct and fragment formation in the spectra (Palmigiano et al., 2018). Briefly, 150 μL of homogenous mixture of NaOH in DMSO was added to the samples and the reaction was initiated by the addition of 150 μL iodomethane. After the rigorous mixing for 40 min at room temperature, the reaction was terminated by the addition of ice-cold water. Afterwards, the permethylated N-glycans were extracted to chloroform, dried and further dissolved in 50 % methanol. Samples were analyzed in reflectron positive ion mode by the UltrafleXtreme MALDI mass spectrometer equipped with the 1000 Hz Smartbeam™-II laser (Bruker Daltonics, MA, USA) with the addition of 20 mg/mL DHB in 30 % ACN + 0.1 % TFA +1 mM NaOH as the matrix solution. Every N-glycan structure was confirmed by the MS/MS (LIFT) analysis and the spectra were interpreted by the ProteinScape (Bruker Daltonics, MA, USA) or GlycoWork Bench (Ceroni et al., 2008) softwares.
3. Results and discussion
3.1. Impact of the succinic acid and asparagine on lactate level, cell concentration, and productivity of the IgA1/2G9 stable cell line
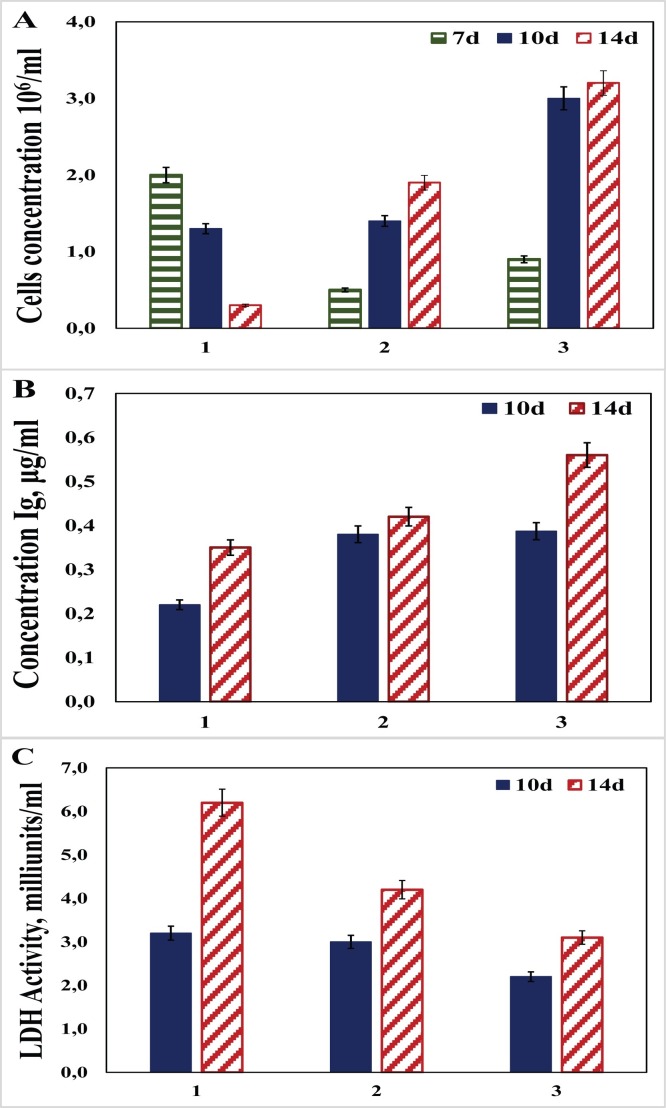
In the first part of this work, the impact of succinic acid or asparagine supplementation on productivity and lactate level of IgA1/2G9 stable cell line was studied. Lactate production is crucial for metabolism of cultured mammalian cells. Lactate is strongly produced during the exponential growth phase. IgA1 producing stable cell line was grown in batch and fed-batch cultures consisting of different media supplements and feedings. The cell culture typically demonstrates low productivity on the log phase (up to 6–8 days) when the cells proliferate exponentially and consume the nutrients in the growth medium. Therefore, we started to measure IgA1 concentration and LDH activity from Day 10. Results shown on Fig. 1 demonstrate influence of supplementation with succinic acid or asparagine in IMDM on cell concentration and productivity. IMDM supplemented with 8 mM l-glutamine was used as control. l-glutamin is a crucial component of culture media. It supports the growth of cells and is therefore used in wide range of serum-free and classical media as source for rapid cells dividing. The concentration of cells grown on basal medium supplemented with 8 mM succinic acid was higher compared to both control and 8 mM asparagine on Days 10 and 14 (Fig. 1A). As shown on Fig. 1B, comparison of productivity for cell line grown on IMDM supplemented with different additives demonstrated that on Day 14 the productivity of the culture supplemented with succinic acid was higher compared to the both control and asparagine. Interestingly, data of productivity for both succinic acid and asparagine was equal on Day 10. In the case of lactate concentration (Fig. 1C), use of succinic acid supplementation led to slightly lower lactate level on Day 10 compared to glutamine and asparagine. On Day 14, lactate level was 1.5 and 2.0 times lower for asparagine and succinic acid supplements, respectively, than for glutamine.
Fig. 1.
Effect of glutamine, succinic acid and asparagine on the cell concentration (A), IgA1 production (B) and lactate level (C). Cell culture was grown on IMDM medium with different supplements: 1 – IMDM +8 mM glutamine (control); 2 – IMDM +8 mM asparagine; 3 - IMDM +8 mM succinic acid.
3.2. Impact of the mannose, galactose and glucose on the lactate level and productivity of the IgA1/2G9 stable cell line
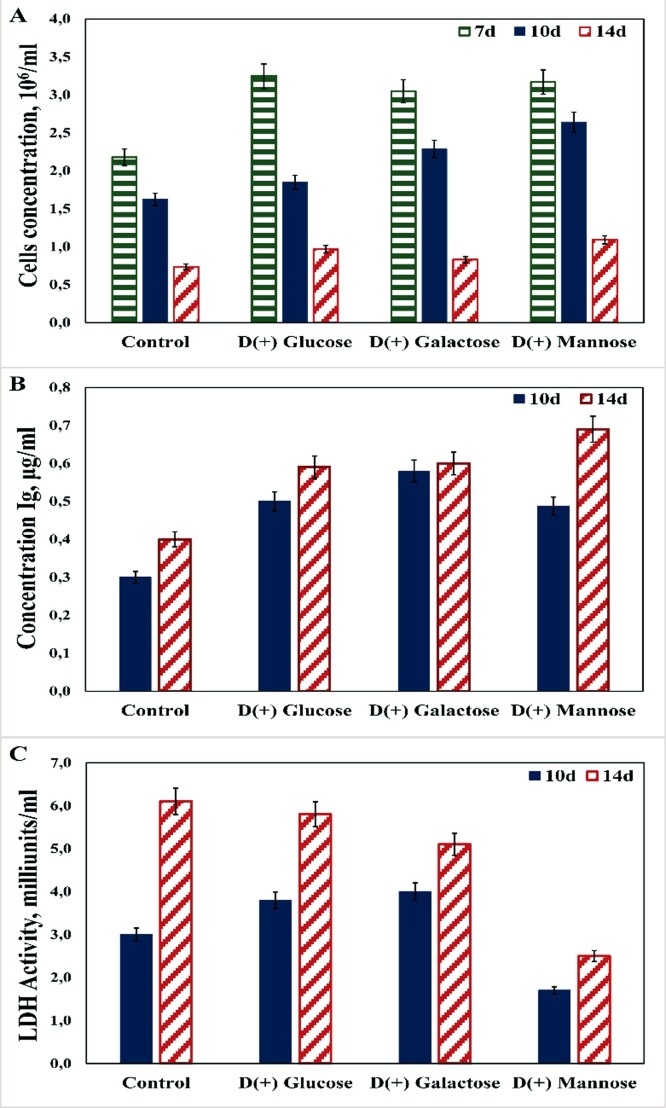
To compare the feeding effect of mannose, galactose and glucose on glycosylation patterns of dimeric forms of IgA1 produced by CHODG44 cells, a fed-batch experiment was carried out. Supplements of d-(+)-glucose (usual feeding component for fed-batch cultures), d-(+)-mannose and d-(+)- galactose were added to each flask in 10 mM final concentration on Days 5 and 7. A control cell culture was grown on basal medium without any feeding. Fig. 2 A shows that cell culture samples fed with glucose, mannose or galactose reached their maximum of cell density between 3,0–3,2 × 106 cell/mL, what is around 1.5 times higher than control on Day 7. However, the sample fed with glucose demonstrated a slightly higher cell density compared to control on Days 10. In contrast, the samples fed with galactose and mannose showed the cell concentration 1.4 and 1.6 times higher, respectively, compared to control on Day 10. On Day 14, the cell concentration was the highest for feeding with mannose and the lowest again for control. Fig. 2B shows that the level of dimeric IgA1 production on Day 14 was the highest for the cell culture fed with mannose, lower for cell cultures grown with glucose and galactose and the lowest for control. The productivity and cell concentration data correlate with lactate level production. Cell cultures fed with glucose and galactose had a higher lactate level than samples fed with mannose. The lactate level production on Day 14 was similar in cultures with glucose and galactose ranging between 5.3–5.8 milliunits/mL. Results presented in Fig. 2C demonstrate that the use of mannose instead of glucose during the fed stage caused the decrease in lactate level twice on Day 14. The fed-batch cell culture grown on the basal medium with glucose or galactose feeding exhibited a typical lactate production way, whereas feeding of cell culture with mannose probably changed the metabolic way reducing lactate level in culture. Culture fed with mannose exhibited a lactate shift from lactate production to lactate consumption. Using of asparagine or succinic acid as basal media supplements also changed metabolism of cells. Such shifting of metabolic pathway could influence the structure of IgA1 glycans.
Fig. 2.
Effects of different carbon sources on the cell concentration (A), IgA1 production (B) and lactate level (C). Cell culture was grown on IMDM medium with different feeding supplements.
3.3. Analysis of glycan profile of IgA1/2G9 stable cell line
For the glycoprofiling of IgA1/2G9 stable cell lines produced at different conditions, two analytical methods were employed. The lectin-based protein microarray allowed screening of biologically available glycans on the surface of IgA1 and comparison of content of certain type of glycan motifs between samples of IgA1 produced at different conditions. MALDI-MS was used for identification of N-glycan structures presented in these samples.
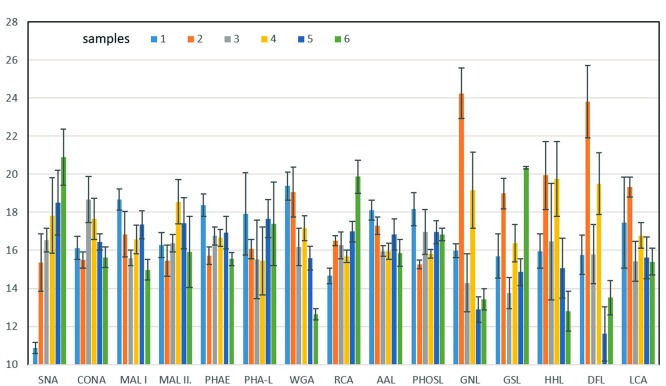
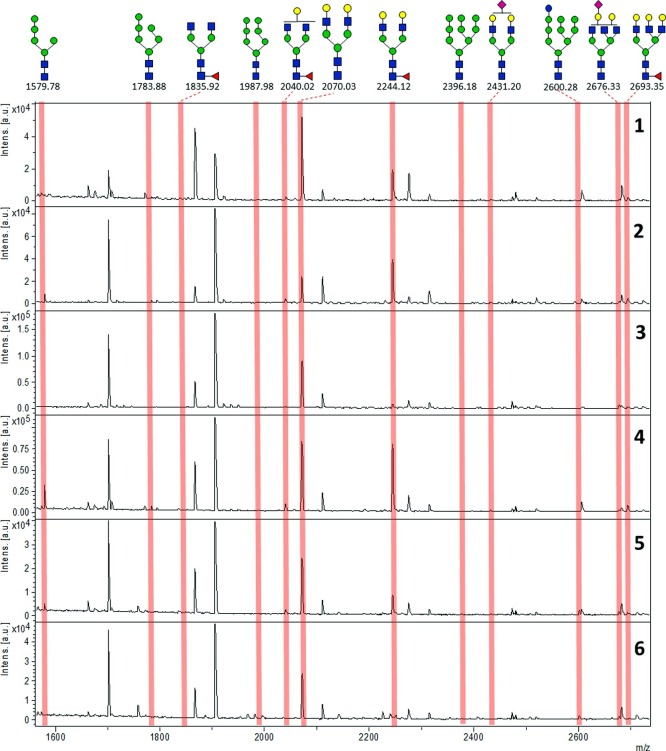
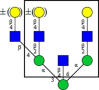
Fig. 3 shows normalized signals of interactions of IgA1/2G9 samples with lectins measured by lectin-based protein microarray. The signals for PNA are not included (SNR < 3). Significant differences for normalized signals of lectin-based microarray analysis between signal of individual sample and average signal for all samples regarding particular lectin were found for some samples and some lectins: for SNA, decreased signal for Sample 1 and increased for Sample 6; for WGA, increased for Samples 1 and 2 and decreased for Sample 6; for RCA, increased for Sample 6; for GNL, increased for Samples 2 and 4 and decreased for Samples 3, 5 and 6; for GSL, increased for Samples 2 and 6 and decreased for Sample 3; for HHL, increased for Samples 2 and 4 and decreased for Sample 6; for DFL, increased for Samples 2 and 4 and decreased for Samples 5 and 6;and for LCA, increased for Sample 2. The important point is that in the lectin-based microarray analysis are not detected all glycan structures presented in the glycoprotein sample, but just “biologically available” ones what means those which are accessible to the lectins. This is a difference from MALDI-MS, where all N-glycans presented in the sample are detected since they are enzymatically released prior to the MS analysis. In Fig. 4 are shown MALDI TOF spectra of N-glycans released from Samples 1−6. Details on the intensity values are shown in the Table S1 in the Supplementary File. Although MALDI mass spectrometry is not quantitative method, remarkable signals of high mannose N-glycans observed in Samples 2 and 4 (while these structures were absent or represented by signals with intensities close to signal-to-noise threshold in the Samples 1, 3, 5 and 6) indicate significantly higher content of high mannose N-glycan structures in Samples 2 and 4 what is in accordance with lectin-based microarray analysis, specifically regarding signals of lectins GNL, HHL and DFL. Various complex N-glycans, usual for immunoglobulins, were observed in all samples without any significant differences in their signal intensities.
Fig. 3.
Normalized signals of interactions of IgA1/2G9 samples with lectins measured by lectin-based protein microarray.
Fig. 4.
Reflectron positive MALDI TOF spectra (m/z 1500 - 2900) of released N-glycans, with representative N-glycan structures found in the samples. Data of intensity values are in the Table S1 in the Supplementary File.
The recombinant monoclonal antibodies produced by CHO cell lines are generally fucosylated and hypogalactosylated, relative to normal human IgG (Jefferis, 2007). It was reported that CHO fed-batch culture producing IFN-γ using glutamine and glucose supplementation facilitate the decrease in sialylation profiles and an increase in the hybrid and high mannose type glycans (Wong et al., 2010). In contrast, cell culture expressing human IgG-Il2 protein demonstrated no difference in glycan profiles for media cultivation with low glucose and glutamine concentrations (Cruz et al., 2000). It was reported that asparagine and glutamine are significant nitrogen and energy sources. Specifically, glutamine and asparagine are mutually related to each other not only in transportation but also in metabolism (Kobayashi et al., 2015; Xu et al., 2014).
In our study we used glutamine, asparagine and succinic acid as media supplementation for cultivation of cell line that produced dimeric IgA1. The supplementation with glutamine without feeding sugars (Sample 1) led to the decrease in sialylation profile (based on signal intensities for interaction with lectin SNA) and increase of accessible GlcNac (WGA). The metabolic shift from glutamine to asparagine could change forms of glycans to hybrid and high mannose type as we observed in Sample 2. The same change in glycan forms, increased high-mannosylation, was observed for the sample supplemented with glutamine and fed with glucose (Sample 4). Increased high-mannose in Samples 2 and 4 was confirmed by both techniques, lectin-based microarray and MALDI-MS as mentioned above. It was suggested that increased high mannose IgG glycosylation produced in CHO was possibly related to osmolality and Golgi/ER pH and increasing productivity (Pacis et al., 2011). Reported was also assumption that high mannose glycosylation during antibody production have multiple mechanisms but the question of why certain clones produce IgG with higher mannose than other clones even with identical culture conditions remains unclear (Slade et al., 2016). It was demonstrated that mannose is a good carbon source for CHO cell growth and immunoglobulin production, readily entering both glycolysis and the TCA Cycle. Usually, proteins produced in CHO cells grown with mannose demonstrate a high increase in total high mannose glycosylation in recombinant IgG, with no effect on cell growth, viability, or titer (Zhang et al., 2019). In contrast, in our study we found an increase in cell viability, productivity and absent of high mannose glycans for cell line produced dimeric form of recombinant IgA1 grown with mannose. Such difference is probably associated with specificity of mannose metabolism for dimeric IgA.
In Sample 2 we detected also higher level of GlcNac (WGA) and Gal forms (GSL). In Sample 3, supplemented with succinic acid, was on the contrary decreased level of accessible Gal structures (GSL). Supplementation with glutamine and feeding with mannose (Sample 6) led to increase of both sialylation (SNA) and galactosylation (GSL, RCA) and decrease of GlcNac structures (WGA) available for interaction with lectin. It is known that the level of antennarity and sialylation depends on cell culture conditions (Hossler et al., 2009) but a disclosure of exact mechanism of these processes will require deeper targeted studies.
4. Conclusions
Therapeutic proteins, dimeric IgA1, were produced by stable cell line IgA1/2G9 on the basal medium at different conditions (different supplementation and feeding). Evaluation of the effect of different conditions on lactate production correlating with IgA productivity reveals lactate metabolism shift in the absence of such main carbon source as glutamine and in the presence of succinic acid or asparagine. A correlation between low lactate level and mannose feeding was found as well. The main aim of this work was to employ high-throughput lectin-based microarray assay for determination of changes in glycan composition of therapeutic proteins and correlation with N-glycan analysis by MALDI-MS. We observed that metabolic shift from glutamine to asparagine changed increase of hybrid and high mannose glycan forms. Supplement of succinic acid led to reduced galactosylation and supplement of glutamine and feeding with mannose led to increase of both sialylation and galactosylation. The explanation of determined changes needs further investigation. Glycan analysis was based mostly on lectin-based microarray assay since MALDI-MS doesn’t allow reliable quantification, however, MALDI-MS confirmed presence of N-glycan structures in accordance with results of lectin-based microarray analysis in the case of all samples. The developed lectin-based microarray platform represents effective tool for the screening and evaluation of glycosylation pattern of IgAs and other therapeutic proteins as well and for the determination of changes in their glycan profile. Moreover, this topic is currently important because of COVID-19 pandemic. Therapeutic IgAs are effective in defense against viruses that use sialic acid as a receptor. Although the binding of SARS-CoV to sialic acids has not been reported so far, if SARS-CoV-2 like other coronaviruses targets sialic acids (Tortorici et al., 2019; Devaux et al., 2020), this interaction could be used as therapeutic target, and it is glycan composition of IgA that has a major impact on antiviral activity of this class of therapeutic antibodies.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the Ministry of Science and Higher Education of Russian Federation [grant number 14.607.21.0177; ID: RFMEFI60717×0177] and by grants VEGA 2/0137/18 and VEGA 2/0130/18 (Slovak Grant Agency for Science VEGA) and APVV-14-0239 (Slovak Research and Development Agency). This work was supported by Ministry of Health of the Slovak Republic under the project registration number 2019/7−CHÚSAV-4. We are grateful to Dr. Yuka Kobayashi from J-Oil mills, Inc. (Japan) for his kind gift of lectin PhoSL.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jbiotec.2020.03.009.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Altamirano C., Paredes C., Cairó J.J., Gòdia F. Improvement of CHO cell culture medium formulation: simultaneous substitution of glucose and glutamine. Biotechnol. Progr. 2000;16:69–75. doi: 10.1021/bp990124j. [DOI] [PubMed] [Google Scholar]
- Altamirano C., Berrios J., Vergara M., Becerra S. Advances in improving mammalian cells metabolism for recombinant protein production. Electron. J. Biotechnol. 2013;16:1–14. doi: 10.2225/vol16-issue3-fulltext-2. [DOI] [Google Scholar]
- Argentova V.V., Aliev T.K., Zarubaev V.V., Klotchenko S.A., Shtro A.A., Sergeeva M.V., Toporova V.A., Dolgikh D.A., Sveshnikov P.G., Vasin A.V., Kirpichnikov M.P. In vitro antiviral activity of recombinant antibodies of IgG and IgA isotypes to hemagglutinin of the influenza A virus. Mol. Biol. 2017;51:804–812. doi: 10.1134/s0026893317060024. [DOI] [PubMed] [Google Scholar]
- Asher M. Hemocytometer counting. In: Kruse P.F. Jr., Patterson M.K. Jr., editors. Tissue Culture Methods and Applications. 1973. pp. 395–397. [DOI] [Google Scholar]
- Blondeel E.J., Braasch K., McGill T., Chang D., Engel C., Spearman M., Butler M., Aucoin M.G. Tuning a MAb glycan profile in cell culture: supplementing N-acetylglucosamine to favour G0 glycans without compromising productivity and cell growth. J. Biotechnol. 2015;214:105–112. doi: 10.1016/j.jbiotec.2015.09.014. [DOI] [PubMed] [Google Scholar]
- Breedveld A., Van Egmond M. IgA and FcαRI: pathological roles and therapeutic opportunities. Front. Immunol. 2019;10:553. doi: 10.3389/fimmu.2019.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceroni A., Maass K., Geyer H., Geyer R., Dell A., Haslam S.M. GlycoWorkbench: a tool for the computer-assisted annotation of mass spectra of glycans. J. Proteome Res. 2008;7:1650–1659. doi: 10.1021/pr7008252. [DOI] [PubMed] [Google Scholar]
- Correa A., Trajtenberg F., Obal G., Pritsch O., Dighiero G., Oppezzo P., Buschiazzo A. Structure of a human IgA1 Fab fragment at 1.55 Å resolution: potential effect of the constant domains on antigen-affinity modulation. Acta Cryst. 2013;69:388–397. doi: 10.1107/S0907444912048664. [DOI] [PubMed] [Google Scholar]
- Cruz H.J., Freitas C.M., Alves P.M., Moreira J.L., Carrondo M.J. Effects of ammonia and lactate on growth, metabolism, and productivity of BHK cells. Enzyme Microb. Technol. 2000;27:43–52. doi: 10.1016/s0141-0229(00)00151-4. [DOI] [PubMed] [Google Scholar]
- Devaux C.A., Rolain J.M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler C., Jarvis D.L. Letter to the Glyco-Forum: effective glycoanalysis with Maackia amurensis lectins requires a clear understanding of their binding specificities. Glycobiology. 2011;21:988–993. doi: 10.1093/glycob/cwr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaderi D., Zhang M., Hurtado-Ziola N., Varki A. Production platforms for biotherapeutic glycoproteins. occurrence, impact, and challenges of non-human sialylation. Biotechnol. Genet. Eng. Rev. 2012;28:147–175. doi: 10.5661/bger-28-147. [DOI] [PubMed] [Google Scholar]
- Goh J.B., Kong S.N. Impact of host cell line choice on glycan profile. Crit. Rev. Biotechnol. 2018;38:851–867. doi: 10.1080/07388551.2017.1416577. [DOI] [PubMed] [Google Scholar]
- Gupta S.K., Srivastava S.K., Sharma A., Nalage V.H.H., Salvi D., Kushwaha H., Chitnis N.B., Shukla P. Metabolic engineering of CHO cells for the development of a robust protein production platform. PLoS One. 2017;12 doi: 10.1371/journal.pone.0181455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi J., Yamada M., Kuno A., Tateno H. Lectin microarrays: concept, principle and applications. Chem. Soc. Rev. 2013;42:4443–4458. doi: 10.1039/c3cs35419a. [DOI] [PubMed] [Google Scholar]
- Hossler P., Khattak S.F., Li Z.J. Optimal and consistent protein glycosylation in mammalian cell culture. Glycobiology. 2009;19:936–949. doi: 10.1093/glycob/cwp079. [DOI] [PubMed] [Google Scholar]
- Jefferis R. Human IgG glycosylation in inflammation and inflammatory disease. Comprehensive Glycoscience. From Chem. Systems Biology. 2007;4:373–392. doi: 10.1016/B978-044451967-2/00099-4. [DOI] [Google Scholar]
- Katrlík J., Švitel J., Gemeiner P., Kožár T., Tkac J. Glycan and lectin microarrays for glycomics and medicinal applications. Med. Res. Rev. 2010;30:394–418. doi: 10.1002/med.20195. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Tateno H., Ogawa H., Yamamoto K., Hirabayashi J. Comprehensive list of lectins: origins, natures, and carbohydrate specificities. In: Hirabayashi J., editor. Lectins. 2014. pp. 555–577. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Motoyoshi N., Itagaki T., Suzuki M., Inokuchi N. Effect of the replacement of aspartic acid/glutamic acid residues with asparagine/glutamine residues in RNase He1 from Hericium erinaceus on inhibition of human leukemia cell line proliferation. Biosci. Biotech. Bioch. 2015;79:211–217. doi: 10.1080/09168451.2014.972327. [DOI] [PubMed] [Google Scholar]
- Lin C.W., Tsai M.H., Li S.T., Tsai T.I., Chu K.C., Liu Y.C., Lai M.Y., Wu C.Y., Tseng Y.C., Shivatare S.S., Wang C.H., Chao P., Wang S.Y., Shih H.W., Zeng Y.F., You T.H., Liao J.Y., Tu Y.C., Lin Y.S., Chuang H.Y., Chen C.L., Tsai C.S., Huang C.C., Lin N.H., Ma C., Wu C.Y., Wong C.H. A common glycan structure on immunoglobulin G for enhancement of effector functions. P. Natl. Acad. Sci. USA. 2015;112:10611–10616. doi: 10.1073/pnas.1513456112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse S., Derer S., Beyer T., Klausz K., Peipp M., Leusen J.H.W., van de Winkel J.G.J., Dechant M., Valerius T. Recombinant dimeric IgA antibodies against the epidermal growth factor receptor mediate effective tumor cell killing. J. Immunol. 2011;186:3770–3778. doi: 10.4049/jimmunol.1003082. [DOI] [PubMed] [Google Scholar]
- Mattu T.S., Pleases R.J., Willis A.C., Kilian M., Wormald R., Lellouch A.C., Rudd P.M., Woof J.M., Dwek R.A. The glycosylation and structure of human serum IgA1, fab, and fc regions and the role of N-Glycosylation on fcα receptor interactions. J. Biol. Chem. 1998;273:2260–2272. doi: 10.1074/jbc.273.4.2260. [DOI] [PubMed] [Google Scholar]
- Maurer M.A., Meyer L., Bianchi M., Turner H.L., Le N.P., Steck M., Wyrzucki A., Orlowski V., Ward A.B., Crispin M., Hangartner L. Glycosylation of human IgA directly inhibits influenza A and other sialic-acid-binding viruses. Cell Rep. 2018;23:90–99. doi: 10.1016/j.celrep.2018.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacis E., Yu M., Autsen J., Bayer R., Li F. Effects of cell culture conditions on antibody N-linked glycosylation—what affects high mannose 5 glycoform. Biotechnol. Bioeng. 2011;108:2348–2358. doi: 10.1002/bit.23200. [DOI] [PubMed] [Google Scholar]
- Palmigiano A., Messina A., Bua R.O., Barone R., Sturiale L., Zappia M., Garozzo D. CSF N-Glycomics using MALDI MS techniques in alzheimer’s disease. Methods Mol. Biol. 2018;1750:75–91. doi: 10.1007/978-1-4939-7704-8_5. [DOI] [PubMed] [Google Scholar]
- Robajac D., Križáková M., Masnikosa R., Miljuš G., Šunderić M., Nedić O., Katrlík J. Sensitive glycoprofiling of insulin-like growth factor receptors isolated from colon tissue of patients with colorectal carcinoma using lectin-based protein microarray. Int. J. Biol. Macromol. 2020;144:932–937. doi: 10.1016/j.ijbiomac.2019.09.170. [DOI] [PubMed] [Google Scholar]
- Roucka M., Zimmermann K., Fido M., Nechansky A. Application of lectin array technology for biobetter characterization: its correlation with FcγRIII binding and ADCC. Microarrays. 2017;6:1. doi: 10.3390/microarrays6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle L., Roos A., Harvey D.J., Wormald M.R., Van Gijlswijk-Janssen D., Redwan E.R.M., Wilson I.A., Daha M.R., Dwek R.A., Rudd P.M. Secretory IgA N-and O-glycans provide a link between the innate and adaptive immune systems. J. Biol. Chem. 2003;278:20140–20153. doi: 10.1074/jbc.M301436200. [DOI] [PubMed] [Google Scholar]
- Schachter H. The joys of HexNAc. The synthesis and function of N and O-glycan branches. Glycoconj. J. 2000;17:465–483. doi: 10.1023/A:1011010206774. [DOI] [PubMed] [Google Scholar]
- Slade P.G., Caspary R.G., Nargund S., Huang C.J. Mannose metabolism in recombinant CHO cells and its effect on IgG glycosylation. Biotechnol. Bioeng. 2016;113:1468–1480. doi: 10.1002/bit.25924. [DOI] [PubMed] [Google Scholar]
- Solá R.J., Griebenow K. Glycosylation of therapeutic proteins: an effective strategy to optimize efficacy. BioDrugs. 2010;24:9–21. doi: 10.2165/11530550-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorici M.A., Walls A.C., Lang Y., Wang C., Li Z., Koerhuis D., Boons G.-J., Bosch B.-J., Rey F.A., de Groot R.J., Veesler D. Structural basis for human coronavirus attachment to sialic acid receptors. Nat. Struct. Mol. Biol. 2019;26:481–489. doi: 10.1038/s41594-019-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderline R.E. Measurement of total lactate dehydrogenase activity. Ann. Clin. Lab. Sci. 1985;15:13–31. [PubMed] [Google Scholar]
- Wong N.S., Wati L., Nissom P.M., Feng H.T., Lee M.M., Yap M.G. An investigation of intracellular glycosylation activities in CHO cells: effects of nucleotide sugar precursor feeding. Biotechnol. Bioeng. 2010;107:321–336. doi: 10.1002/bit.22812. [DOI] [PubMed] [Google Scholar]
- Wuhrer M., Stam J.C., van de Geijn F.E., Koeleman C.A., Verrips C.T., Dolhain R.J., Hokke C.H., Deelder A.M. Glycosylation profiling of immunoglobulin G (IgG) subclasses from human serum. Proteomics. 2007;7:4070–4081. doi: 10.1002/pmic.200700289. [DOI] [PubMed] [Google Scholar]
- Xu P., Dai X.P., Graf E., Martel R., Russell R. Effects of glutamine and asparagine on recombinant antibody production using CHO-GS cell lines. Biotechnol. Prog. 2014;30:1457–1468. doi: 10.1002/btpr.1957. [DOI] [PubMed] [Google Scholar]
- Yu H., Shu J., Li Z. Lectin microarrays for glycoproteomics: an overview of their use and potential. Expert Rev. Proteomics. 2020;17:27–39. doi: 10.1080/14789450.2020.1720512. [DOI] [PubMed] [Google Scholar]
- Zagari F., Jordan M., Stettler M., Broly H., Wurm F.M. Lactate metabolism shift in CHO cell culture: the role of mitochondrial oxidative activity. N. Biotechnol. 2013;30:238–245. doi: 10.1016/j.nbt.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Zámorová M., Holazová A., Miljuš G., Robajac D., Šunderić M., Malenković V., Đukanović B., Gemeiner P., Katrlík J., Nedić O. Analysis of changes in the glycan composition of serum, cytosol and membrane glycoprotein biomarkers of colorectal cancer using a lectin-based protein microarray. Anal. Methods. 2017;9:2660–2666. doi: 10.1039/C7AY00159B. [DOI] [Google Scholar]
- Zhang L., Luo S., Zhang B. Glycan analysis of therapeutic glycoproteins. MAbs. 2016;8:205–215. doi: 10.1080/19420862.2015.1117719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Luo S., Zhang B. The use of lectin microarray for assessing glycosylation of therapeutic proteins. MAbs. 2016;8:524–535. doi: 10.1080/19420862.2016.1149662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Castan A., Stevenson J., Chatzissavidou N., Vilaplana F., Chotteau V. Combined effects of glycosylation precursors and lactate on the glycoprofile of IgG produced by CHO cells. J. Biotechnol. 2019;289:71–79. doi: 10.1016/j.jbiotec.2018.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.