As many as 25% of patients diagnosed with ulcerative colitis are hospitalized with an episode of acute severe ulcerative colitis (ASUC).1 The standard of care for patients hospitalized with ASUC relies on rapid induction with intravenous (IV) corticosteroids. Up to 30% of patients do not respond to corticosteroids alone.2 Rescue therapy with infliximab or cyclosporine has been shown to reduce rates of colectomy to 20% by 90 days.3,4 This still represents a significant rate of treatment failure, which leads to an unplanned and irreversible surgery. In recent years, increasing numbers of patients admitted with ASUC have already failed infliximab therapy, highlighting the need for additional treatment options for these patients. Tofacitinib is a rapidly acting, oral, small-molecule Janus kinase inhibitor that was recently approved by the Food and Drug Administration for treatment of ulcerative colitis.5 We present the first reported use of off-label, high-intensity tofacitinib in 4 patients admitted to our institution with ASUC predicted to fail medical management.
Methods
All 4 patients with ASUC had a high likelihood of failing IV corticosteroid monotherapy based on severe Truelove and Witt’s criteria, C-reactive protein (CRP) >100 mg/L at presentation, endoscopic features during admission (Figure 1A–1D), and prior failure of IV corticosteroids or infliximab therapy (Supplementary Table 1).6,7 Clostridium difficile infection and cytomegalovirus colitis were excluded. Patients were offered total abdominal colectomy or off-label, high-intensity tofacitinib in a shared decision-making process. Three of the 4 patients received IV methylprednisolone 60 mg daily per our institutional severe ulcerative colitis protocol in addition to tofacitinib 10 mg 3 times daily for 9 doses. A dose of 10 mg 3 times daily was chosen based on the short half-life (~3.2 hours) and because of the reported efficacy of 30 mg daily in a phase 2 trial.5 Systemic corticosteroids were avoided in 1 patient because of previous corticosteroid-induced exacerbation of psychiatric illness. This patient received tofacitinib 10 mg 3 times daily for 9 doses in addition to budesonide. Early use of tofacitinib was supported by recently published data demonstrating that tofacitinib induction produces rapid clinical improvements by day 3 of therapy.8 Institutional review board approval was obtained for this retrospective case series.
Figure 1.
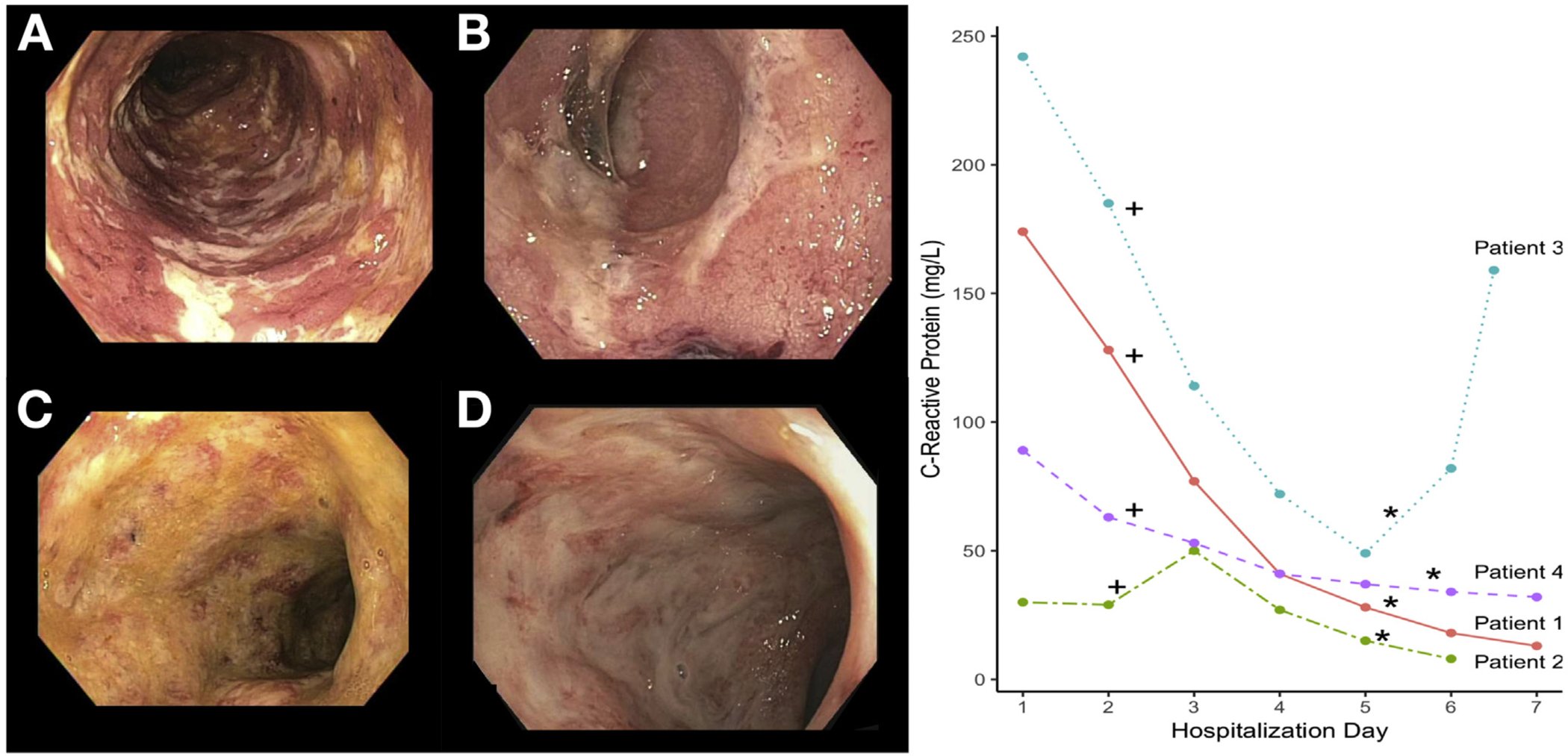
(A–D) Endoscopic appearance of patients before receiving tofacitinib (Image order corresponds to patient order where A, Patient 1; B, Patient 2; C, Patient-3; D, Patient 4). (Right) CRP improvement after initiation of tofacitinib. +Indicates when tofacitinib was initiated. *Indicates when the dose of tofacitinib was reduced.
Results
After receiving tofacitinib, all 4 patients had a rapid improvement in clinical symptoms and decline in CRP (Figure 1, right). Patient 1 and patient 2 achieved clinical remission with a combination of tofacitinib 10 mg 3 times daily for 9 doses and IV corticosteroids, whereas patient 4 achieved clinical remission with tofacitinib 10 mg 3 times daily for 9 doses and budesonide. Of these patients, patient 2 ultimately required elective colectomy 6 months after their index hospitalization for multifocal dysplasia. Only patient 3 was unable to achieve clinical remission. Of note, this patient had the most elevated CRP (242 mg/L) and colonic dilation despite previously receiving more than 7 days of IV corticosteroids at an outside hospital. Despite these high-risk features, this patient had an initial rapid improvement in symptoms and CRP until tofacitinib was reduced to maintenance doses of 5 mg 2 times daily on day 5. This dose adjustment was accompanied by a rapid rise in CRP and return of severe symptoms necessitating urgent colectomy. Patient 4, with the contraindication to systemic corticosteroids, provides proof of concept that tofacitinib therapy (with budesonide) may be an effective induction agent in the treatment of ASUC even without systemic corticosteroids. No major adverse effects directly attributable to the use of tofacitinib were reported during the induction phase of drug administration or up to 18 months of reported follow-up. Patient 4 developed a nonspecific truncal maculopapular rash prompting tofacitinib exchange for vedolizumab.
Discussion
This case series represents the first reported evaluation of off-label, high-intensity tofacitinib in the treatment of ASUC. Despite the limitations of this small, single arm, retrospective case series, the rapid improvement in clinical symptoms and inflammatory biomarkers after 10 mg 3 times daily of tofacitinib therapy, including patient 4 who did not receive systemic corticosteroids, provides convincing support that tofacitinib could be an effective therapeutic augmentation option for patients with ASUC likely to fail first-line medical management. In addition, the rapid onset of action supports early use of tofacitinib rescue and the rapid plasma clearance has the theoretical benefit of minimizing intraoperative and postoperative complications because it can be cleared before colectomy even in urgent cases. Despite these initial encouraging findings, there is currently not sufficient data to change standard clinical practice; however, our findings provide justification for more rigorous clinical trials in the future. Additional trials are needed to identify the optimal dose, frequency, duration, and safety of high-intensity Janus kinase inhibitor therapy in patients presenting with ASUC.
Supplementary Material
Acknowledgments
The patients included in this report verbally consented to the inclusion of all deidentified patient information and images.
Funding
Jeffrey A. Berinstein is supported by T32 DK062708.
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2018.11.022.
Conflicts of interest
These authors disclosure the following: Jami A. R. Kinnucan serves on advisory boards for Janssen, AbbVie, and Pfizer. Peter D. R. Higgins received consulting fees from AbbVie, Amgen, Genentech, JBR Pharma, and Lycera. The remaining authors disclose no conflicts.
References
- 1.Dinesen LC, Walsh AJ, Protic MN, et al. The pattern and outcome of acute severe colitis. J Crohns Colitis 2010;4:431–437. [DOI] [PubMed] [Google Scholar]
- 2.Turner D, Walsh CM, Steinhart AH, et al. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol 2007;5:103–110. [DOI] [PubMed] [Google Scholar]
- 3.Laharie D, Bourreille A, Branche J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet 2012;380:1909–1915. [DOI] [PubMed] [Google Scholar]
- 4.Gibson DJ, Heetun ZS, Redmond CE, et al. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol 2015;13:330–335. [DOI] [PubMed] [Google Scholar]
- 5.Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723–1736. [DOI] [PubMed] [Google Scholar]
- 6.Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J 1955;2:1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Travis SP, Farrant JM, Ricketts C, et al. Predicting outcome in severe ulcerative colitis. Gut 1996;38:905–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanauer S, Panaccione R, Danese S, et al. Tofacitinib induction therapy reduces symptoms within 3 days for patients with ulcerative colitis. Clin Gastroenterol Hepatol 2019; 17:139–147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


