Abstract
The full impact of the coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), on the field of hematopoietic cell transplantation (HCT) is unknown. This perspective paper reviews the following: current COVID-19 epidemiology, diagnosis, and potential therapies; care considerations unique to HCT recipients; and the concept of a learning network to assimilate emerging guidelines and best practices and to optimize patient outcomes through facilitating shared learning and experience across transplantation centers.
Key Words: SARS-CoV-2, Coronavirus, COVID-19, Bone marrow transplantation, Cell therapy, Hematopoietic cell transplantation, Immunocompromise, Severe acute respiratory syndrome
INTRODUCTION
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel single-strand RNA beta-coronavirus, has caused the current coronavirus disease 2019 (COVID-19) pandemic. As of April 13, 2020, the World Health Organization (WHO) had reported 1,773,084 laboratory-confirmed cases and 111,652 deaths globally [1]. The ultimate impact of COVID-19 on the field of hematopoietic cell transplantation (HCT) is currently unknown. This perspective paper anticipates the significant influence of COVID-19 on HCT recipients given their immunocompromised state, presence of other medical comorbidities, and concerns for higher infection-related severity and mortality, and also reviews the substantial impact of COVID-19 on the HCT-related healthcare system. To address these challenges, novel care approaches and ways to assimilate and share information in the background of rapid change are critically needed. Therefore, the concept of learning networks as interactive platforms for effectively assimilating and distributing evolving information to transplantation centers is introduced [2].
COVID-19: AN OVERVIEW
Epidemiology and Transmissibility
COVID-19 was first recognized in persons presenting with pneumonia of unknown etiology in Wuhan City, China in December 2019 [3,4]. The origin of the virus causing COVID-19, SARS-CoV-2, is currently unconfirmed, although emergence from an animal reservoir has been proposed [5,6]. Metagenomic next-generation sequencing of bronchoalveolar lavage (BAL) specimens from affected patients identified a previously unobserved coronavirus (CoV), initially referred to as novel coronavirus 2019 (2019-nCoV) [5]. The genome sequence of this 2019-nCoV was confirmed to be structurally related to other CoVs, including 89% identical to the bat severe acute respiratory syndrome (SARS-like CoV) and 82% identical to human SARS-CoV-1; thus, the virus was renamed SARS-CoV-2 7, 8, 9. SARS-CoV-2 is also similar to other zoonotic CoVs causing global outbreaks of severe respiratory illnesses, such as SARS-CoV-1 in 2002 and Middle East respiratory syndrome (MERS) in 2012, and has been confirmed to be transmitted from human to human [10].
In contrast to SARS-CoV-1 and MERS, the community spread of SARS-CoV-2 has been global, likely reflecting differences not only in how the virus may be transmitted [11], but also in its association with high viral loads in the upper respiratory tract [12] and significant asymptomatic carriage [13]. In this regard, the reported household transmission rate is estimated to be 15% [14]. The virus’s basic reproduction number (R0), an epidemiologic metric describing transmissibility in terms of the average number of cases that could be caused by 1 infected patient in a susceptible population [15], has been estimated as 1.4 to 3.0 [16], compared with ∼1.2 to 3 for seasonal influenza R0, ∼12 to 17 for pertussis R0, and ∼12 to 18 for measles R0 [15,17]. The COVID-19 case fatality risk (CFR) is estimated to be in the range of .25% to 4.7% [18,19], with the highest mortality reported in older adults with comorbidities and varying by geographic location [20]. Preliminary data from the United States suggest high fatality in persons age ≥85 years (CFR ∼10% to 27%), lower CFR in persons age 65 to 84 years (∼3% to 11%), and further declines in younger age groups (CFR ∼1% to 3% in 55- to 64-year-olds, <1% in 20- to 54-year-olds) [21,22]. A striking finding in these data is the proportion of severe disease and hospitalization in younger adults (age 45 to 64 years), which contrasts with the Chinese experience [21].
Clinical Manifestations
After an estimated median incubation period of 5 days (range, 2 to 14 days), patients may present with fever (77% to 98%) and nonspecific symptoms similar to an influenza-like illness (eg, fever, cough, myalgia, fatigue) [4,23]. Although the spectrum of COVID-19 varies from asymptomatic infection to mild and severe disease, the most well-described clinical manifestation is lower respiratory tract disease (LRTD) that presents with shortness of breath and can progress to pneumonia and acute respiratory distress syndrome (ARDS) in 17% to 29% of patients [8,20,23,24]. Progression to LRTD generally occurs around 10 days after illness onset [4].
Although the risk factors for severe COVID-19 disease have not been fully elucidated, older age and medical comorbidities, including diabetes, cardiovascular disease, and pulmonary disease in adults, have been associated with severe disease [23,25, 26, 27], which often requires intensive care unit (ICU) care [24], with 47% to 71% of ICU patients requiring mechanical ventilation [24,28]. Additional complications include secondary infections (10%) and development of shock and multiorgan dysfunction [3].
Laboratory and Radiographic Findings
Lymphopenia is frequently reported with COVID-19, occurring in approximately 63% to 83% of patients [10,20,23,29]. Notably, decreases in CD8+ T cells and B cells in adults have been associated with severe COVID-19 and poor response to therapy [30], whereas CD8+ T cell and B cell recovery has been associated with moderate disease [31]. Finally, decreases in regulatory T cells also have been associated with a hyperinflammatory response in adults [32], necessitating the use of monoclonal blocking antibodies, such as tocilizumab [33].
Given these preliminary observations, lymphopenia and alloreactivity associated with HCT may portend a worse prognosis for COVID-19 in HCT recipients, similar to lymphopenia associating with LRTD progression and higher mortality from community respiratory viruses 34, 35, 36. Interestingly, risk factors for endemic human CoV strains (eg, 229E, NL63, OC43, HKU1) vary based on age. Although endemic CoV was frequently detected in pediatric HCT recipients, low absolute lymphocyte count was not associated with progression to LRTD or severe LRTD, but level of immunosuppression was [37]. Among adult HCT recipients with endemic CoV, 34 of 112 (30%) progressed to LRTD, with graft-versus-host disease (GVHD), corticosteroids, hypoalbuminemia, and older age associated with disease progression [38].
Other notable COVID-19 laboratory findings include leukopenia (9% to 25%) or leukocytosis (24% to 30%) and elevated transaminases (37%) [20,24]. The most common radiologic findings in patients with COVID-19 are unilateral or bilateral ground-glass opacities [39,40]. The most severe lung abnormalities occurred at approximately 10 days after symptom onset in patients who underwent sequential imaging [41,42].
Diagnosis
Viral Dynamics
The viral dynamics of SARS-CoV-2 are still being elucidated in real time [28,43]. SARS-CoV-2 has been detected in blood, urine, stool, upper and lower respiratory tract, and saliva using real-time polymerase chain reaction (RT-PCR) [20,23,44,45]. The performance characteristics of the SARS-CoV-2 PCR are unknown, and the diagnostic yield of the SARS-CoV-2 RT-PCR may be site-specific [43], may depend on clinical manifestations and disease severity [46,47], and may be influenced by variations in assay sensitivity and specificity [48]. As a result, the diagnostic capability of PCR may be suboptimal compared with other modalities such as chest imaging [49], and a negative PCR result does not conclusively exclude COVID-19.
Preliminary data suggest that SARS-CoV-2 viral loads are higher in BAL and sputum samples, followed by nasopharyngeal (NP) and throat specimens [12,28]. The median duration of viral detection from oropharyngeal specimens is 20 days (range, 8 to 37 days) [50]. In addition, SARS-CoV-2 viral loads from NP sites have been found to be similar in both asymptomatic and symptomatic patients [12]. Furthermore, SARS-CoV-2 transmission occurs from asymptomatic persons, who likely contribute to rapid viral dissemination [13]. The significance of ongoing viral detection by PCR in asymptomatic persons, including whether detection equates to viable/transmissible virus, remains an ongoing area of research. As serologic testing becomes more widely available, data will evolve regarding immunologic correlates of protection against SARS-CoV-2 infection [43,51,52].
Recommendations
Establishing the diagnosis of COVID-19 is important for understanding a disease in its evolution and instituting appropriate infection prevention precautions. Clinical judgment to guide testing is based on the presence of signs/symptoms compatible with COVID-19, disease severity, and local epidemiologic patterns of the disease. Clinicians should have a high index of suspicion to test patients who present with fever and/or lower respiratory tract (LRT) symptoms and have either recently traveled from an area of high community SARS-CoV-2 prevalence or have been exposed to a close contact with confirmed or suspected COVID-19 in the previous 14 days [53,54]. A close contact is defined as being within 6 feet (2 meters) of a COVID-19 case for a prolonged period or having direct contact with infectious secretions of a COVID-19 case [55]. In addition, patients with severe LRT disease of unclear etiology should be considered for testing. A coronavirus self-checker is now available to help guide patients through the process [56]. If a patient is considered a person under investigation (PUI) based on geographic [55] or healthcare [57] exposure, clinicians should immediately institute appropriate infection precautions (see details below) and notify their state and local health departments.
Current commercially available multiplex PCRs that detect endemic CoV do not detect SARS-CoV-2. Therefore, RT-PCR assays have been developed to improve the sensitivity of diagnostic testing; however, these also have limitations, as reviewed previously. For initial testing, the Centers for Disease Control and Prevention (CDC) recommends testing upper respiratory tract specimens (NP swabs) in all PUIs. In patients with a productive cough, sputum may be tested, but procedures that generate aerosols are discouraged. For example, BAL sampling is considered high-risk for aerosol dissemination and is not recommended in patients known to be positive for SARS-CoV-2 unless a co-infection is suspected. In patients receiving mechanical ventilation, LRT aspirate can be obtained. Chest imaging should be considered in patients positive for SARS-CoV-2 with LRT symptoms. Research is ongoing to improve diagnostic strategies, including more rapid, point-of-care PCR tests [58] and serologic assays to identify patients still at risk [59].
Treatment
Supportive care is the mainstay of COVID-19 treatment. Updated, interim clinical guidance for patients with confirmed COVID-19 is available from the CDC and WHO 60, 61, 62. At this time, there are no proven effective therapies against SARS-CoV-2 [63]. The safety and efficacy of other treatment options for COVID-19 are currently being evaluated (Table 1 ) [64,65]. Recently, the WHO announced the “Solidarity Trial,” a multicountry clinical research study to evaluate multiple potential treatment options against SARS-CoV-2—remdesivir, hydroxychloroquine, lopinavir/ritonavir, and lopinavir/ritonavir plus interferon-beta—compared with supportive care measures alone [66].
Table 1.
Current investigational therapies being evaluated for COVID-19.
| Agent | Data from Previous Studies | ClinicalTrials.gov Identifier, Other Sources |
|---|---|---|
| Remdesivir | Ebola, MERS | NCT04280705 |
| NCT04302766 | ||
| NCT04292899 | ||
| NCT04292730 | ||
| NCT04252664 | ||
| NCT04257656 | ||
| Favipiravir | Ebola | NCT04310228 (In Japan) |
| NCT04303299 | ||
| Lopinavir/ritonavir 141, 142, 143, 144 | SARS-CoV, MERS | NCT04261907 |
| NCT04276688 | ||
| NCT04307693 | ||
| Chloroquine [145,146] | SARS-CoV | NCT04307693 |
| NCT04315896 | ||
| Interferon-alpha 2B [147,148] | MERS | NCT04293887 |
| Camostat mesylate | SARS-CoV | Approved in Japan, no previous human testing |
| Nitazoxanide [149] | Coronavirus | |
| Intravenous immunoglobulin (IGIV) from COVID-19 patients | N/A | NCT04264858 |
| NCT04261426 | ||
| Mesenchymal stem cells | N/A | NCT04288102 |
| NCT04293692 | ||
| NCT04273646 | ||
| Carrimycin | N/A | NCT04286503 |
| Bevacizumab | Acute lung injury, ARDS | NCT04275414 |
| NCT04305106 | ||
| Tocilizumab | N/A | NCT04317092 |
| NCT04310228 | ||
| Chinese National Health Commission guidelines [42] | ||
| Recombinant human angiotensin-converting enzyme 2 | N/A | NCT04287686 |
N/A indicates not applicable.
A complete list of COVID-19 clinical trials is available at ClinicalTrials.gov (https://clinicaltrials.gov/ct2/results?cond=COVID-19).
Remdesivir is an intravenous investigational novel nucleoside analog in development that has previously been used to treat Ebola and MERS infections [67,68] and has demonstrated in vitro activity against SARS-CoV-2 [69]. Remdesivir has been approved by the Food and Drug Administration (FDA) for a National Institutes of Health-sponsored randomized controlled clinical trial for hospitalized COVID-19 patients with advanced symptoms (ClinicalTrials.gov identifier NCT04280705). Remdesivir may be available for individuals via compassionate use requests (children or pregnant women) or under an expanded access program for severely ill patients (https://rdvcu.gilead.com/). Recent findings from a compassionate use trial using remdesivir in patients with severe COVID-19 demonstrated clinical improvement in 68% (36/53) of patients [70].
The use of convalescent plasma collected from patients who have recovered from COVID-19 is being evaluated as a treatment based on its use in other severe viral infections [71,72]. A recent limited intervention study noted that convalescent plasma therapy in adults with severe COVID-19 who were receiving multiple other investigational therapies was well tolerated (primary endpoint) and demonstrated potential clinical efficacy as measured by improvements in clinical symptoms, laboratory abnormalities, and radiographic imaging (secondary endpoints) [73]. Clinical trials are now available for adults to assess safety and efficacy of convalescent plasma based on virologic and clinical endpoints (https://clinicaltrials.gov).
Given the increasing data supporting a hyperinflammatory state in adults with severe COVID-19 [74], clinical research trials are ongoing to evaluate the effect of IL-6 blockade on COVID-19 outcomes [75,76]. Treatment with systemic corticosteroids is not routinely recommended, given a lack of survival benefit in SARS-CoV-1, possible adverse events, and concerns for prolonging viral replication and delaying viral clearance, unless clearly required for other indications (eg, ARDS, septic shock) [77].
Prevention
Three basic but effective strategies should be implemented by all inpatient bone marrow transplantation units and outpatient clinics (Table 2 ): (1) maintaining proper and frequent handwashing, including before and after patient encounters, as a primary tool to prevent the spread of respiratory illness; 78, 79, 80 (2) implementing standardized visitor screening that is limited to direct caregivers only [79,81]; and (3) mandating that sick employees stay home [78,79]. Transplantation centers should initiate these strategies immediately and identify mechanisms to reliably improve adherence to these best practices.
Table 2.
Process and Practice Interventions Associated with Improved Compliance
| process | Potential Mechanisms for Process Measurement | Practice Interventions Associated with Improved Compliance |
|---|---|---|
| Handwashing [79,80] | Direct observation [150,151] | Patient, caregiver, and hospital staff handwashing education initiatives [153] |
| Physician and staff financial incentives [153] | ||
| Frequent reminders [152] | ||
| Timely and frequent audits [154] | ||
| Individual feedback to promote accountability [154] | ||
| Amount of soap used [152] | ||
| Screen patients and visitors for symptoms of acute respiratory illness[79] | Direct assessment for all persons entering the transplantation unit | Consider highly reliable interventions 155, 156, 157, 158 |
| Screening station at entry of unit | ||
| Unit door locks for entry to limit entry onto unit without screening | ||
| Encourage sick or at-risk employees to stay home [78,79] | Number of employees self-reporting | Staff education of when self-reporting should be completed [159] Adoption of a just culture for self-reporting [160]; self-reporting is encouraged and rewarded and focuses not on individual blame, but on improving health |
Given that nosocomial transmission of SARS-CoV-2 has been reported [28,82], best practices in hospital infection control must be implemented to prevent SARS-CoV-2 transmission to other patients and healthcare personnel [81]. Even after environmental contamination by SARS-CoV-2 [83], effective infection control practices and environmental cleaning can prevent nosocomial transmission of SARS-CoV-2 [84,85]. Infection prevention measures should be immediately instituted for any PUI, including masking the patient and moving him or her into a private room, optimally with airborne isolation. Hospital personnel should follow standard, contact, and airborne precautions, including the use of appropriate personal protective equipment (PPE) and eye protection [81] The COVID-19 pandemic has highlighted the continuing burden and challenges to healthcare resources that necessitate redirection of timely efforts to conserve, optimize, and restock equipment, including PPE [86].
In the ambulatory setting, preventative measures should be provided to HCT recipients and their household contacts (Table 3 ). Stable patients and PUI should contact their healthcare provider if they develop worsening symptoms and before presenting to medical facilities. In a medical emergency, they should inform emergency medical personnel of their immunocompromised status and possible COVID-19 exposure [78]. In addition, continued public health efforts should be maintained to mitigate community transmission of COVID-19 [87].
Table 3.
Recommendations for Patients in the Ambulatory Setting to Prevent COVID-19
| • Wash hands often with soap and water for 20 seconds (singing “Happy Birthday” to yourself twice while washing your hands = 20 seconds). If soap and water are not available and hands are not visibly dirty, use an alcohol-based hand sanitizer that contains ≥60% alcohol. |
| • Avoid touching your eyes, nose, or mouth. |
| • Avoid or at least maintain a distance of 6 feet (2 meters) away from anyone who has respiratory symptoms (cough or sneezing). |
| • Stay home if you feel sick or have cold-like or flu-like symptoms, including fever, cough, sore throat, headache, or body aches; contact your healthcare professional should your symptoms worsen before presenting for medical attention, if possible. |
| • Practice good cough hygiene, including covering your coughs and sneezes with a tissue and performing good hand hygiene. |
| • Avoid any unnecessary travel or travel to high-risk areas for COVID-19. |
| • Contact your healthcare professional if you think you may have come in contact with another person with suspected or confirmed SARS-CoV-2. |
| • Clean and disinfect any objects and surfaces that you touch frequently using a regular household cleaning spray or wipe. |
| • Refer to reputable information sources for additional details to prevent COVID-19 (https://www.cdc.gov/coronavirus/2019-ncov/community/index.html). |
Multiple vaccine candidates have been identified [88], and clinical trials are underway [89], including an open-label phase 1 trial of differing doses of mRNA-1273 to assess their safety and efficacy against SARS-CoV-2 [90] and microneedle array-delivered SARS-CoV-2 subunit vaccines, which can be rapidly produced and generate potent antibody responses [91]. Together with rapid diagnostic and serologic testing, vaccines will serve as the foundation for current and future protection against SARS-CoV-2 [92].
COVID-19: CONSIDERATIONS IN UNIQUE PATIENT POPULATIONS
Children
Children with SARS-CoV-1 tend to have milder disease and more favorable outcomes compared with adults 93, 94, 95. The epidemiology of SARS-CoV-2 in children is evolving, but early observations suggest that immunocompetent children experience milder COVID-19 than adults [28,45,96, 97, 98], even though they have high viral loads [99] and prolonged respiratory and fecal viral shedding after COVID-19 [45,100]. Children at higher risk for severe COVID-19, defined as necessitating hospitalization or intensive care, include those with cardiovascular and chronic lung disease, infants age <1 year, and immunocompromised patients receiving immunosuppressive therapy [101]. Radiographic changes in children are reportedly similar to those seen in adults, with ground-glass and patchy opacities the most common finding [102,103]. Given their milder disease but prolonged viral shedding, children may act as significant reservoirs for SARS-CoV-2 in the community.
Immunocompromised Patients
Unlike their immunocompetent counterparts, immunocompromised patients likely have different COVID-19 features, including but not limited to the following: viral incubation period and duration of shedding, onset and duration of clinical signs and symptoms, viral detection and associated laboratory features, risk factors for progression to severe disease, risk for concomitant and secondary infections, and response to supportive care or future antiviral therapies. Such additional data on viral dynamics and immune response are critical in defining the risk of SARS-CoV-2 transmission from potential HCT donors to recipients and the influence on transplantation outcomes.
Immunocompromised patient populations are at elevated risk for severe infection from SARS-CoV-2 (Figure 1 ). At the time of this report, the literature on COVID-19 in immunocompromised patients is very limited, consisting of 2 case reports of COVID-19 in adult renal transplantation recipients [104,105], 1 case report of COVID-19 in a renal transplantation and HCT recipient [106], and 2 case series on COVID-19 in adult cancer patients [107,108].
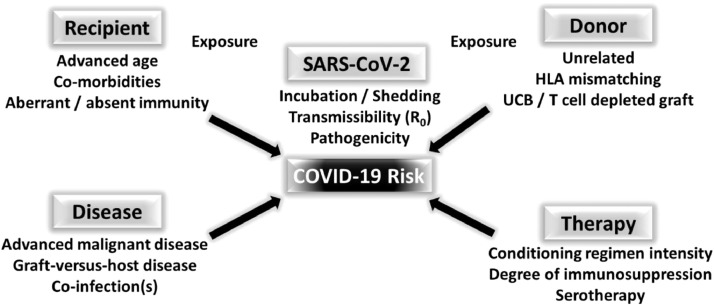
Figure 1.
Presumed risk factors for COVID-19 in HCT recipients. The risk for developing COVID-19 is likely a composite of donor- and recipient-derived factors, underlying disease, and therapy received, in addition to exposure of both donor and recipient to SARS-CoV-2. In addition, factors inherent to the SARS-CoV-2 virus, including transmissibility (R0), incubation period, and duration of shedding, also confer risk to the immunocompromised patient. UCB, umbilical cord blood.
The single case report of COVID-19 in a HCT recipient describes a 51-year-old Chinese male who underwent allogeneic HCT for acute myelogenous leukemia in June 2019 and developed COVID-19 22 days after traveling to Wuhan in February 2020 [106]. The details of the patient's HCT are scanty, but he was receiving maintenance cyclosporine and was lymphopenic at the time of COVID-19 infection, which ultimately progressed within 10 days of symptom onset. He required mechanical ventilation and died 22 days after symptom onset, seemingly from nosocomial bacterial infection. As his SARS-CoV-2 RNA was negative after treatment with lopinavir/ritonavir and methylprednisolone and discontinuation of cyclosporine [106].
Liang et al [107] reported on 18 adult cancer patients out of 1590 patients with COVID-19, most of whom were cancer survivors (n = 12; 67%) not receiving cancer-directed therapy. After adjusting for risk factors (age, smoking, and other comorbidities), cancer was associated with an increased risk for severe events (odds ratio, 5.34; 95% confidence interval [CI], 1.80 to 16.18; P = .0026), including more rapid clinical deterioration requiring ICU admission (hazard ratio, 3.56; 95% CI, 1.65 to 7.69) [107]. Yu et al [108] reported on their institutional experience with 12 of 1524 cancer patients who developed COVID-19 pneumonia during the COVID-19 outbreak in China. Similar to Liang et al [107], they found that cancer patients had a higher risk for developing SARS-CoV-2 infection (odds ratio, 2.31; 95% CI, 1.89 to 3.02) compared with the general community, and that older patients (>60 years) with non-small cell lung cancer (NSCLC) were at especially high risk [108]. Both groups concluded that cancer-directed therapies should be delayed and the frequency of hospital visits reduced, if possible, and that cancer patients with COVID-19 require more vigilant surveillance and likely more aggressive treatment.
In personal communications from the Italian experience in a pediatric hematology-oncology department in Lombardia, Italy on March 14, 2020, Balduzzi et al [109] reported that SARS-CoV-2 was not detected in any pediatric hematology-oncology or HCT patient, nor had any positive cases in these patients been reported elsewhere within Italy. The Infectious Diseases Working Party of the European Society for Blood and Marrow Transplantation (EBMT) provided an update on their ongoing “prospective survey on impact of COVID-19 on stem cell transplant recipients” (https://www.ebmt.org/covid-19-and-bmt) at a webinar on March 20, 2020. Dr Per Ljungman reported that 15 patients (12 allogeneic HCT recipients and 3 autologous HCT recipients), with a median age of 59 years, developed COVID-19 post-HCT. Ten patients were diagnosed with an upper respiratory tract infection, and 5 patients were diagnosed with a lower respiratory tract infection. One of the 15 patients had died from COVID-19.
HCT Donors and Recipients
The COVID-19 pandemic poses unique challenges for the field of HCT related to donors, recipients, and cell products, all in the background of rapid change and stress to health care systems, suppliers, government agencies, and workforces. Reflecting such changes, guidelines for HCT from the American Society of Transplantation and Cellular Therapy (ASTCT) and the EBMT (summarized in Table 4 ) continue to evolve [110,111]. For the most up-to-date care guidelines and healthcare policies, the reader is directed to the Web-based resources listed in Table 5 .
Table 4.
Evolving ASTCT and EBMT Guidelines for Autologous and Allogeneic HCT Donors and Recipients During the COVID-19 Pandemic1-2., 3., 4., 5.
| Low-Risk Disease | High-Risk Disease | Information/Recommendations | |
|---|---|---|---|
| Recipients | Avoid exposure to COVID-19 | ||
| Refrain from travel | |||
| Practice good hygiene | |||
| Confirmed COVID-19 | Defer HCT for 3 mo | Defer HCT until asymptomatic and at least 2 negative weekly PCRs | |
| Exposed COVID-19 | Defer HCT for at least 14 d, preferably 21 d | Deferral based on clinical judgement | Follow SARS-CoV-2 testing per local guidelines |
| SARS-CoV-2 PCR screen with symptoms | SARS-CoV-2 PCR screen with symptoms | ASTCT: Screen all recipients at initial evaluation and 2 d before conditioning | |
| Respiratory symptoms | Multiplex respiratory PCR | Multiplex respiratory PCR | If SARS-CoV-2 detected, defer as feasible. Chest imaging recommended for lower respiratory tract symptoms. |
| SARS-Cov-2 PCR if available (NP sampling) | SARS-Cov-2 PCR if available (NP sampling) | ||
| Donors | Avoid exposure to COVID-19 | ||
| Refrain from travel | |||
| Practice good hygiene | |||
| Confirmed COVID-19 | Exclude from donation | Exclude from donation | Unclear when to donate in future |
| Exposed COVID-19 | Defer donation for 28 d | SARS-CoV-2 PCR screen | Follow SARS-CoV-2 testing per local guidelines |
| Monitor for COVID-19 | Monitor for COVID-19 | ||
| Respiratory symptoms | Multiplex respiratory PCR | Multiplex respiratory PCR | Defer donation if SARS-CoV-2 positive |
| SARS-Cov-2 PCR if consistent (NP sampling) | SARS-Cov-2 PCR if consistent (NP sampling) | ||
| Product | |||
| Do not collect | Collect and freeze if possible | Acquire and freeze product before start of conditioning | |
| If unable to freeze product, arrange for alternative donor |
Guidelines compiled from the ASTCT Interim Guidelines for COVID-19 Management in Hematopoietic Cell Transplant and Cellular Therapy Patients (version 1.2, March 18, 2020), the EBMT recommendations update (April 7, 2020), and National Marrow Donor Program's “New TC requirement for unrelated donor products” (March 23, 2020).
Exposure includes living in or traveling from high-risk areas (WHO level 2 and 3) or exposed to close contacts with COVID-19.
Repeat negative SARS-CoV-2 PCR screen if clinical suspicion for COVID-19 given variable screening test sensitivities (ie, false-negative rates).
Bronchoalveolar lavage (BAL) sampling is discouraged if the patient is known to be SARS-CoV-2-positive unless coinfection is suspected.
5. Donor-to-recipient transmission of MERS- or SARS-CoV in blood/cell products has not been reported.
Table 5.
Resources for the COVID-19 Pandemic Pertinent to HCT and Cell Therapy
Donor Considerations
In addition to standard infectious disease marker screening for donor clearance, screening of the donor for exposure to COVID-19 is essential to prevent potential transmission of SARS-CoV-2 to the HCT recipient as well as to avoid undo harm to the donor. Specifically, donor screening by symptoms and exposure should be done at the time of donor clearance and before product collection. Donor exclusion is based on the donor having COVID-19 at the time of screening or product collection. Given the notable overlap in symptoms among community respiratory viruses [112], respiratory multiplex PCR testing in addition to SARS-CoV-2 testing should be performed if the donor manifests respiratory symptoms. Additional considerations for donors include access to screening and collection centers, which may be impeded by travel restrictions and closures. Therefore, a donor backup plan is important, and frequent communication with the collection center is vital to ensure donor eligibility and to plan for alternative donors as needed [113]. The use of alternative donors, including umbilical cord blood and haploidentical donors, may be worth considering, particularly given the similar outcomes with these sources as with matched unrelated donor transplants.
Recipient Considerations
HCT recipients should be screened for COVID-19 exposure during the pretransplantation workup and up to and including the day before admission for transplantation. In the event of exposure to COVID-19 before transplantation, an HCT candidate with low-risk disease should have the procedure deferred for at least 14 days (preferably 21 days) while being monitored for symptoms. In an HCT candidate with high-risk disease, deferral of transplantation is based on clinical judgment.
Before transplantation, patients who develop respiratory symptoms should have the procedure postponed and undergo both community respiratory virus multiplex and SARS-CoV-2 PCR testing. In patients positive for COVID-19, autologous HCT should be deferred for at least 3 months, and allogeneic HCT should be deferred until the recipient is asymptomatic and has had at least 2 negative consecutive weekly PCR tests.
Transplantation Considerations
All elective HCTs for nonmalignant, nonurgent conditions should be delayed. However, more urgent HCT for high-risk malignant diseases may need to proceed despite donor and recipient exposure, as explained above. The conditioning regimen should not be initiated until the HCT donor and recipient have been cleared and the donor product has been deemed acceptable for use and is readily available. For unrelated donor grafts, the graft must be cryopreserved and on site before the start of conditioning.
Blood and Medication Considerations
According to the FDA, no cases of transfusion-transmitted respiratory viruses, including MERS and SARS-CoV, have been reported to date [114]. In addition, no transfusion-transmitted infections of SARS-CoV-2 have been reported by the AABB [115].
Interruptions in the blood supply have occurred, and there is a high likelihood that blood donors will either contract or be exposed to COVID-19. In this regard, SARS-CoV-2 RNA was detected by RT-PCR in 4 (3 whole blood, 1 platelets) out of 2430 total donor blood products (774 whole blood, 1656 platelets) collected at the Wuhan Blood Center, but no definite viral transmission was noted [116]. The AABB Interorganizational Task Force on Domestic Disasters and Acts of Terrorism is encouraging to donating blood to maintain an adequate blood supply. The American Red Cross is also encouraging blood donation while recommending that individuals postpone donation if they have traveled to a pandemic area, been diagnosed with COVID-19, or been in contact with a person infected with COVID-19 [117]. Judicious use of blood products through the use of more stringent transfusion criteria should now be applied.
With respect to the availability of pharmaceutical agents, including biological and immunosuppressive therapies, the American Society of Health-System Pharmacists has not posted any restrictions at this time [118]. However, interruptions in medication supplies are anticipated, given the significant amount of overseas drug manufacturing [119].
Research Considerations
Federal agencies and HCT-related research consortia have provided some guidance for patients enrolled in clinical trials with respect to anticipated deviations in sample acquisition, data reporting, and follow-up, as well as site visits by study moderators [120,121]. Such directions will need to anticipate disruptions caused by COVID-19 at the institutional level with respect to limitations on the clinical research office workforce. Most importantly, patient safety must be prioritized during the COVID-19 pandemic. To this end, central and institutional review boards will need to review protocol changes and offer suggestions [122].
ADDRESSING NEEDS OF PATIENTS AND HEALTHCARE PROVIDERS DURING COVID-19: A HOLISTIC CARE MODEL
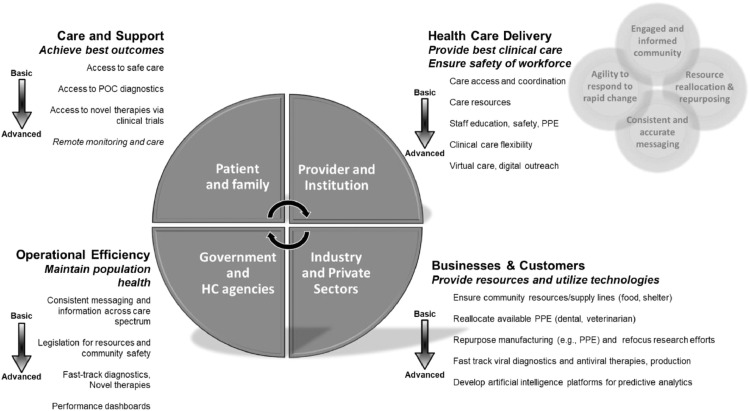
The COVID-19 pandemic requires a multitiered approach to address boots-on-the-ground and system-level needs of HCT recipients and healthcare providers [123,124]. To this end, a holistic care model has been proposed with 4 interdependent focus areas: HCT patients and families, healthcare providers and institutions, government and regulatory agencies, and industry and the private sector (Figure 2 ). This care model functions by each focus area identifying and prioritizing goals that address basic to advanced needs within and across areas then working together to achieve those goals. Four vital components are needed to ensure that the model functions effectively: (1) agility to address and respond to rapid changes inherent to the COVID-19 pandemic; (2) consistent and accurate messaging at government, state or region, and local levels of care to provide unified recommendations for directing the public and industry; (3) reallocation and repurposing of available resources at the federal and local government and private sector levels to provide essential needs, including but not limited to diagnostics, therapeutics, finances, and essential goods; and (4) engaged and informed communities that understand their personal health needs, as well as the needs of the healthcare community at large.
Figure 2.
Proposed holistic care model for patients and healthcare providers during COVID-19. To address the COVID-19 pandemic, a holistic care model is needed that addresses 4 key areas—patients and families, healthcare providers and institutions, government and regulatory agencies, and the industrial and private sectors—through interdependent collaboration. Each focus area must identify and prioritize goals that address basic to advanced needs within and across areas. Finally, 4 key components are needed to ensure functionality of the model: agility to respond to changing needs, consistent and accurate messaging, resource reallocation and repurposing, and an engaged and informed community. HC, healthcare; POC, point of care.
To illustrate how this model might work, patients need access to faster point-of-care diagnostics and novel forms of therapy and prevention during COVID-19 [125]. To address this need, government and healthcare agencies must provide fast-tracking for these diagnostics and therapies by working with industry to facilitate product testing and production. One form of government support could be incentivizing industry with a fast-track approval process. Likewise, healthcare institutions need to provide access to these products for patients, but they can only do so by ensuring a safe work environment for care providers. In response, reallocation of resources and repurposing of manufacturing for PPE production would be supported by the government that could incentivize industry to create PPE garments and masks through grants or tax breaks. The effectiveness of this model hinges on a supportive community that receives consistent and reliable information to perform the needed tasks and to provide the needed resources by acting as an inclusive workforce (government, business, and healthcare industry).
Facilitating system learning is essential for the model's success, as health care providers need to apply successful best practices across institutions facing the COVID-19 pandemic, particularly as resources become increasingly scarce. Therefore, developing a transplantation-specific learning network potentially could enable goal-directed practice changes through information assimilation and sharing. The learning network could function similarly to address some of the goals in each focus area.
LEARNING NETWORKS: QUALITY IMPROVEMENT INITIATIVES TO DEFINE BEST PRACTICES
The Institute of Medicine defines learning health systems as networks that align scientific and cultural tools, leading to knowledge generation to improve healthcare as a result of daily practice [126]. Specifically, learning health networks are multicenter collaborations consisting of healthcare providers, researchers, patients, and families aimed at driving healthcare innovation and improving outcomes [2,127]. Institutions and individuals engaged in a learning health network work together to solve complex problems impacting patient care by sharing best practices, data, and new knowledge efficiently in real time 128, 129, 130. Recent evidence demonstrates that collaborative learning health networks can achieve marked improvement in the quality of care [127,131, 132, 133, 134].
Characteristics and Examples of Learning Networks
Learning network leaders facilitate alignment of the community around a common goal and have standards, processes, policies, and infrastructure in place to enable collaboration [135,136]. They use a platform for sharing ideas and resources that includes best practice materials and pertinent tools to address the mission of the network [137]. Members of the network receive regular reports on network functioning and key process and outcome measures that correlate with the mission of the network.
The Partnership of HIV-Free Survival is a learning health network comprising 6 countries with the goal of improving the survival of infants born to mothers with HIV [138]. Through collaborative efforts, this network has demonstrated effective best practices mechanisms for preventing mother-to-child transmission [139]. The primary purpose for the 112 pediatric hospitals participating in the Children's Hospitals’ Solutions for Patient Safety collaborative is to eliminate serious healthcare-associated harm. From 2013 to 2019, by following best practices, approximately 14,000 children were protected from serious harm, with an estimated healthcare savings of $249.4 million and a consistent upward trend in harm prevented each month [140].
Transplant-Associated Learning Network Team
The Transplant-Associated Learning Network Team (TALNT), a learning network of the ASTCT, is a collaborative composed of transplantation and cellular therapy academic practitioners. The purpose of the TALNT is to improve the outcomes of adult and pediatric transplantation and cellular therapy recipients by building a sustainable collaborative network and operationalizing multi-institutional clinical, translational, and basic science research with quality improvement methodology to implement best practices.
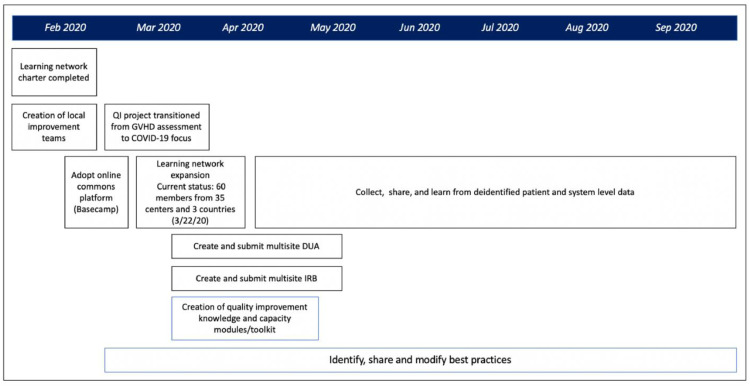
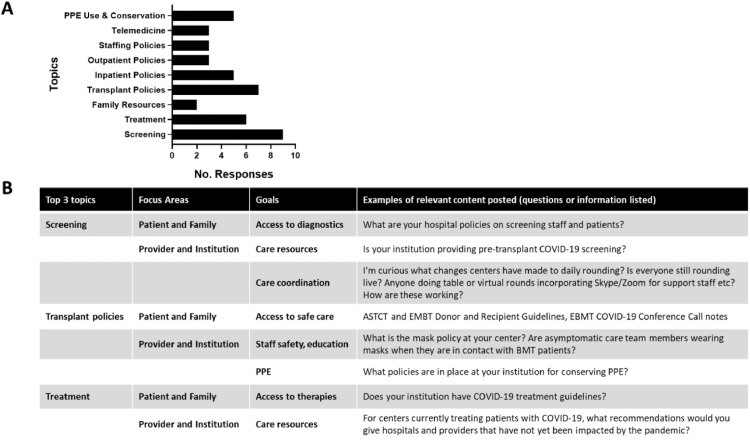
Although still in its infancy, the TALNT has expanded dramatically, coinciding with the impact of the COVID-19 pandemic (Figure 3 ). The network started with 16 members and now includes 102 individuals from 35 hospitals from Belgium, Brazil, China, Lithuania, Spain, and the United States. An online platform with rules for participation, posting, and participation contains nearly 100 COVID-19-related documents, along with a message board covering 35 topics, which includes timely recommendations and insight from our colleagues in China. Use has increased substantially, with an average of 20 posts per day, which are automatically summarized into daily “latest activity” by discussion category (eg, PPE conservation), with associated responses and documents posted. After responding to a survey defining initial focus areas (Figure 4 A), TALNT users are now providing substantive experiences, documents, and protocols relevant to focus areas and associated goals of the proposed holistic care model (Figure 4B). The network will continue its initial efforts on COVID-19, while longer-term goals include continued expansion, creation of best practices, and production of a quality improvement toolkit that includes online learning modules. In addition, the TALNT has the capacity to collect and share deidentified patient and systems-level data, and so learning among participants can translate into improved outcomes in HCT recipients.
Figure 3.
Timeline showing relevant activities of the TALNT, including membership profile and short-term and long-term goals. DUA, data use agreement; GVHD, graft-versus-host disease; IRB, institutional review board; QI, quality improvement.
Figure 4.
TALNT survey results and subsequent content shared through an online platform relevant to care model focus areas and goals. (A) TALNT membership survey results. Members were asked to rank which topics would be most helpful for addressing COVID-19. The top 3 topics became the focus for future interaction among the membership. (B) The top 3 topics and their relevance to focus areas and goals of the proposed holistic care model needed to confront the COVID-19 pandemic. Examples of content posted on the online platform are provided, including questions as well as publications.
We believe that learning networks can be transformative tools for the transplantation community given the COVID-19 pandemic. Our TALNT learning health network is aligned to decrease morbidity and mortality from COVID-19 in the HCT population and to optimize our healthcare delivery by enabling real-time collaboration and learning among practitioners and institutions.
CONCLUSIONS
COVID-19 has a significant impact in immunocompromised hosts, particularly HCT recipients, as well as HCT donors and medical caregivers. The present report extrapolates from previously published CoV data and experiences, summarizes current SARS-CoV-2 data, and offers both care considerations and recommendations based on current data. Ongoing modifications will be necessary with the increasing availability of data about COVID-19 in immunocompromised patients that will better inform individual and system-level care. Clinical personnel and transplantation centers must keep abreast of changing dynamics, evolving SARS-CoV-2 data, and governmental policies and recommendations within the context of their local epidemiology. Open collaboration and communication among institutional infectious disease, infection control, and prevention teams within transplantation centers and local and state health departments will be vital in providing optimal care to all patients, especially those at greatest risk for severe COVID-19.
REFERENCES
- 1.World Health Organization. Coronavirus disease (COVID-2019) situation reports. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
- 2.Britto M.T., Fuller S.C., Kaplan H.C. Using a network organisational architecture to support the development of Learning Healthcare Systems. BMJ Qual Saf. 2018;27:937–946. doi: 10.1136/bmjqs-2017-007219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Available at: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf
- 4.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan J.F., Kok K.H., Zhu Z. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J. Pathogenicity and transmissibility of 2019-nCoV–a quick overview and comparison with other emerging viruses. Microbes Infect. 2020;22:69–71. doi: 10.1016/j.micinf.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu F., Zhao S., Yu B. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan J.F., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Doremalen N., Bushmaker T., Morris D.H. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou L., Ruan F., Huang M. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2). Science. doi: 10.1126/science.abb3221. [e-pub ahead of print] [DOI] [PMC free article] [PubMed]
- 14.Bi Q, Wu Y, Mei S, et al. Epidemiology and transmission of COVID-19 in Shenzhen China: analysis of 391 cases and 1,286 of their close contacts. medRxiv. 2020. doi: 10.1101/2020.03.03.20028423. [DOI] [PMC free article] [PubMed]
- 15.Dietz K. The estimation of the basic reproduction number for infectious diseases. Stat Methods Med Res. 1993;2:23–41. doi: 10.1177/096228029300200103. [DOI] [PubMed] [Google Scholar]
- 16.Sanche S., Lin Y.T., Xu C., Romero-Severson E, Hengartner N., Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome Coronavirus 2. Emerg Infect Dis J. 2020:26:1-19. doi: 10.3201/eid2607.200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowell G., Nishiura H. Transmission dynamics and control of Ebola virus disease (EVD): a review. BMC Med. 2014;12:196. doi: 10.1186/s12916-014-0196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson N., Kvalsvig A., Barnard L.T., Baker M.G. Case-fatality risk estimates for COVID-19 calculated by using a lag time for fatality. Emerg Infect Dis. 2020:26. doi: 10.3201/eid2606.200320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omer SB, Malani P, Del Rio C. The COVID-19 pandemic in the US: a clinical update. JAMA. doi: 10.1001/jama.2020.5788. [e-pub ahead of print] [DOI] [PubMed]
- 20.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CDC COVID-19 Response Team Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [e-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fauci A.S., Lane H.C., Redfield R.R. Covid-19—navigating the uncharted. N Engl J Med. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of Covid-19—studies needed. N Engl J Med. 2020;382:1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 27.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. doi: 10.1007/s00134-020-05991. [DOI] [PMC free article] [PubMed]
- 28.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. doi: 10.1001/jama.2020.1585. [e-pub ahead of print] [DOI] [PMC free article] [PubMed]
- 29.Fan BE, Chong VCL, Chan SSW, et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. doi: 10.1002/ajh.25774. [e-pub ahead of print] [DOI] [PubMed]
- 30.Wang F, Nie J, Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. doi: 10.1093/infdis/jiaa150. [e-pub ahead of print] [DOI] [PMC free article] [PubMed]
- 31.Thevarajan I., Nguyen T.H.O., Koutsakos M. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med. 2020;26:453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. doi: 10.1093/cid/ciaa248. [e-pub ahead of print] [DOI] [PMC free article] [PubMed]
- 33.Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah D.P., Ghantoji S.S., Ariza-Heredia E.J. Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood. 2014;123:3263–3268. doi: 10.1182/blood-2013-12-541359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chemaly R.F., Shah D.P., Boeckh M.J. Management of respiratory viral infections in hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 2014;59(Suppl 5):S344–S351. doi: 10.1093/cid/ciu623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Y.J., Guthrie K.A., Waghmare A. Respiratory syncytial virus in hematopoietic cell transplant recipients: factors determining progression to lower respiratory tract disease. J Infect Dis. 2014;209:1195–1204. doi: 10.1093/infdis/jit832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogimi C., Englund J.A., Bradford M.C., Qin X., Boeckh M., Waghmare A. Characteristics and outcomes of coronavirus infection in children: the role of viral factors and an immunocompromised state. J Pediatric Infect Dis Soc. 2019;8:21–28. doi: 10.1093/jpids/pix093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eichenberger E.M., Soave R., Zappetti D. Incidence, significance, and persistence of human coronavirus infection in hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2019;54:1058–1066. doi: 10.1038/s41409-018-0386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dai W.C., Zhang H.W., Yu J. CT imaging and differential diagnosis of COVID-19. Can Assoc Radiol J. 2020;71:195–200. doi: 10.1177/0846537120913033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia, Radiology. 10.1148/radiol.2020200370. [e-pub ahead of print]. [DOI]
- 42.National Health Commission & State Administration of Traditional Chinese Medicine. Diagnosis and treatment protocol for novel coronavirus pneumonia. Released on March 3, 2020, trial version 7. WHO translation. https://www.chinalawtranslate.com/wp-content/uploads/2020/03/Who-translation.pdf
- 43.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized cases of COVID-2019. Nature. doi: 10.1038/s41586-020-2196. [e-pub ahead of print] [DOI] [PubMed]
- 44.To KK, Tsang OT, Chik-Yan Yip C, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. doi: 10.1093/cid/ciaa149. [e-pub ahead of print]. [DOI] [PMC free article] [PubMed]
- 45.Cai J, Xu J, Lin D, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. doi: 10.1093/cid/ciaa198. [e-pub ahead of print] [DOI] [PMC free article] [PubMed]
- 46.Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. doi: 10.1016/S1473-3099(20)30232-2. [e-pub ahead of print]. [DOI] [PMC free article] [PubMed]
- 47.Cheng M.P., Papenburg J., Desjardins M. Diagnostic testing for severe acute respiratory syndrome-related coronavirus-2: a narrative review. Ann Intern Med. April 13, 2020 doi: 10.7326/M20-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogels C.B.F., Brito A.F., Wyllie A.L. Analytical sensitivity and efficiency comparisons of SARS-COV-2 qRT-PCR assays. medRxiv. 2020 doi: 10.1101/2020.03.30.20048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. doi: 10.1148/radiol.2020200642. [e-pub ahead of print] [DOI] [PMC free article] [PubMed]
- 50.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu F, Wang A, Liu M, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv. doi: 10.1101/2020.03.30.20047365. [DOI]
- 52.Jiang S, Hillyer C, Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. doi: 10.1016/j.it.2020.03.007. [e-pub ahead of print] [DOI] [PMC free article] [PubMed]
- 53.Centers for Disease Control and Prevention. Coronavirus disease 2019: Travel. Available at: https://www.cdc.gov/coronavirus/2019-ncov/travelers/index.html.
- 54.Centers for Disease Control and Prevention. Coronavirus disease 2019: evaluating and reporting persons under investigation (PUI). Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html.
- 55.Centers for Disease Control and Prevention. Coronavirus disease 2019: Interim US guidance for risk assessment and public health management of persons with potential coronavirus disease 2019 (COVID-19) exposures: geographic risk and contacts of laboratory-confirmed cases. Available at: https://www.cdc.gov/coronavirus/2019-ncov/php/risk-assessment.html.
- 56.Centers for Disease Control and Prevention. Coronavirus disease 2019: Self-checker. Available at: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/index.html.
- 57.Centers for Disease Control and Prevention. Coronavirus disease 2019: Interim US guidance for risk assessment and public health management of healthcare personnel with potential exposure in a healthcare setting to patients with coronavirus disease (COVID-19). Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html.
- 58.US Food and Drug Administration. Coronavirus (COVID-19) update: FDA issues first emergency use authorization for point of care diagnostic. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-first-emergency-use-authorization-point-care-diagnostic.
- 59.Amant F., Stadlbauer D. A serological assay to detect SARS-CoV-2 seroconversion in humans. medRxiv. 2020 doi: 10.1101/2020.03.17.20037713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Centers for Disease Control and Prevention. Coronavirus disease 2019: Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html.
- 61.World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 is suspected. Available at: https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf?sfvrsn=bc7da517_2.
- 62.World Health Organization. Coronavirus disease (COVID-19) technical guidance: Patient management. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/patient-management.
- 63.Infectious Diseases Society of America. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19.Available at: https://www.idsociety.org/COVID19guidelines. [DOI] [PMC free article] [PubMed]
- 64.World Health Organization (WHO). WHO R&D Blueprint: Informal consultation on prioritization of candidate therapeutic agens for use in novel coronavirus 2019 infection. Available at: https://apps.who.int/iris/bitstream/handle/10665/330680/WHO-HEO-RDBlueprint%28nCoV%29-2020.1-eng.pdf.
- 65.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 66.World Health Organization. WHO launches global megatrial of the four most promising coronavirus treatments. Available at: https://www.sciencemag.org/news/2020/03/who-launches-global-megatrial-four-most-promising-coronavirus-treatments.
- 67.Mulangu S., Dodd L.E., Davey R.T., Jr A randomized, controlled trial of ebola virus disease therapeutics. N Engl J Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sheahan T.P., Sims A.C., Leist S.R. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. doi: 10.1056/NEJMoa2007016. [e-pub ahead of print] [DOI] [PMC free article] [PubMed]
- 71.Casadevall A., Pirofski L.A. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130:1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. doi: 10.1073/pnas.2004168117. [e-pub ahead of print] [DOI] [PMC free article] [PubMed]
- 74.Thevarajan I., Torresi J., Simmons C. Exploring the role of a recently licensed dengue vaccine in Australian travellers. Med J Aust. 2020;212:102–103. doi: 10.5694/mja2.50471. e1. [DOI] [PubMed] [Google Scholar]
- 75.Biopharma-reporter.com. Roche's treatment for coronavirus enters phase III. Available at: https://www.biopharma-reporter.com/Article/2020/03/19/Roche-enters-Phase-III-for-COVID-19-treatment.
- 76.Biopharma-reporter.com. Sanofi, Regeneron start late-stage trials for coronavirus treatment. Available at: https://www.biopharma-reporter.com/Article/2020/03/18/Sanofi-and-Regeneron-advance-candidate-against-coronavirus.
- 77.Auyeung T.W., Lee J.S., Lai W.K. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J Infect. 2005;51:98–102. doi: 10.1016/j.jinf.2004.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Centers for Disease Control and Prevention. COVID-19 prevention and treatment. Available at: https://www.cdc.gov/coronavirus/2019-ncov/about/prevention-treatment.html.
- 79.Centers for Disease Control and Prevention. Coronavirus disease 2019: Resources for healthcare professionals. Available at: https://www.cdc.gov/coronavirus/2019-ncov/healthcare-facilities/index.html.
- 80.Centers for Disease Control and Prevention. Handwashing: clean hands save lives. Available at: https://www.cdc.gov/handwashing/.
- 81.Centers for Disease Control and Prevention. Coronavirus disease 2019: Infection control guidance. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html.
- 82.Centers for Disease Control and Prevention COVID-19 Response Team. Characteristics of health care personnel with COVID-19—United States, February 12-April 9. MMWR Morb Mortal Wkly Rep. 2020;69:477-481. [DOI] [PMC free article] [PubMed]
- 83.Guo ZD, Wang ZY, Zhang SF, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. doi: 10.3201/eid2607.200885. [e-pub ahead of print] [DOI] [PMC free article] [PubMed]
- 84.Cheng VCC, Wong SC, Chen JHK, et al. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect Control Hosp Epidemiol. doi: 10.1017/ice.2020.5. [e-pub ahead of print] [DOI] [PMC free article] [PubMed]
- 85.Ong SWX, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed]
- 86.Centers for Disease Control and Prevention. Coronavirus disease 2019: Optimize PPE supply. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/index.html.
- 87.Cowling BJ, Aiello A.Public health measures to slow community spread of COVID-19. J Infect Dis. doi: 10.1093/infdis/jiaa123. [DOI] [PMC free article] [PubMed]
- 88.Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52:583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nature: News Explainer. Coronavirus vaccines: five key questions as trials begin. Available at: https://www.nature.com/articles/d41586-020-00798-8. [DOI] [PubMed]
- 90.Precision Vaccinations. Coronavirus vaccines. Available at: https://www.precisionvaccinations.com/vaccines/coronavirus-vaccines. Accessed March 23, 2020
- 91.Kim E, Erdos G, Huang S, et al. Microneedle array delivered recombinant coronavirus vaccines: immunogenicity and rapid translational development. EBioMedicine. doi: 10.1016/j.ebiom.2020.102743. [e-pub ahead of print] [DOI] [PMC free article] [PubMed]
- 92.Gottlieb S, Rivers C, McClellan MB, Silvis L, Watson C.; American Enterprise Institute. National coronavirus response: a roadmap to reopening. March 29, 2020. https://www.aei.org/wp-content/uploads/2020/03/National-Coronavirus-Response-a-Road-Map-to-Recovering-2.pdf.
- 93.Hon K.L., Leung C.W., Cheng W.T. Clinical presentations and outcome of severe acute respiratory syndrome in children. Lancet. 2003;361:1701–1703. doi: 10.1016/S0140-6736(03)13364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bitnun A., Allen U., Heurter H. Children hospitalized with severe acute respiratory syndrome-related illness in Toronto. Pediatrics. 2003;112:e261. doi: 10.1542/peds.112.4.e261. [DOI] [PubMed] [Google Scholar]
- 95.Li A.M., Ng P.C. Severe acute respiratory syndrome (SARS) in neonates and children. Arch Dis Child Fetal Neonatal Ed. 2005;90:F461–F465. doi: 10.1136/adc.2005.075309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu Z, McGoogan J.M.Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. doi: 10.1001/jama.2020.2648. [e-pub ahead of print] [DOI]
- 97.Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang ZJ. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. doi: 10.1001/jama.2020.2131. [DOI] [PMC free article] [PubMed]
- 98.Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. doi: 10.1542/peds.2020-0702. [e-pub ahead of print] [DOI]
- 99.Kam KQ, Yung CF, Cui L, et al. A well infant with coronavirus disease 2019 (COVID-19) with high viral load. Clin Infect Dis. doi: 10.1093/cid/ciaa201. [e-pub ahead of print] [DOI] [PMC free article] [PubMed]
- 100.Xu Y., Li X., Zhu B. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Centers for Disease Control and Prevention COVID-19 Response Team. Coronavirus disease 2019 in children—United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422-426. [DOI] [PMC free article] [PubMed]
- 102.Liu W., Zhang Q., Chen J. Detection of COVID-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020;382:1370–1371. doi: 10.1056/NEJMc2003717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lu X., Zhang L., Du H. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guillen E, Pineiro GJ, Revuelta I, et al. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? Am J Transplant. doi: 10.1111/ajt.15874. [e-pub ahead of print] [DOI] [PMC free article] [PubMed]
- 105.Zhu L, Xu X, Ma K, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. doi: 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed]
- 106.Huang J, Lin H, Wu Y, et al. COVID-19 in post-transplantation patients - report of two cases. Am J Transplant. doi: 10.1111/ajt.15896. [e-pub ahead of print] [DOI]
- 107.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed]
- 109.Balduzzi A, Brivio E, Rovelli A, et al. Lessons after the early management of the COVID-19 outbreak in a pediatric transplant and hemato-oncology center embedded within a COVID-19 dedicated hospital in Lombardia, Italy. Bone Marrow Transplant. doi: 10.1038/s41409-020-0895-4. [e-pub ahead of print] [DOI] [PMC free article] [PubMed]
- 110.American Society for Transplantation and Cellular Therapy. COVID-19 interim patient care guidelines. Available at: https://www.astct.org/connect/astct-response-to-covid-19.
- 111.European Society for Blood and Marrow Transplantation. Coronavirus disease COVID-19: EBMT recommendations update March 16, 2020. Available at: https://www.ebmt.org/sites/default/files/2020-03/EBMT%20COVID-19%20guidelines%20v.3.2%20%282020-03-16%29.pdf.
- 112.Shah N. Higher co-infection rates in COVID19. Available at: https://medium.com/@nigam/higher-co-infection-rates-in-covid19-b24965088333.
- 113.Be the Match. Response to COVID-19: up-to-date information for all Network partners on coronavirus impacts. Available at: https://network.bethematchclinical.org/news/nmdp/be-the-match-response-to-covid-19/.
- 114.US Food and Drug Administration. Important information for blood establishments regarding the novel coronavirus outbreak. Available at: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/important-information-blood-establishments-regarding-novel-coronavirus-outbreak.
- 115.AABB. Update: Impact of 2019 novel coronavirus and blood safety. Available at: http://www.aabb.org/advocacy/regulatorygovernment/Documents/Impact-of-2019-Novel-Coronavirus-on-Blood-Donation.pdf.
- 116.Chang L, Zhao L, Gong H, Wang L, Wang L. Severe acute respiratory syndrome coronavirus 2 RNA detected in blood donations. Emerg Infect Dis. doi: 10.3201/eid2607.200839. [e-pub ahead of print] [DOI] [PMC free article] [PubMed]
- 117.American Red Cross. Red Cross media statement on coronavirus disease 2019. Available at: https://www.redcross.org/about-us/news-and-events/press-release/2020/red-cross-media-statement-on-2019-novel-coronavirus.html.
- 118.American Society of Health System Pharmacists. ASHP COVID-19 resource center. Available at: https://www.ashp.org/Pharmacy-Practice/Resource-Centers/Coronavirus.
- 119.US Food and Drug Administration. FDA drug shortages. Available at: https://www.accessdata.fda.gov/scripts/drugshortages/default.cfm.
- 120.CIRB for the National Cancer Institute. Memorandum on Interim guidance for patients on clinical trials supported by the NCI Cancer Therapy Evaluation Program and the NCI Community Oncology Research Program (NCORP). Available at: https://www.ncicirb.org/announcements/memorandum-interim-guidance-patients-clinical-trials-supported-nci-cancer-therapy.
- 121.Horowitz M, Devine S, National Marrow Donor Program (NMDP) Mendizabal A.BMT CTN Responses to the COVID-19 Pandemic. March 20, 2020.
- 122.King RJ. Memo COVID-001: National Marrow Donor Program (NMDP) IRB Guidance for Research Protocols Impacted by COVID-19. March 20, 2020.
- 123.Szer J, Weisdorf D, Querol S, Foeken L, Madrigal A.The impact of COVID-19 on the provision of donor hematopoietic stem cell products worldwide: collateral damage. Bone Marrow Transplant. doi: 10.1038/s41409-020-0873. [DOI] [PMC free article] [PubMed]
- 124.Ueda M, Martins R, Hendrie PC, et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward a common goal. J Natl Compr Canc Netw. doi: 10.6004/jnccn.2020.7560. [e-pub ahead of print] [DOI] [PubMed]
- 125.Lurie N, Saville M, Hatchett R, Halton J.Developing Covid-19 vaccines at pandemic speed. N Engl J Med. doi: 10.1056/NEJMp2005630. [e-pub ahead of print] [DOI] [PubMed]
- 126.Grossman C., Powers B., Mcginnis J.M., editors. Digital Infrastructure for the Learning Health System: The Foundation for Continuous Improvement in Health and Health Care. Institute of Medicine; Washington, DC: 2011. [PubMed] [Google Scholar]
- 127.Seid M., Dellal G., Peterson L.E. Co-designing a Collaborative Chronic Care Network (C3N) for inflammatory bowel disease: development of methods. JMIR Hum Factors. 2018;5:e8. doi: 10.2196/humanfactors.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang Y. Real-time development of patient-specific alarm algorithms for critical care. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:4351–4354. doi: 10.1109/IEMBS.2007.4353300. [DOI] [PubMed] [Google Scholar]
- 129.Margolis P.A., Peterson L.E., Seid M. Collaborative Chronic Care Networks (C3Ns) to transform chronic illness care. Pediatrics. 2013;131(suppl 4):S219–S223. doi: 10.1542/peds.2012-3786J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Forrest C.B., Margolis P., Seid M., Colletti R.B. PEDSnet: how a prototype pediatric learning health system is being expanded into a national network. Health Aff (Millwood) 2014;33:1171–1177. doi: 10.1377/hlthaff.2014.0127. [DOI] [PubMed] [Google Scholar]
- 131.Gaur A.H., Bundy D.G., Werner E.J. A prospective, holistic, multicenter approach to tracking and understanding bloodstream infections in pediatric hematology-oncology patients. Infect Control Hosp Epidemiol. 2017;38:690–696. doi: 10.1017/ice.2017.57. [DOI] [PubMed] [Google Scholar]
- 132.Bundy D.G., Gaur A.H., Billett A.L., He B., Colantuoni E.A., Miller M.R. Preventing CLABSIs among pediatric hematology/oncology inpatients: national collaborative results. Pediatrics. 2014;134:e1678–e1685. doi: 10.1542/peds.2014-0582. [DOI] [PubMed] [Google Scholar]
- 133.Wong Quiles C.I., Gottsch S., Thakrar U., Fraile B., Billett A.L. Health care institutional charges associated with ambulatory bloodstream infections in pediatric oncology and stem cell transplant patients. Pediatr Blood Cancer. 2017;64:324–329. doi: 10.1002/pbc.26194. [DOI] [PubMed] [Google Scholar]
- 134.Linam W.M., Margolis P.A., Atherton H., Connelly B.L. Quality-improvement initiative sustains improvement in pediatric health care worker hand hygiene. Pediatrics. 2011;128:e689–e698. doi: 10.1542/peds.2010-3587. [DOI] [PubMed] [Google Scholar]
- 135.Lannon C.M., Peterson L.E. Pediatric collaborative networks for quality improvement and research. Acad Pediatr. 2013;13(6 suppl):S69–S74. doi: 10.1016/j.acap.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 136.Lannon C.M., Peterson L.E. Pediatric collaborative improvement networks: background and overview. Pediatrics. 2013;131(suppl 4):S189–S195. doi: 10.1542/peds.2012-3786E. [DOI] [PubMed] [Google Scholar]
- 137.Aaboud M., Aad G., Abbott B. Search for doubly charged scalar bosons decaying into same-sign W boson pairs with the ATLAS detector. Eur Phys J C Part Fields. 2019;79:58. doi: 10.1140/epjc/s10052-018-6500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Webster P.D., Deka S., Ismail A., Stern A.F., Barker P.M. Using a multicountry learning network to harvest and rapidly spread implementation knowledge across programs aimed to reduce mother-to-child transmission of HIV and improve nutrition: perspectives and lessons learned for similar large-scale initiatives. J Int Assoc Provid AIDS Care. 2019;18 doi: 10.1177/2325958219847452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Barker P., Quick T., Agins B., Rollins N., Sint T.T., Stern A.F. A 6-country collaborative quality improvement initiative to improve nutrition and decrease mother-to-child transmission of HIV in mother-infant pairs. J Int Assoc Provid AIDS Care. 2019;18 doi: 10.1177/2325958219855625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Children's Hospitals’ solutions for patient safety. Available at: https://www.solutionsforpatientsafety.org.
- 141.Yao TT, Qian JD, Zhu WY, Wang Y, Wang GQ. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-a possible reference for coronavirus disease-19 treatment option. J Med Virol. doi: 10.1002/jmv.25729. [e-pub ahead of print] [DOI] [PMC free article] [PubMed]
- 142.Chu C.M., Cheng V.C., Hung I.F. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Park S.Y., Lee J.S., Son J.S. Post-exposure prophylaxis for Middle East respiratory syndrome in healthcare workers. J Hosp Infect. 2019;101:42–46. doi: 10.1016/j.jhin.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. doi: 10.1056/nejmoa2001282. [e-pub ahead of print]. [DOI] [PMC free article] [PubMed]
- 145.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19-associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 146.Colson P., Rolain J.M., Lagier J.C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Falzarano D., de Wit E., Rasmussen A.L. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19:1313–1317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Falzarano D., de Wit E., Martellaro C., Callison J., Munster V.J., Feldmann H. Inhibition of novel β coronavirus replication by a combination of interferon-α2b and ribavirin. Sci Rep. 2013;3:1686. doi: 10.1038/srep01686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gamiño-Arroyo A.E., Guerrero M.L., McCarthy S. Efficacy and safety of nitazoxanide in addition to standard of care for the treatment of severe acute respiratory illness. Clin Infect Dis. 2019;69:1903–1911. doi: 10.1093/cid/ciz100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Haverstick S., Goodrich C., Freeman R., James S., Kullar R., Ahrens M. Patients' hand washing and reducing hospital-acquired infection. Crit Care Nurse. 2017;37:e1–e8. doi: 10.4037/ccn2017694. [DOI] [PubMed] [Google Scholar]
- 151.Hummel A.T., Vleck K., Greenough W.B.3r.d. A quality improvement initiative for improving hospital visitor hand hygiene. J Hosp Infect. 2019;101:422–423. doi: 10.1016/j.jhin.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 152.Segal S., Harris H.M., Gunawan A., Schumann R. A simple method for estimating hand hygiene use among anesthesia personnel: development, validation, and use in a quality improvement project. Anesth Analg. 2019;129:1549–1556. doi: 10.1213/ANE.0000000000004106. [DOI] [PubMed] [Google Scholar]
- 153.Rosenbluth G., Garritson S., Green A.L. Achieving hand hygiene success with a partnership between graduate medical education, hospital leadership, and physicians. Am J Med Qual. 2016;31:577–583. doi: 10.1177/1062860615596567. [DOI] [PubMed] [Google Scholar]
- 154.Scherer A.M., Reisinger H.S., Goto M. Testing a novel audit and feedback method for hand hygiene compliance: a multicenter quality improvement study. Infect Control Hosp Epidemiol. 2019;40:89–94. doi: 10.1017/ice.2018.277. [DOI] [PubMed] [Google Scholar]
- 155.Pronovost P.J., Berenholtz S.M., Goeschel C.A. Creating high reliability in health care organizations. Health Serv Res. 2006;41(4 Pt 2):1599–1617. doi: 10.1111/j.1475-6773.2006.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Benneyan J.C., Lloyd R.C., Plsek P.E. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458–464. doi: 10.1136/qhc.12.6.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Institute of Medicine. Committee on Quality of Health Care in America . National Academies Press; Washington, DC: 2001. Crossing the Quality Chasm: A New Health System for the 21st Century. [Google Scholar]
- 158.Langley G.J., Moen R.D., Nolan K.M., Nolan T.W., Norman C.L., Provost L.P. 2nd ed. Jossey-Bass; San Francisco, CA: 2009. The Improvement Guide: a Practical Approach to Enhancing Organizational Performance. [Google Scholar]
- 159.Institute for Healthcare Improvement. Number of self-reported medication errors. Available at: http://www.ihi.org/resources/Pages/Measures/NumberofSelfReportedMedicationErrors.aspx.
- 160.Singh I., Goorah N., Singh S., Hughes B. Working together for a just culture. Br J Hosp Med (Lond) 2019;80:562–563. doi: 10.12968/hmed.2019.80.10.562. [DOI] [PubMed] [Google Scholar]