Abstract
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2, has affected virtually all aspects of patient care. Health-care systems around the world are trying simultaneously to treat patients with COVID-19, prepare for its long-term impacts, and treat patients with other acute and chronic diseases. There are multiple ways that the COVID-19 pandemic will directly affect patients with fibrotic interstitial lung disease (ILD), particularly given their common risk factors for poor outcomes. Major issues for patients with ILD will include restricted access to key components of the diagnostic process, new uncertainties in the use of common ILD pharmacotherapies, limited ability to monitor both disease severity and the presence of medication adverse effects, and significantly curtailed research activities. The purpose of this review is to summarize how COVID-19 has impacted key components of the diagnosis and management of fibrotic ILD as well as to provide strategies to mitigate these challenges. We further review major obstacles for researchers and identify priority areas for future ILD research related to COVID-19. Our goals are to provide practical considerations to support the care of patients with ILD during the COVID-19 pandemic and to provide a road map for clinicians caring for these patients during future infectious disease outbreaks.
Key Words: coronavirus disease, diagnosis, interstitial lung disease, treatment
Abbreviations: ACE-2, angiotensin-converting enzyme 2; COVID-19, coronavirus disease 2019; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; MDD, multidisciplinary discussion; PFT, pulmonary function test; PPE, personal protective equipment; RT-PCR, reverse transcription-polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SLB, surgical lung biopsy
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), rapidly escalated to a pandemic in the span of 2 months and has compromised health-care systems around the world. There is rationing of health-care resources such as coronavirus test kits and personal protective equipment (PPE) in many health regions. Health care for all patients has been affected, including decreased access to primary care and specialist physicians, as well as limited access to investigations, procedures, and elective surgeries. Lack of access to critical resources, most notably ventilators, has directly contributed to patient deaths.1
COVID-19 has similarly impacted all aspects of care for patients with interstitial lung disease (ILD), including essential components of the diagnostic process, with further implications for the initiation, maintenance, and monitoring of ILD therapy. The objective of this review is to discuss practical considerations for the diagnosis and management of fibrotic ILD during the COVID-19 pandemic, with a secondary purpose to describe the impact of the COVID-19 pandemic on clinical and translational research activities. This is a rapidly evolving situation, with limited evidence to guide specific recommendations. We therefore focused on general practical considerations, with the intent that these apply to health-care settings with substantial risk of community spread and will be modified as further evidence on COVID-19 is generated.
Diagnosis
The diagnosis of ILD requires integration of clinical, radiologic, and pathologic information that is best accomplished through face-to-face multidisciplinary discussion at an experienced ILD center.2 The standard approach to diagnosis includes a history and physical examination, blood work (eg, autoimmune serologies), pulmonary function tests (PFTs), high-resolution CT imaging of the lungs, and possibly bronchoscopy and/or surgical lung biopsy. The median delay in diagnosis from symptom onset is typically over 1 year,3 , 4 which will likely worsen with reduced access to diagnostic tests during the COVID-19 pandemic.
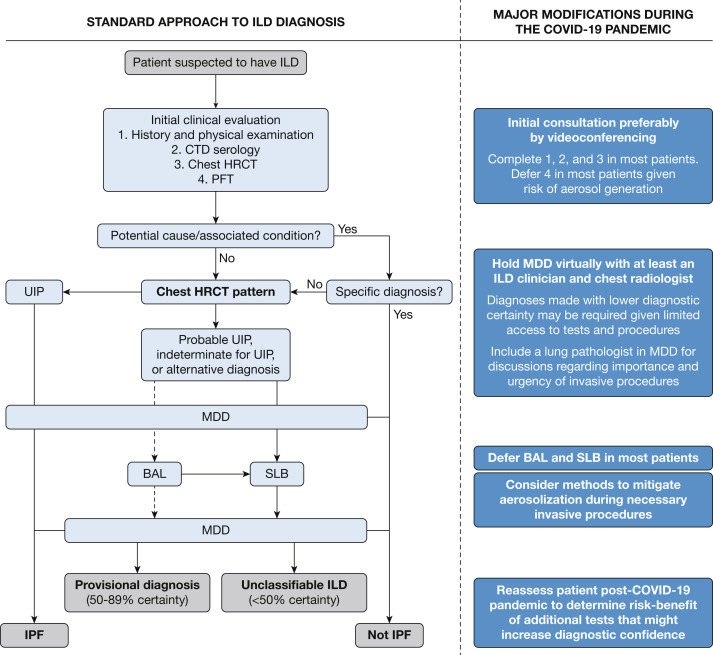
Several modifications are appropriate for an initial ILD assessment in the context of the COVID-19 pandemic (Fig 1 ),5 with the overarching goal of minimizing delays in diagnosis and management while also avoiding nonessential contact with the health-care system. In areas with confirmed or anticipated community spread of COVID-19, it is appropriate to minimize in-person contact between health-care workers and patients for each component of an initial ILD assessment. For example, patients should obtain blood work at outpatient laboratories during off-peak hours, make online reservations to minimize time in the waiting room, and use mobile laboratory services, if available. Most hospital laboratories have closed or reduced access for outpatients, and are best avoided if there is a feasible alternative. PFTs have a higher risk of spreading infection to both patients and health-care workers compared with many diagnostic tests, and most PFT laboratories have therefore restricted access. PFTs are likely still appropriate in some situations, but it is essential to ensure that the results of a PFT will impact patient treatment and include only the components required (eg, spirometry or diffusing capacity of the lungs for carbon monoxide only).6 For example, if all surgeries, including lung cancer resection, have been postponed due to critical resource constraints, then postponing a presurgical PFT would be justified. Routine PFTs for patients with symptomatically stable disease are not appropriate in regions with a high community burden of COVID-19. Imaging studies should similarly be performed only when these are needed to inform short-term management decisions.
Figure 1.
Proposed algorithms for standard and COVID-19-modified approach to ILD diagnosis. Standard approach adapted with permission from Raghu et al.5 Adapted with permission of the American Thoracic Society. Copyright © 2020 American Thoracic Society. All rights reserved. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society. Readers are encouraged to read the entire article for the correct context at https://doi.org/10.1164/rccm.201807-1255ST. The authors, editors, and The American Thoracic Society are not responsible for errors or omissions in adaptations. COVID-19 = coronavirus disease 2019; CTD = connective tissue disease; HRCT = high-resolution CT; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis; MDD = multidisciplinary discussion; PFT = pulmonary function test; SLB = surgical lung biopsy; UIP = usual interstitial pneumonia.
Decisions to pursue more invasive tests such as bronchoscopy or surgical lung biopsy (SLB) are more difficult in the context of the COVID-19 pandemic given the infection control concerns; however, the same principle holds that these tests should be performed if there is a reasonable likelihood that their results will directly inform urgent management decisions. Ideally, this assessment is based on a virtual multidisciplinary discussion (MDD) that specifically considers whether the result of this test is expected to impact short-term management decisions.7 If a patient already has a confident diagnosis (> 90% certainty), then further testing is unlikely to alter management decisions and should therefore not be pursued. In patients with a provisional diagnosis (50%-89% certainty), it is sometimes appropriate to pursue additional investigations; however, this threshold has likely shifted in the context of the COVID-19 pandemic, and it may be appropriate to observe or consider empiric treatment for such patients with a working diagnosis. In such patients, it is important to ensure that appropriate diagnostic confidence has been reached and to complete necessary additional studies if required after the COVID-19 pandemic resolves. Patients with an uncertain diagnosis (< 50% certainty) could be observed if stable, empirically treated, or considered for more invasive diagnostic procedures if available and urgently required for important short-term therapeutic decisions.
The potential diagnostic usefulness of bronchoscopy and SLB should always be weighed against their possible complications, with additional specific considerations during the COVID-19 pandemic. There are several known risk factors for poor outcomes following invasive procedures,8 , 9 particularly for SLB. These risks are likely amplified in the context of the COVID-19 pandemic, with a shift in the safety threshold for some of these procedures. For example, SLB is typically not pursued in patients > 75 years old,8 but a younger age threshold may now be more appropriate given the more dramatic association of increased age with poor outcomes with COVID-19.10 , 11 There is also a risk of health-care workers being exposed to infectious patients during these aerosol-generating medical procedures, which is of particular concern given the demonstration of viral transmission when patients have mild or no symptoms.12, 13, 14 Given these risks, most regions with community spread of COVID-19 have limited both bronchoscopy and SLB to patients who require the performance of these tests within the next 4 to 12 weeks. Such patients should have symptomatic and temperature screening to identify mild COVID-19,15 and ideally would undergo COVID-19 testing 1 to 2 days before the procedure.
Treatment
The complete pathogenesis of SARS-CoV-2 is unknown. SARS-CoV-2 predominantly invades alveolar epithelial cells by binding to the angiotensin-converting enzyme 2 (ACE-2) receptor and then replicating within cells. New viral particles are then released and activate the cellular and humoral immune response.16 Immune effector cells also release many cytokines and chemokines that can result in a cytokine storm and subsequent ARDS.16 , 17 This pathogenesis has implications for both immunomodulatory and antifibrotic medications in patients with underlying ILD.
Immunomodulatory Medications
Prednisone, mycophenolate mofetil, azathioprine, cyclophosphamide, and rituximab are immunomodulatory medications often used to treat ILD. There are limited data on the impact these therapies have during infectious disease outbreaks; however, common community-acquired respiratory viruses such as adenovirus, rhinovirus, and influenza can result in more severe disease in immunocompromised patients.18 Despite this concern, immunosuppressed patients did not appear to be at higher risk of severe illness during past coronavirus outbreaks, including severe acute respiratory syndrome in 2002 and Middle East respiratory syndrome in 2013.19 Immunosuppressed patients similarly do not appear to have an increased risk for or severity of COVID-19,19 with the exception that corticosteroids may be harmful in early stages of infection.20 , 21 This has raised the question of whether severe COVID-19 is in part related to an exaggerated immune response.22, 23, 24 Given the absence of robust data, it remains unknown if there are unique considerations for the initiation or maintenance of immunomodulatory therapies for patients with fibrotic ILD during the COVID-19 pandemic.
In this context of limited data, a balanced approach to the initiation of immunomodulatory therapy is appropriate. In patients with progressive disease who would typically be offered immunomodulatory therapy, it remains reasonable to use such therapies in most patients, preferably limiting the use of corticosteroids and prioritizing steroid-sparing therapies where possible. There are no data to guide dosing of immunosuppressive therapies in the context of the COVID-19 pandemic, and a moderate approach is also reasonable until further data are available. Treatment initiation should be considered on a case-by-case basis, and it may be appropriate to delay initiation of nonurgent therapy if there is limited likelihood of short-term progression.
For patients already receiving immunomodulatory therapy, there are again limited data on whether to continue current doses, consider dose reduction, or discontinue therapy. Early or overly rapid taper of therapy may result in a flare of the underlying disease, particularly in patients with connective tissue disease, which can subsequently precipitate the need for more aggressive immunosuppression or hospitalization to regain disease control. For this reason, it is likely most appropriate to maintain patients on at least low doses of immunomodulatory therapy, again prioritizing steroid-sparing medications over prednisone.
Antifibrotic Medications
Multiple additional considerations influence decisions to start antifibrotic medications during the COVID-19 pandemic. There is no evidence that antifibrotic therapies (nintedanib and pirfenidone) impact the risk or severity of COVID-19. However, there is overlap between adverse effects of these medications and symptoms of COVID-19 (eg, diarrhea, fatigue, loss of appetite), which can confound early identification and lead to worse manifestations of COVID-19. In addition, many regions have criteria for medication coverage or reimbursement that include PFT values (eg, FVC > 50%) and/or confirmation of an idiopathic pulmonary fibrosis (IPF) diagnosis via MDD, which were major inclusion criteria in previous clinical trials.25, 26, 27 The current difficulty in obtaining PFTs and conducting MDDs in places with a high burden of COVID-19 will limit fulfillment of these criteria. Some health authorities have consequently modified eligibility, forgoing or extending deadlines for completion of PFTs and allowing alternative methods of diagnostic confirmation. This approach is supported by the consistent benefits of antifibrotic therapy across severities of IPF and in patients with non-IPF fibrotic ILD,28, 29, 30, 31, 32, 33, 34, 35 suggesting that temporary loosening of previous criteria may be preferable to restricting medication access.
Monitoring of Pharmacologic Therapies
An additional consideration in the initiation or continuation of therapy is the need for ongoing blood-work monitoring for many ILD medications, and specifically the impact that this monitoring will have on physical isolation. In patients receiving chronic ILD therapy, it may be appropriate to decrease monitoring frequency during the COVID-19 pandemic to minimize patient contact with the health-care system, while still ensuring that patients receive appropriate medical care. In patients starting on ILD therapy, the potential for significant complications (eg, hepatotoxicity) will mandate blood-work monitoring on a regular basis over the short term. Given the potential for patients with COVID-19 to have no or minimal symptoms, it is possible that incidental detection of lymphopenia may be the first finding during the acute phase of infection, which may also confound or exacerbate lymphopenia that can be attributed to immunomodulatory therapy.36 Routine PFTs for the purpose of ensuring ongoing medication coverage (ie, “stopping” criteria) are not justified in areas with a high burden of COVID-19 and should be deferred, and symptomatic stability should instead be used as evidence of disease stability.
Nonpharmacologic Therapies and Supports
Physical (social) distancing is critical, with many viable strategies (Table 1 ). Patients should be encouraged to continue nonpharmacologic therapies such as exercise and smoking cessation. The provision of home oxygen should not be delayed for patients who require continuous oxygen. Some oxygen programs have stopped providing oxygen for patients with isolated exertional hypoxemia; however, the more severe exertional desaturation in fibrotic ILD compared with other chronic lung diseases may warrant exemption from such restrictions in some patients.37 Many ILD programs have clinical nurse specialists, who remain crucial in addressing patient concerns and supporting ongoing management at home, thus potentially reducing unnecessary emergency room visits. Patients should also be encouraged to maintain social support through virtual means (eg, online patient support groups or phone/video calls with friends and family).
Table 1.
Summary of Strategies Used to Reduce Potential Exposure to COVID-19 for Patients With Fibrotic Interstitial Lung Disease
| Physical distancing |
| • Advise patients to follow local public health recommendations and to stay informed, using credible resources |
| • Use telephone and/or video appointments whenever feasible, including with ILD nursing support if available |
| • Use family/friend/professional assistance for delivery services of groceries and pharmaceuticals; maintain 2-m (6.5-ft) distance from others when leaving the home |
| • Encourage ongoing social engagement with family and online support groups via phone or video |
| • Advise patients to remain active at home and to avoid deconditioning, potentially using online patient resources for exercises that can be done safely at home |
| Hygiene practices |
| • Emphasize importance of frequent hand washing (20 s with soap and warm water) and not touching their face if they must leave their home |
| • Disinfect frequently touched surfaces and products brought into the home (eg, deliveries) |
| • Wash hands after handling delivered goods to avoid transmission from contaminated surfaces |
| • Using a mask is of uncertain benefit/risk and is not an adequate replacement for appropriate hand hygiene and physical distancing measures |
| Investigations |
| • Defer nonessential blood work |
| • Consider less frequent routine blood-work monitoring and/or use of scheduled and/or mobile laboratory services if available |
| • Avoid nonurgent pulmonary function testing and ensure proper decontamination of equipment if performed |
| • Avoid nonurgent imaging including chest radiography and CT imaging |
| • Avoid procedures that would not change immediate treatment or could result in hospitalization (bronchoscopy and surgical lung biopsy) |
| Treatment |
| • Support virtual pulmonary rehabilitation and educational initiatives |
| • Consider short-term elimination of need for repeat testing of oxygen supplementation criteria |
| • Consider how COVID-19 impacts risks and benefits of initiating or continuing ILD therapies |
| • Modify drug eligibility and funding criteria to reflect limited access to pulmonary function tests and multidisciplinary review |
| • Consider early discussions on advanced care directives and end-of-life planning, with referral to palliative care services when appropriate |
COVID-19 = coronavirus disease 2019; ILD = interstitial lung disease.
Lung transplantation can be a life-saving procedure for patients with end-stage ILD; however, there are several specific considerations regarding lung transplant during the COVID-19 pandemic. From the standpoint of the recipient, there are significant risks including the risk of acquiring COVID-19 in the postoperative period and reduced access to essential monitoring of posttransplant blood work, spirometry, and bronchoscopy. From the perspective of health-care systems, lung transplants use critical health-care resources such as operating room time, critical care beds, PPE, and health-care worker time. For these reasons, many programs are choosing to postpone all but the most urgent transplants.
COVID-19 in Patients With ILD
The majority of patients with COVID-19 (81%) present with mild symptoms (fever, cough, and dyspnea), while 14% have respiratory distress and hypoxemia, and 5% will develop respiratory failure.38 It is unknown whether patients with ILD have different or more severe manifestations. Patients with ILD who notice a new fever or mild change in respiratory symptoms should have a lower threshold than the general population for assessment, potentially using telemedicine to determine whether an emergency room visit is necessary. Urgent medical attention should be sought for patients with ILD who have more than mild symptoms or objectively worsened features (eg, decline in oxygenation if home pulse oximetry is available). Patients with risk factors for severe COVID-19 (eg, older age, cardiovascular disease, and diabetes)10 , 11 should have a lower threshold for a more comprehensive assessment of COVID-19 and for other causes of respiratory worsening.39 In particular, thrombosis should be considered given the increased risk of coagulopathy with COVID-19 and ILD.40 , 41
With currently available technologies, the diagnosis of COVID-19 often requires multiple tests, and sometimes sampling of multiple anatomic sites or supplementation with chest imaging findings.39 A study of 1,070 specimens found that the detection of SARS-CoV-2 by RT-PCR was highest in BAL fluid (14 of 15, 93%), sputum (75 of 104, 72%), and nasal swabs (5 of 8, 63%).42 In another study, the combination of pharyngeal swab RT-PCR and CT imaging for detecting COVID-19 was also highly sensitive (91.9%).43 Given the imperfect sensitivity of nasal swabs in isolation,42 , 44 COVID-19 should remain in the differential diagnosis for patients without a clearly identified alternative etiology, even with a negative initial RT-PCR result.
There are limited data to support routine use of systemic corticosteroids in mechanically ventilated patients with COVID-19 who have ARDS.45 An uncontrolled case series of 84 patients with ARDS secondary to COVID-19 reported a decreased risk of death in those treated with methylprednisolone (hazard ratio, 0.38; 95% CI, 0.20-0.72).10 However, early use of hydrocortisone was associated with higher plasma viral load and delayed viral clearance for severe acute respiratory syndrome,46 with similar results in Middle East respiratory syndrome.47 There is also little evidence to guide the use of corticosteroids during an acute exacerbation of ILD, although these are prescribed by the majority of clinicians.48 Given the available data, it seems prudent to avoid early use of corticosteroids in patients with ILD and COVID-19; however, corticosteroids are likely appropriate in the presence of ongoing inflammatory findings on chest imaging after resolution of the initial infection. The risks of delayed virus clearance, secondary infections, and drug interactions will lead to temporary discontinuation of other immunomodulatory therapies in most patients during acute COVID-19. Other medications are being investigated (eg, hydroxychloroquine, IV immunoglobulin, tocilizumab, remdesivir); however, these are currently of uncertain usefulness and should be used primarily in the context of a clinical trial.
Low lung volume and low driving pressure ventilation strategies are typically used for mechanically ventilated patients with ARDS or an acute exacerbation of ILD. The use of higher positive end-expiratory pressure (> 10 cm H2O), with close monitoring for barotrauma, is recommended in COVID-1945; however, there is concern that this could worsen hemodynamic impairment.49 A higher positive end-expiratory pressure strategy is associated with increased 1-year mortality (hazard ratio, 4.72; 95% CI, 2.06-11.15) in patients with fibrotic ILD and is preferably avoided.50 The in-hospital mortality for patients with an exacerbation of IPF is estimated at 50%51; while the mortality rate for patients with COVID-19 requiring mechanical ventilation has ranged from 61%52 to as high as 97%.11 These data suggest that mechanical ventilation in patients with fibrotic ILD and concurrent COVID-19 may not be appropriate, particularly when urgent lung transplantation is not an option or when resources are significantly constrained. Establishing an advanced care directive while patients are stable is exceedingly important to ensure that physicians and family members make decisions that are aligned with patient values.
Research in ILD During the COVID-19 Pandemic
The COVID-19 pandemic has significantly impacted the conduct of clinical trials (Table 2 ). Research centers in areas with a high burden of community spread of COVID-19 are no longer opening new clinical trials, while ongoing trials are adapting to ensure patient safety while attempting to maintain trial integrity. As with clinical care, the major change is movement to virtual study assessments and suspension of noncritical efficacy testing that requires in-person patient contact with the health-care system. Most study sponsors have provided clear protocol modifications to maintain consistency across study sites and indicate the priority of patient safety. As a result, most studies are no longer enrolling new patients, limiting access to novel therapies that are available only through clinical trials. This will also delay completion of clinical trials and slow translation of findings into the clinical setting. An additional important impact of clinical trial interruption is the decreased revenue to study sites and compromised ability to retain salary support for key personnel.
Table 2.
Impact of the COVID-19 Pandemic on Interstitial Lung Disease Research
| Impact | Potential Strategies for Mitigation |
|---|---|
| Work-from-home mandates for nonessential employees |
|
| Restrictions on in-person study visits |
|
| Interrupted recruitment |
|
| Reduced access to study medications |
|
| Decreased clinical trial revenue and risk to research staff salaries |
|
| Cancelled/postponed grant competitions |
|
See Table 1 legend for expansion of abbreviation.
Investigator-led research (eg, ILD registries, translational research) has also been suspended in most cases, resulting in a period of missing data and dropouts that will complicate future analyses. Access to awards and grants may be delayed, and use of previous funding will similarly be limited for most research. Strategies to support remote work for research staff are important to mitigate lost productivity and to support ongoing career development for trainees and young faculty. This may necessitate modifying short-term research priorities that can be advanced or even fully completed remotely, as well as establishment of virtual lab meetings that support dynamic and collaborative discussions that advance research ideas.
There are significant uncertainties on how COVID-19 will specifically impact patients with ILD, and many research ethics boards have acknowledged the importance and urgency of these research questions by expediting reviews of such proposals (Table 3 ).53 , 54 A better understanding of the risk factors and disease course for severe COVID-19 in ILD is needed to guide management and support informed decisions for physicians and patients. In particular, there is uncertainty about the significance of the ACE-2 receptor and the role of ACE inhibitors in treating patients with COVID-19.55 , 56 The ACE-2 receptor appears to be protective against some types of pulmonary fibrosis,53 but is also used by SARS-CoV-2 to enter cells. Considering these effects, it is unknown if ACE-2 receptors impact susceptibility to SARS-CoV-2 infection in ILD, and whether this susceptibility might be different across ILD subtypes (eg, sarcoidosis) or genetic/racial backgrounds. Similarly, the generation of data to better inform decisions on immunomodulatory therapy is also urgently required given the potential for both benefits and risks. Long-term studies will be needed to determine the natural history of COVID-19 in ILD, including its impact on lung function and ILD progression. It is also unknown whether a subset of patients with severe COVID-19 will develop progressive fibrotic changes that may warrant future therapy.
Table 3.
New Interstitial Lung Disease Research Priorities During the COVID-19 Pandemic
| Major Research Priorities | Selected Key Questions |
|---|---|
| Impact of COVID-19 |
|
| Impact of COVID-19 in ILD |
|
| Biology of COVID-19 and ILD | |
| Impact of ILD medications in COVID-19 |
|
Conclusions
The COVID-19 pandemic has caused a major upheaval in health-care systems around the world, precipitating the most rapid change in health-care delivery that we will likely see in our lifetimes. For patients with ILD, COVID-19 has compromised the ability to comprehensively evaluate patients with newly identified ILD, and has impacted management decisions by altering access to various medications, impairing the ability to monitor adverse effects, and reducing access to lung transplantation. Some of these changes also present opportunities for improvement on previous approaches, such as widespread adoption of virtual care that can extend expertise to remote communities. The COVID-19 pandemic has also brought together medical communities from around the world with a concentrated focus on rapidly vetting and disseminating literature on COVID-19. Although there are many challenges ahead for patients with ILD and the world in general, COVID-19 has connected the global community in a remarkable way, leaving hope that we can successfully navigate the current crisis and serve patients even better in the future.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: V. M. reports grants from the University of Saskatchewan, the Royal University Hospital Foundation (Saskatoon, SK), and the Canadian Pulmonary Fibrosis Foundation; grants and personal fees from Boehringer-Ingelheim Canada; and personal fees from Hoffman-La Roche Ltd. and AstraZeneca, outside the submitted work. K. A. J. reports personal fees and other from Boehringer-Ingelheim, Hoffman La Roche Ltd, Theravance, and Blade Therapeutics; grants from the Chest Foundation, University of Calgary School of Medicine, the Pulmonary Fibrosis Society of Calgary, UCB Biopharma SPRL, and other from the Three Lakes Foundation, outside the submitted work. D. A. reports grants and personal fees from Boehringer-Ingelheim, and personal fees from Hoffman-La Roche Ltd. and AstraZeneca, outside the submitted work. J. H. F. reports grants from Boehringer-Ingelheim and the Canadian Pulmonary Fibrosis Foundation, outside the submitted work. N. H. reports grants and personal fees from Actelion, Bayer, Boehringer-Ingelheim, and Hoffman-La Roche Ltd. and personal fees from Novartis, outside the submitted work. M. K. reports grants and personal fees from Boehringer-Ingelheim and Hoffman-La Roche Ltd., GSK, Gilead, and Prometic; grants from Actelion, Respivert, Alkermes, and Pharmaxis; and personal fees from Genoa, Indalo, and Third Pole, outside the submitted work. J. M. reports personal fees from Boehringer-Ingelheim and Hoffman-La Roche Ltd, outside the submitted work. S. S. reports grants and personal fees from Boehringer-Ingelheim and Hoffman-La Roche Ltd. and personal fees from AstraZeneca, outside the submitted work. C. J. R. reports grants and personal fees from Boehringer-Ingelheim and Hoffman-La Roche Ltd., outside the submitted work. None declared (A. W. W., L. F.).
References
- 1.Emanuel E.J., Persad G., Upshur R. Fair allocation of scarce medical resources in the time of COVID-19. N Engl J Med. 2020;382(21):2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 2.Travis W.D., Costabel U., Hansell D.M. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoyer N., Prior T.S., Bendstrup E., Wilcke T., Shaker S.B. Risk factors for diagnostic delay in idiopathic pulmonary fibrosis. Respir Res. 2019;20(1):103. doi: 10.1186/s12931-019-1076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamas D.J., Kawut S.M., Bagiella E., Philip N., Arcasoy S.M., Lederer D.J. Delayed access and survival in idiopathic pulmonary fibrosis: a cohort study. Am J Respir Crit Care Med. 2011;184(7):842–847. doi: 10.1164/rccm.201104-0668OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raghu G., Remy-Jardin M., Myers J.L. Diagnosis of idiopathic pulmonary fibrosis: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 6.American Thoracic Society Pulmonary function laboratories: advice regarding COVID-19. 2020. https://www.thoracic.org/professionals/clinical-resources/disease-related-resources/pulmonary-function-laboratories.php
- 7.Ryerson C.J., Corte T.J., Lee J.S. A standardized diagnostic ontology for fibrotic interstitial lung disease. an international working group perspective. Am J Respir Crit Care Med. 2017;196(10):1249–1254. doi: 10.1164/rccm.201702-0400PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutchinson J.P., McKeever T.M., Fogarty A.W., Navaratnam V., Hubbard R.B. Surgical lung biopsy for the diagnosis of interstitial lung disease in England: 1997-2008. Eur Respir J. 2016;48(5):1453–1461. doi: 10.1183/13993003.00378-2016. [DOI] [PubMed] [Google Scholar]
- 9.Fisher J.H., Shapera S., To T., Marras T.K., Gershon A., Dell S. Procedure volume and mortality after surgical lung biopsy in interstitial lung disease. Eur Respir J. 2019;53(2) doi: 10.1183/13993003.01164-2018. [DOI] [PubMed] [Google Scholar]
- 10.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China [published online ahead of print March 13, 2020]. JAMA Intern Med. 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed]
- 11.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai Y., Yao L., Wei T. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan J.F., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention Coronavirus Disease 2019 (COVID-19) 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html#Asymptomatic
- 16.Sarzi-Puttini P., Giorgi V., Sirotti S. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol. 2020;38(2):337–342. [PubMed] [Google Scholar]
- 17.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaltsas A., Sepkowitz K. Community acquired respiratory and gastrointestinal viral infections: challenges in the immunocompromised host. Curr Opin Infect Dis. 2012;25(4):423–430. doi: 10.1097/QCO.0b013e328355660b. [DOI] [PubMed] [Google Scholar]
- 19.D’Antiga L. Coronaviruses and immunosuppressed patients: The facts during the third epidemic. Liver Transpl. 2020;26(6):832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 20.Lee N., Allen Chan K.C., Hui D.S. Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H., Ding Y., Li X., Yang L., Zhang W., Kang W. Fatal aspergillosis in a patient with SARS who was treated with corticosteroids. N Engl J Med. 2003;349(5):507–508. doi: 10.1056/NEJM200307313490519. [DOI] [PubMed] [Google Scholar]
- 22.Hui D.S.C., Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect Dis Clin North Am. 2019;33(4):869–889. doi: 10.1016/j.idc.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zumla A., Hui D.S., Azhar E.I., Memish Z.A., Maeurer M. Reducing mortality from 2019-nCoV: host-directed therapies should be an option. Lancet. 2020;395(10224):e35–e36. doi: 10.1016/S0140-6736(20)30305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King T.E., Jr., Bradford W.Z., Castro-Bernardini S. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 26.Noble P.W., Albera C., Bradford W.Z. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 27.Richeldi L., du Bois R.M., Raghu G. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 28.Brown K.K., Flaherty K.R., Cottin V. Lung function outcomes in the INPULSIS® trials of nintedanib in idiopathic pulmonary fibrosis. Respir Med. 2019;146:42–48. doi: 10.1016/j.rmed.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Distler O., Highland K.B., Gahlemann M. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med. 2019;380(26):2518–2528. doi: 10.1056/NEJMoa1903076. [DOI] [PubMed] [Google Scholar]
- 30.Flaherty K.R., Wells A.U., Cottin V. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381(18):1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 31.Maher T.M., Corte T.J., Fischer A. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2020;8(2):147–157. doi: 10.1016/S2213-2600(19)30341-8. [DOI] [PubMed] [Google Scholar]
- 32.Maher T.M., Lancaster L.H., Jouneau S. Pirfenidone treatment in individuals with idiopathic pulmonary fibrosis: impact of timing of treatment initiation. Ann Am Thorac Soc. 2019;16(7):927–930. doi: 10.1513/AnnalsATS.201810-720RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nathan S.D., Costabel U., Albera C. Pirfenidone in patients with idiopathic pulmonary fibrosis and more advanced lung function impairment. Respir Med. 2019;153:44–51. doi: 10.1016/j.rmed.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 34.Raghu G., Wells A.U., Nicholson A.G. Effect of nintedanib in subgroups of idiopathic pulmonary fibrosis by diagnostic criteria. Am J Respir Crit Care Med. 2017;195(1):78–85. doi: 10.1164/rccm.201602-0402OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wuyts W.A., Kolb M., Stowasser S., Stansen W., Huggins J.T., Raghu G. First data on efficacy and safety of nintedanib in patients with idiopathic pulmonary fibrosis and forced vital capacity of ≤50% of predicted value. Lung. 2016;194(5):739–743. doi: 10.1007/s00408-016-9912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection: a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9(1):727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du Plessis J.P., Fernandes S., Jamal R. Exertional hypoxemia is more severe in fibrotic interstitial lung disease than in COPD. Respirology. 2018;23(4):392–398. doi: 10.1111/resp.13226. [DOI] [PubMed] [Google Scholar]
- 38.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 39.Rubin G, Ryerson CJ, Haramati L, et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner Society [published online ahead of print April 7, 2020]. Chest. 10.1016/j.chest.2020.04.003. [DOI] [PMC free article] [PubMed]
- 40.Sprunger D.B., Olson A.L., Huie T.J. Pulmonary fibrosis is associated with an elevated risk of thromboembolic disease. Eur Respir J. 2012;39(1):125–132. doi: 10.1183/09031936.00041411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. [DOI] [PMC free article] [PubMed]
- 42.Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843-1844. [DOI] [PMC free article] [PubMed]
- 43.Ren X, Liu Y, Chen H, et al. Application and optimization of RT-PCR in diagnosis of SARS-CoV-2 infection [published online ahead of print February 25, 2020]. Lancet. https://doi.org/10.2139/ssrn.3546086.
- 44.Long C., Xu H., Shen Q. Diagnosis of the coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol. 2020;126:108961. doi: 10.1016/j.ejrad.2020.108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. In 2020;46(5):854-887. [DOI] [PMC free article] [PubMed]
- 46.Hui D.S. Systemic corticosteroid therapy may delay viral clearance in patients with Middle East respiratory syndrome coronavirus infection. Am J Respir Crit Care Med. 2018;197(6):700–701. doi: 10.1164/rccm.201712-2371ED. [DOI] [PubMed] [Google Scholar]
- 47.Arabi Y.M., Mandourah Y., Al-Hameed F. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 48.Kreuter M, Polke M, Walsh SLF, et al. Acute exacerbation of idiopathic pulmonary fibrosis: international survey and call for harmonisation. Eur Respir J. 2020;55(4):1901760. [DOI] [PubMed]
- 49.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 201(10):1299-1300. [DOI] [PMC free article] [PubMed]
- 50.Fernandez-Perez E.R., Yilmaz M., Jenad H. Ventilator settings and outcome of respiratory failure in chronic interstitial lung disease. Chest. 2008;133(5):1113–1119. doi: 10.1378/chest.07-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leuschner G., Behr J. Acute exacerbation in interstitial lung disease. Front Med (Lausanne) 2017;4:176. doi: 10.3389/fmed.2017.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-481. [DOI] [PMC free article] [PubMed]
- 53.Li X., Molina-Molina M., Abdul-Hafez A., Uhal V., Xaubet A., Uhal B.D. Angiotensin converting enzyme-2 is protective but downregulated in human and experimental lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;295(1):L178–L185. doi: 10.1152/ajplung.00009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khoury M, Cuenca J, Cruz FF, Figueroa FE, Rocco PR, Weiss DJ. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur Respir J. 2020;55(6):2000858. [DOI] [PMC free article] [PubMed]
- 55.Patel AB, Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA. 2020;23(18):1769-1777. [DOI] [PubMed]
- 56.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics [published online ahead of print March 4, 2020]. Drug Dev Res. https://doi.org/10.1002/ddr.21656. [DOI] [PMC free article] [PubMed]