Abstract
Use of convalescent plasma transfusions could be of great value in the current pandemic of coronavirus disease (COVID-19), given the lack of specific preventative and therapeutic options. This convalescent plasma therapy is of particular interest when a vaccine or specific therapy is not yet available for emerging viruses, such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes COVID-19. This report summarizes existing literature around convalescent plasma as a therapeutic option for COVID-19. It also includes recommendations for establishing a convalescent plasma program, enhancement considerations for convalescent plasma, and considerations around pathogen reduction treatment of convalescent plasma. Time is of the essence to set up protocols for collection, preparation, and administration of apheresis-collected convalescent plasma in response to the current pandemic. The immediate use of convalescent plasma provides prompt availability of a promising treatment while specific vaccines and treatments are evaluated and brought to scale. Further development of improved convalescent plasma, vaccines and other therapeutics depends on quick generation of additional data on pathogenesis and immune response. Additionally, given the lack of information around the natural history of this disease, PRT should be considered to add a layer of safety to protect recipients of convalescent plasma.
Keywords: Convalescent plasma, COVID-19, SARS-CoV-2, PRT, Pathogen reduction
1. Introduction
On average 5.3 viruses per year, of which 60%–70% are human pathogens, have emerged from 1940 to 2004 [1]. In a rapidly evolving pandemic, therapeutic options must be available quickly. Use of convalescent plasma transfusions could be of great value in the current pandemic of coronavirus disease (COVID-19), given the lack of specific preventative and therapeutic options. This convalescent plasma therapy is of particular interest when a vaccine or specific therapy is not yet available for emerging viruses, such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes COVID-19. Response to emerging and re-emerging infectious diseases throughout history has included rapid scientific collaborations to develop specific vaccines or therapies. To that end, currently, there is a large global trial supported by the World Health Organization (WHO), SOLIDARITY, to investigate existing therapies for COVID-19, including remdesivir, chloroquine and hydroxychloroquine, lopinavir and ritonavir, and lopinavir + ritonavir + interferon-beta. In addition, there is broad interest to leverage convalescent plasma from recovered COVID-19 patients as treatment or for prophylaxis of health care workers and other caregivers. The United States Food and Drug Administration (US FDA) has released guidance for investigation of convalescent plasma in the United States for COVID-19 [2]. Additionally, historic data has reported safety and efficacy of convalescent plasma for use in other infectious diseases, and there is also new data on convalescent plasma use in the current global public health emergency specifically to treat COVID-19.
Time is of the essence to set up protocols for collection, preparation, and administration of apheresis-collected convalescent plasma in response to the current pandemic. Additionally, optimization of known potential benefits of convalescent plasma may improve efficacy to support the medical needs of the widespread impact of COVID-19.
2. Clinical use of convalescent plasma
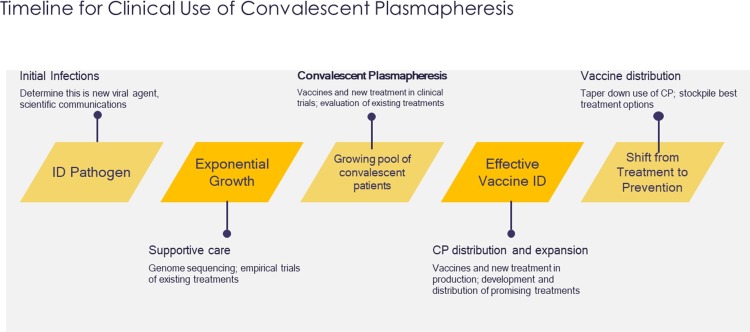
The transfusion of convalescent blood products is not a new clinical tool in emerging infectious disease outbreaks (Fig. 1]). Historically, passive immune therapy has involved convalescent whole blood, convalescent plasma, pooled human immunoglobulin for intravenous or intramuscular administration, high-titer human immunoglobulin, and polyclonal or monoclonal antibodies; however, plasma collected by apheresis is currently the preferred therapy [3]. Use of blood products from recovered patients dates back to the late 1800s [4]. The Spanish influenza (pandemic of 1918–1920) was the first viral infection for which convalescent blood products were found to be potentially effective during clinical studies [[5], [6], [7], [8], [9], [10], [11]] A meta-analysis of 8 studies of the Spanish flu (1703 patients) showed reduced mortality from treatment with convalescent blood products [12]. The possibility of using convalescent plasma for prevention and/or treatment was of interest during the recent West African Ebola outbreak due to the lack of vaccines and therapeutics, highly infectious nature of the virus, and high associated case-fatality rate [13]. Several other emerging infectious diseases, such as West Nile Virus, MERS-CoV, SARS-CoV-1, and H1N1 have also been the target of possible passive immunity with convalescent plasma. Despite a long history of convalescent plasma usage, clinical efficacy has not been studied robustly and conclusions are weak, likely because convalescent plasma was used in critical situations, during massive epidemic/pandemic outbreaks, requiring immediate actions. The effectiveness of this convalescent plasma therapy appears to differ depending on the pathogen and treatment protocols (eg, timing, volume, and dosing of administration). There have been several publications on convalescent plasma use for SARS-CoV-1 and MERS, including reviews/editorials, observational studies (retrospective, prospective, or case series), and a systematic literature review [[14], [15], [16], [17], [18]].
Fig. 1.
Clinical Response and Research for Emerging Infectious Diseases.
A 2015 systematic review and exploratory post-hoc meta-analysis of convalescent plasma and immunoglobulin for the treatment of severe acute respiratory viral infections reported a statistically significant reduction (75 %) in the odds of mortality [19]. Seven studies of convalescent plasma or intravenous immunoglobulin for SARS were inconclusive [20]. One feasibility intervention study of convalescent plasma treatment for MERS failed to identify sufficient high-titer plasma from patients [21]. In Kuo-Ming Yeh et al., experience using convalescent plasma with a serum antibody titer of >1:640 for SARS treatment reported that severely ill patients survived after this treatment. [15].
It is important to understand the antibody characteristics and titers able to affect the course of disease as well as the role of the recipients' immune response when considering therapeutic options such as convalescent plasma and vaccine candidates for new emerging viruses. Convalescent plasma has demonstrated safety and shown promise without knowledge of viral serotypes or antibody titers.
2.1. Clinical use of convalescent plasma for COVID-19
SARS-CoV-2 causes COVID-19 and is the new emerging virus responsible for the current global pandemic. [22,23] There are no vaccines, monoclonal antibodies, or drugs available for SARS-CoV-2, although a human monoclonal antibody has shown promise of cross-neutralization to target a communal epitope on these SARS-CoV-1 and SARS-CoV-2 viruses and has potential for prevention and treatment of COVID-19; however, this possible therapy is not likely to be available soon [24].
Another line of investigation focuses on possible mechanism of actions of the virus. Ling Lin et al. hypothesize that the virus first attacks the organs in patients that express angiotensin converting enzyme 2 (ACE2) receptors and that there is a second attack around 7–14 days after symptom onset. [23] The virus may cause B lymphocyte reduction (and IL-6 reduction) early in the disease, which may affect antibody production. Lymphocytes may continue to decline over the course of disease, and inflammatory cytokines increase. Hence, therapies should focus on 1) enhancement of the immune function of patients and 2) inhibiting the formation of inflammatory cytokine storms. Additional data on pathogenesis and immune response will inform further development of improved convalescent plasma as well as development of vaccines and other therapies.
The immediate use of convalescent plasma provides prompt availability of a promising treatment while specific vaccines and treatments are evaluated and brought to scale. Convalescent plasma from donors who have recovered from COVID-19 may be most promising when used as prophylaxis or when administered shortly after symptom onset (within 14 days). Protection may last from weeks to months [16,17,19,25]. There are more than 1.5 million and over 300,000 recovered COVID-19 patients, who could present a valuable resource of convalescent plasma.
There have been 3 case series from China for the use of convalescent plasma to treat COVID-19 (19 total patients, Table 1 ) [16,26,27]. Some seriously ill patients in China who received convalescent plasma therapy resulted in improved oxygenation and reduced inflammation and viral load [16,27]. In this pilot study, 9 patients received 1 dose of 200 mL convalescent plasma with neutralizing antibody titers of >1:640. The median time from onset of illness to convalescent plasma transfusion was 16.5 days. Within 3 days, the patients showed improved clinical symptoms along with increase of oxyhemoglobin saturation. Lymphocyte counts increased from 0.65 × 109/L to 0.76 × 109/mL and C-reactive protein decreased from 55.98 mg/L vs. 18.13 mg/L. The neutralizing antibody in recipients increased in all patients and the viral load was undetectable in 7 of the 9 patients who had previous viremia.
Table 1.
Use of Convalescent Plasma for COVID-19.
| Reference | Infectious Agent | Patient Condition Study Design |
# Patients | Timing of Administration | Volume Transfused | Antibody Titer | Patient Outcomes |
|---|---|---|---|---|---|---|---|
| Convalescent Plasma for COVID-19 | |||||||
| Shen et al., 2020 [14] | SARS-CoV-2 | Critically Ill Case Series |
5 | 10–22 days (range) | 200 mL (x2) | >1:1000 | Body temperature normalized within 3 days in 4/5 patients SOFA score decreased, and PAO2/FIO2 increased within 12 days ARDS resolved in 4 patients at 12 days |
| Duan et al., 2020 [16] | SARS-CoV-2 | Severely Ill Case Series |
10 | 16.5 days (median) | 200 mL (x1) | >1:640 | Improved oxygenation and reduced inflammation and viral load |
| Zhang et al., 2020 [30] | SARS-CoV-2 | Critically Ill Case Series |
4 | 15.5 days (mean) | 200–2400 mL (1–8 infusions) | – | All 4 patients recovered |
| Convalescent Plasma for SARS-CoV-1 and MERS | |||||||
| Wong et al., 2003 [31] | SARS-CoV-1 | Stable Case Report |
1 | 14–16 days (range) | 200 mL (x2) | – | Uneventful recovery |
| Soo et al., 2004 [18] | SARS-CoV-1 | Progressive disease Retrospective non-randomized comparison |
40 (19 CP) |
11.4 days (mean) | 200–400 mL | – | Patients who received CP had a shorter hospital stay (p < 0.001) and lower mortality (p = 0.049) than the comparator group who received continued methylprednisone (no CP) |
| Yeh et al., 2005 [32] | SARS-CoV-1 | Severely Ill Case Series |
3 | 10.5 days (mean) | 500 mL | >1:640 | Infected healthcare workers had progressed severely and had failed to respond to available treatment All survived after CP transfusion |
| Cheng et al., 2005 [33] | SARS-CoV-1 | Patients whose condition continued to deteriorate, as defined by SaO2 <90% on 0.5 FiO2 were given CP (depending on availability and clinical judgement) Case Series |
80 | 7–30 days (range) | Mean: 279.3 mL (± 127.1) | 1:160–2560 | Patients with progressive SARS (after ribavirin + methylprednisolone) had a higher discharge rate by day 22 when CP was administered before day 14. Patients receiving CP after day 14 had a longer hospital stay and a higher mortality rate (discharge rate 58.3 % vs 15.6 %; P < 0.001) Higher Day 22 discharge rate observed among those who were PCR positive and seronegative for coronavirus at the time of CP infusion (66.7 % vs 20 %; P = 0.001) |
| Chun et al., 2016 [34] | MERS-CoV | Case Report | 1 | 19 days | 250 mL | – | The patient developed respiratory distress within two hours after transfusion (TRALI) |
| Ko et al., 2018 [35] | MERS-CoV | 3/13 MERS patients received CP Case Series |
3 | 8–18 days (range) | – | >1:40 or 1:80 | 2/3 patients showed neutralizing antibody activity (no response with 1:40 titer infusion) |
Abbreviations: CP = Convalescent Plasma; MERS = Middle Eastern Respiratory Syndrome; mL = milliliters; SARS = Severe Acute Respiratory Syndrome.
Convalescent plasma reduced viral load and was safe when administered in China in this current outbreak [16,26]. There were no severe adverse effects reported from transfusion of convalescent plasma in these recipients. Individuals with pulmonary disease have a risk for transfusion-related acute lung injury (TRALI) from plasma transfusion [28,29]. Additionally, several viral diseases, such as Dengue virus and SARS, may cause immune enhancement of pathogenicity in the presence of certain antibodies [22,24]. However, there is safety data from the use of convalescent plasma in patients with SARS and MERS, and anecdotal evidence from its use in 245 patients with COVID-19 [19,26]. This should be a consideration in the risk-benefit assessment.
Effective convalescent plasma should contain high-titer specific antibodies which bind to SARSCoV-2 and neutralize the viral particles, block access to uninfected cells, and activate potent effector mechanisms [25].
There are scientific, operational and logistical considerations for availability to obtain plasma in recovered patients and convert this to a therapy [25,36]. The following elements will be essential parts of a convalescent plasma program:
-
1
Available donors who have recovered from the disease and meet eligibility criteria to donate convalescent serum; special attention will be necessary to assure that plasma donation will be safe for the recovering patient/donor
-
2Develop approach to screening recovered COVID patients to identify potential donors
-
aRecovery will need to be demonstrated with appropriate standardized viral nucleic acid and antibody screening which is important because severe cases have tested positive for SARS-CoV-2 at or beyond day 10 post-onset [37]
-
a
-
3Recently approved serological assays are necessary to detect SARS-CoV-2 in serum and virologic assays to measure viral neutralization [38]
-
aInfrastructure and personnel to perform antibody titers in eligible donors
-
bUnderstanding of type of antibody being measured
-
a
-
4Selection of desired antibody level in donors, preferably with high neutralizing antibody titers
-
aFDA has recommended a titer of >1:320 for eIND [2]
-
a
-
5Identify blood banking facilities to process the plasma donations
-
aExperience with plasmapheresis
-
a
-
6Select specific product to be prepared (eg FFP, fresh plasma, or lyophilized plasma)
-
aDetermine and standardize amount of plasma to be collected and product volume
-
a
-
7
Establish a dosage schedule based on knowledge of SARS-CoV-2 antibodies
-
8
Establishment of a registry for possible future donations should be considered
If potential prophylaxis and therapeutic protocols for administration of convalescent plasma program are established, they must be carried out as part of a clinical trial to assess the efficacy of the intervention and to measure immune responses. It may be advisable to consider an adaptive design to adjust for rapid generation of new evidence in this pandemic, to evaluate effective neutralizing titer and timing of administration, and to assure regulatory compliance including IRB approval, and multiple centers.
Patient/donors can be selected for high titers of convalescent plasma donations but variability in antibody titers, characteristics, and persistence may occur in different patients. This information will facilitate optimization of convalescent plasma to consider both prophylactic and therapeutic dosing. Depending on the volumes needed and the neutralizing activity of donated convalescent plasma, preparations could be pooled or used individually, and the plasma may be treated with pathogen reduction technology (PRT).
Given the lack of information around the natural history of this disease, PRT may add a layer of safety to protect recipients of convalescent plasma from residual antigen, variants of virus, or other possible co-infections in the recovered patient/donor. This PRT protection may be even more important if convalescent plasma is considered for prophylactic administration.
Protocols for local preparation of convalescent plasma may be available quickly for emergency use in this pandemic [2]. Protocols to produce convalescent plasma —gaining purified preparations with high titers of neutralizing antibodies —are preferred and should be urgently considered so the products could be available should a second wave of infections occur in several months [22].
2.2. Pathogen reduction of convalescent plasma
Available PRTs are effective in reducing infectious pathogen load in blood products [3,39]. Generally, PRT is intended to reduce or eliminate detectable infectious organisms, including bacteria, viruses, and parasites, from blood components intended for transfusion. Two widely used examples of PRT include the INTERCEPT Blood System (Cerus) and the Mirasol PRT System (Terumo BCT) and are briefly reviewed here [40]. In the United States, the INTERCEPT Blood System for Platelets is approved to reduce the risk of transfusion-transmitted infections from platelet transfusions. The Mirasol PRT System is currently undergoing a clinical study under an IDE with the US FDA for the treatment of platelets. Both of these PRTs are used for treatment of plasma in some countries outside of the United States.
Mirasol treatment efficiently reduces EBOV titers to below limits of detection in both serum and whole blood and immunoglobulin concentrations and antibody titers remain within reference ranges [39,41]. Mirasol treatment also effectively inactivates MERS (> 4 log reduction, internal data on file).
The INTERCEPT Blood System has been used to treat convalescent plasma from EBOV survivors [3,39]. Solvent/detergent for use on single-donor plasma or mini-pools of plasma has also been used as a PRT method but this process uses large pools of plasma and takes several months to complete so would not likely be of value in this current urgent situation.
The use of PRT may add a layer of safety for the use of convalescent plasma, particularly with emerging viruses for which the pathogenesis and immune response is not fully understood. Further, the use of PRT of convalescent plasma may also support use of pools, or less strict donor selection criteria in this devastating and rapidly evolving pandemic. Convalescent plasma treatment for the West African EBOV outbreak is an example in which the WHO has proposed a PRT-based approach to increase the donor pool and add a layer of safety when there is a lack of information on pathogenesis [13].
3. Summary
Convalescent plasma has the potential to provide an immediate promising treatment option while evaluating existing drugs and developing new specific vaccines and therapies. Time is of the essence to 1. identify donor criteria and eligible donors, 2. adjust blood processing facilities and testing capabilities, 3. develop sufficient serologic assays for screening, and 4. identify dosing protocols for convalescent plasma from apheresis collection to respond to the current pandemic. Given the lack of information around the natural history of this COVID virus, PRT should be considered to add a layer of safety to protect recipients of convalescent plasma.
Funding disclosures
Jeffrey McCullough receives advisory fees from Fresenius Kabi, Haemonetics and Terumo BCT.
References
- 1.Stramer S.L. Current perspectives in transfusion-transmitted infectious diseases: emerging and re-emerging infections. ISBT Sci Ser. 2014;9(1):30–36. doi: 10.1111/voxs.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center for Biologics Evaluation and Research Investigational COVID-19 convalescent plasma - emergency INDs. https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/investigational-covid-19-convalescent-plasma-emergency-inds 26 March 2020. Available at:
- 3.Marano G., Vaglio S., Pupella S. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016;14(2):152–157. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon J. Emil Behring’s medical culture: from disinfection to serotherapy. Med Hist. 2007;51(2):201–218. doi: 10.1017/s0025727300001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FB B. Influenza pneumonia treated by blood transfusions. NY Med J. 1919;109:765. [Google Scholar]
- 6.Francis F.D.H.M., Gaines A.R. Early use of convalescent serum in influenza. Mil Surg. 1920;47:177–179. [Google Scholar]
- 7.Jacobaeus Treatment of influenza pneumonia with serum from convalescents. Svenska Lakartidnin. 1920;18:385–399. [Google Scholar]
- 8.Lesne E.B.P., Saint-Girons F. Plasma therapy in influenza. Presse Med. 1919;27:181–182. [Google Scholar]
- 9.Miller O.O.M.W. Report of influenza treated with serum from recovered cases. Ky Med J. 1919;17:218–219. [Google Scholar]
- 10.PM C. Injection of whole blood in influenza. Br Med J Acoust Soc Am. 1919;1:698. [Google Scholar]
- 11.WR R. Treatment of influenza-pneumonia by use of convalescent human serum. Boston Med Surg J. 1919;161:688–691. [Google Scholar]
- 12.Luke T.C., Kilbane E.M., Jackson J.L., Hoffman S.L. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145(8):599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization . World Health Organization; Geneva: 2014. Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease for transfusion, as an empirical treatment during outbreaks. Interim guidance for national health authorities and blood transfusion services. September. Available at: http://apps.who.int/iris/bitstream/10665/135591/1/WHO_HIS_SDS_2014.8_eng.pdf?ua=1. Accessed on 21/05/2015. [Google Scholar]
- 14.Shen C., Wang Z., Zhao F. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020 doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuo-Ming Yeh T.-S.C., Siu L.K., Lin Jung-Chung, Chan Paul K.S., Peng Ming-Yieh, Wan Hsiang-Lin. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56:919–922. doi: 10.1093/jac/dki346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan K. 2020. The feasibility of convalescent plasma therapy in severe COVID-19 patients: a pilot study. [DOI] [Google Scholar]
- 17.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soo Y.O., Cheng Y., Wong R. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10(7):676–678. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arabi Y., Balkhy H., Hajeer A.H., Bouchama A., Hayden F.G., Al-Omari A. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. Springerplus. 2015;4(November):709. doi: 10.1186/s40064-015-1490-9. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casadevall A., Pirofski L.A. The convalescent sera option for containing COVID-19. J Clin Invest. 2020 doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9(1):727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Chunyan, Li W., Drabek Dubravka, Okbada Nisreen M.A., Haperen Rienvan, Osterhaus Albert D.M.E. A human monoclonal antibody blocking SARS-CoV-2 infection. bioRxiv. 2020 doi: 10.1101/20200311987958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunningham A.C., Goh H.P., Koh D. Treatment of COVID-19: old tricks for new challenges. Crit Care. 2020;24(1):91. doi: 10.1186/s13054-020-2818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.China puts 245 COVID-19 patients on convalescent plasma therapy. News release. Xinhua. February 28, 2020. Available at: http://www.xinhuanet.com/english/2020-02/28/c_138828177.htm. Accessed March 10, 2020.
- 27.China Seeks Plasma From Recovered Patients to Treat Virus. Time. Available from: https://time.com/5784286/covid-19-china-plasma-treatment/. [cited 2020 Feb 16].
- 28.Afessa B., Gajic O., Keegan M.T. Severity of illness and organ failure assessment in adult intensive care units. Crit Care Clin. 2007;23(3):639–658. doi: 10.1016/j.ccc.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Menis M., Sridhar G., Selvam N. Hyperimmune globulins and same-day thrombotic adverse events as recorded in a large healthcare database during 2008-2011. Am J Hematol. 2013;88(12):1035–1040. doi: 10.1002/ajh.23559. [DOI] [PubMed] [Google Scholar]
- 30.Zhang B., Liu S., Tan T. Treatment with convalescent plasma for critically ill patients with SARS-CoV-2 infection. Chest. 2020 doi: 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong V.W., Dai D., Wu A.K., Sung J.J. Treatment of severe acute respiratory syndrome with convalescent plasma. Hong Kong Med J. 2003;9(3):199–201. [PubMed] [Google Scholar]
- 32.Yeh K.M., Chiueh T.S., Siu L.K. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56(5):919–922. doi: 10.1093/jac/dki346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng Y., Wong R., Soo Y.O. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chun S., Chung C.R., Ha Y.E. Possible transfusion-related acute lung injury following convalescent plasma transfusion in a patient with middle east respiratory syndrome. Ann Lab Med. 2016;36(4):393–395. doi: 10.3343/alm.2016.36.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ko J.H., Seok H., Cho S.Y. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir Ther (Lond) 2018;23(7):617–622. doi: 10.3851/IMP3243. [DOI] [PubMed] [Google Scholar]
- 36.Dong L., Bouey J.P. Ublic mental health crisis during COVID-19 pandemic, China. Emerg Infect Dis. 2020;26(7) doi: 10.3201/eid2607.200407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin Z., Liu S., Liu X. An exceptional peroxide birefringent material resulting from d-pi interactions. Angew Chem Int Ed Engl. 2020 doi: 10.1002/anie.202002828. [DOI] [PubMed] [Google Scholar]
- 38.Okba N.M.A., Widjaja I., Li W. Serologic detection of middle east respiratory syndrome coronavirus functional antibodies. Emerg Infect Dis. 2020;26(5) doi: 10.3201/eid2605.190921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dean C.L., Hooper J.W., Dye J.M. Characterization of Ebola convalescent plasma donor immune response and psoralen treated plasma in the United States. Transfusion. 2020 doi: 10.1111/trf.15739. [DOI] [PubMed] [Google Scholar]
- 40.Letowska M., Przybylska Z., Piotrowski D. Hemovigilance survey of pathogen-reduced blood components in the Warsaw Region in the 2009 to 2013 period. Transfusion. 2016;56(Suppl. 1):S39–44. doi: 10.1111/trf.13330. [DOI] [PubMed] [Google Scholar]
- 41.Cap A.P., Pidcoke H.F., Keil S.D. Treatment of blood with a pathogen reduction technology using ultraviolet light and riboflavin inactivates Ebola virus in vitro. Transfusion. 2016;56(Suppl. 1):S6–15. doi: 10.1111/trf.13393. [DOI] [PMC free article] [PubMed] [Google Scholar]