INTRODUCTION
A rare disease is defined as any life-limiting or chronically debilitating disease affecting <1 person in 2000, with many rare diseases affecting <1 person per 100 000. There are approximately 8000 rare diseases, with recent analysis suggesting a conservative prevalence of 3.5–5.9%.1 Over the last decade, tremendous advances have been made in rare disease identification, treatment, and support. This has been largely driven by an increasingly vehement consolidated patient voice and an EU directive (2009) that all member states required a rare disease plan/strategy by 2013. In 2013, the UK strategy for rare disease was published,2 followed by the launch of rare disease implementation plans for the devolved nations (Scotland in 2014;3 Northern Ireland in 2015;4 Wales in 20175).
DIFFICULTIES WITH DIAGNOSIS
Rare diseases have major unmet medical needs. Healthcare professionals and rare disease charities often hear tragic stories of patients with rare life-threatening diseases where responsible doctors have been unable to help as they have never heard of the condition, nor seen a similar case presentation, nor found relevant knowledge and expertise online. More than 80% of individuals report difficulty in accessing relevant information and >70% of GPs struggle to identify or manage rare diseases.
A long diagnostic odyssey is common, with patients often feeling misunderstood, isolated, vulnerable, receiving incorrect diagnoses, and feeling marginalised in decision making. The average time to receive an accurate rare disease diagnosis is 5 years, with half of patients with rare disease receiving at least one misdiagnosis. Even with a diagnosis, more than half of rare diseases do not have a dedicated support group. As a result, rare disease patients and families often feel self-advocacy is essential so they become experts in their own conditions, conducting extensive research and travelling worldwide to speak to professional experts and attend relevant conferences.
RESOURCES FOR GPs
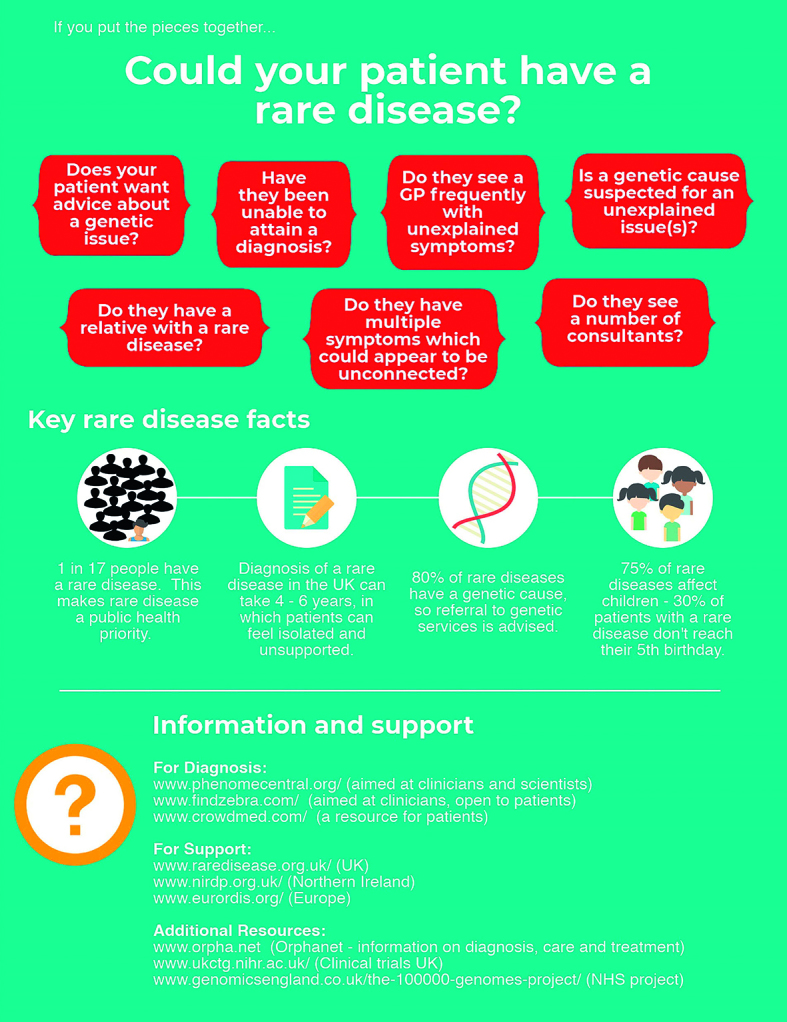
Despite GPs having considerable expertise managing multisystem disease and (often uniquely) having oversight of long-term patient healthcare records, few have the resources to thoroughly research rare diseases and many report becoming overwhelmed as patients attend with detailed reports about rare conditions. Doctors often seek information on the internet, but it is difficult to know what information is trustworthy and where to direct patients for further support. Approximately 80% of rare diseases have a genetic cause, with others including rare cancers, autoimmune diseases, and toxic and infectious diseases; the molecular basis is known for one-third of these rare genetic conditions, so referral to a local genetics service is advised. Patients with a rare disease often present to their GP with unusual symptoms or signs that are difficult to diagnose, multiple diagnoses that in combination seem unlikely, and/or multiple ‘umbrella’ diagnoses (Figure 1). Useful resources include:
Orphanet (https://www.orpha.net) is an internationally recognised one-stop online portal for collating high-quality knowledge and information on rare disease diagnosis, care, and treatment. It contains rare disease literature, reviewed by medical experts, that any member of the public may search through, as well as information on orphan drugs, patient organisations, experts in particular conditions, research expert centres, and medical laboratories that provide diagnostic testing.
Royal College of General Practitioners genomics webinars (https://www.rcgp.org.uk/clinical-and-research/our-programmes/genomics-webinars.aspx) illustrate how genomics impacts primary care.
Launched in March 2017, European Reference Networks (ERNs; https://ec.europa.eu/health/ern_en) are an excellent resource providing cross-border, high-quality virtual access to expert healthcare providers across Europe, particularly where highly specialised treatment is required.
Professional resources exist for enhancing global collaboration and matching signs and symptoms to patients with similar phenotypes. PhenomeCentral (www.phenomecentral.org/) is aimed at helping clinicians and scientists make diagnoses, while FindZebra (www.findzebra.com) significantly outperforms Google6 in helping match phenotypes to rare diseases for doctors and patients. Mendelian (www.mendelian.co) helps doctors match phenotypes and genotypes. CrowdMed (www.crowdmed.com) is a patient-oriented resource where medical detectives have helped solve challenging cases and provided many diagnoses within the rare disease community.
Local, national, and international charities offer support and access to healthcare professionals with expertise in specific rare diseases. Unique (https://www.rarechromo.org/) is a small UK charity that has provided information and resources for families and healthcare professionals focused on chromosome abnormalities for over 30 years, and many of their disorder guides are available in several languages. Genetic Alliance (www.geneticalliance.org.uk/) brings together patient organisations working on rare diseases with genetic inheritance and includes a national campaign (Rare Disease UK; www.raredisease.org.uk), while also running a support network for families with undiagnosed genetic conditions in the UK (SWAN UK, Syndromes Without A Name; www.undiagnosed.org.uk). Northern Ireland has an alliance bringing together patients, carers, families, healthcare providers, and policymakers within the Northern Ireland Rare Disease Partnership (www.nirdp.org.uk). Several charities have resource modules developed with the Royal College of General Practitioners to help improve diagnosis and treatment of rare diseases; for example, the motor neurone disease red-flag toolkit (www.rcgp.org.uk/-/media/ Files/CIRC/Rare-Diseases/RCGP-Red-Flags-Final-Nov-13.ashx?la=en) and online learning tool (http://elearning.rcgp.org.uk/course/info.php?popup=0&id=160), and Muscular Dystrophy UK’s GP online learning tool for neuromuscular conditions (http://elearning.rcgp.org.uk/course/info.php?popup=0&id=183).
Figure 1.
Rare disease diagnosis and management support.
THE FUTURE OF TREATMENT
‘Big data’ approaches offer significant potential to improve rare disease identification and visibility; however, this means that careful data entry to electronic health and social care systems is essential. One strategy to improve information standards and data retrieval is using enhanced coding nomenclature in information systems such as using an ORPHA number or updated 11th revision of the international classification of diseases (ICD-11) codes. Information systems still need upgrading to make using these codes simple and efficient.
An increasing number of medications and clinical trials are available to the rare disease community. Although designing an orphan drug may not be intuitively competitive for pharmaceutical companies, rare disease drugs often have a faster path to commercialisation and attractive regulatory incentives. The US Food and Drug Administration Office of Orphan Products Development currently has 4699 listed treatments approved for rare diseases (orphan drugs), while the UK clinical trial gateway collates information to help informed decision making. Scientific advances such as international registries and biorepositories for rare diseases, improved molecular characterisation through initiatives such as the UK 100 000 genomes project, genetic editing, induced pluripotent stem cells (iPSCs), and RNA interference (RNAi) technologies are paving the way for the development of accessible treatments for patients with a rare disease.
Funding
Ashleen Crowe: PhD studentship from the Department for the Economy, Northern Ireland. Medical Research Council—Northern Ireland Executive supports the Northern Ireland Genomic Medicine Centre though Belfast Health and Social Care Trust.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Nguengang Wakap S, Lambert DM, Olry A, et al. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur J Hum Genet. 2020;28:165–173. doi: 10.1038/s41431-019-0508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Department of Health The UK strategy for rare diseases. 2013 https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/260562/UK_Strategy_for_Rare_Diseases.pdf (accessed 16 Apr 2020). [Google Scholar]
- 3.Scottish Government It’s not rare to have a rare disease: the implementation plan for rare diseases in Scotland. 2014 https://www.gov.scot/Publications/2014/07/4751 (accessed 16 Apr 2020). [Google Scholar]
- 4.Department of Health, Social Services and Public Safety Providing high quality care for people affected by rare diseases — the Northern Ireland implementation plan for rare diseases. 2015 https://www.health-ni.gov.uk/sites/default/files/publications/dhssps/ni-rare-diseases-implementation-plan-oct-2015.pdf (accessed 16 Apr 2020). [Google Scholar]
- 5.Welsh Government Welsh rare disease implementation plan. 2017 https://gov.wales/sites/default/files/publications/2019-01/welsh-rare-diseases-implementation-plan-july-2017_0.pdf (accessed 16 Apr 2020).
- 6.Dragusin R, Petcu P, Lioma C, et al. FindZebra: a search engine for rare diseases. Int J Med Inform. 2013;82(6):528–538. doi: 10.1016/j.ijmedinf.2013.01.005. [DOI] [PubMed] [Google Scholar]


