Highlights
-
•
Emerging data shows a correlation between serum IL-6 and COVID-19 infection severity.
-
•
Tocilizumab, an IL-6 inhibitor, is a potential supportive treatment for COVID-19.
-
•
Preliminary investigations are showing benefits with tocilizumab for COVID-19.
-
•
Ongoing RCTs will clarify tocilizumab’s place in therapy for COVID-19.
1. Introduction
In December of 2019, an outbreak of inexplicable pneumonia cases in several patients appeared in Wuhan, China. What is now known as coronavirus disease 2019 (COVID-19) has spread widely since then and, as of this writing, has infected over 1.8 million people worldwide and has claimed the lives of over 100,000 patients [1]. The disease, COVID-19, is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 has many similarities to SARS-CoV-1 and Middle East respiratory syndrome coronavirus (MERS-CoV), both of which have also been fatal [2]. The number of cases of COVID-19 continues to increase but there is not an approved vaccine or medication that can be used for treatment. Thus, there is an immense need for treatment strategies to help combat this contagious disease. In previous studies, severe patients that have been hospitalized for COVID-19 have had laboratory results that show an increased level of cytokines, specifically interleukin 6 (IL-6) [3]. This increase may be attributed to the cytokine release syndrome (CRS) that is triggered by a variety of factors such as infections [4]. Since IL-6 plays an important role in CRS, it serves as a possible mechanism of treatment in severe patients. Tocilizumab (TCZ) is a recombinant humanized monoclonal antibody that has an antagonist effect on the IL-6 receptor. It is currently used in the treatment of rheumatoid arthritis, but could also play a key role in treatment for severely ill patients with COVID-19. We performed a systematic review to evaluate outcomes associated with TCZ treatment in patients with COVID-19.
2. Methods
A systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A literature search was conducted on PubMed, Embase, Scopus, and Web of Science for articles published until April 9, 2020 using keywords such as “COVID-19,” “tocilizumab,” “interleukin 6,” or “IL-6.” We utilized the following inclusion criteria for article selection: (1) involve humans with COVID-19 receiving TCZ; (2) be either a randomized controlled trial, prospective trial, retrospective analysis, case series or case report; (3) include clinical findings at presentation. Saeed K. Alzghari (SKA) formulated the search strategy. SKA identified relevant articles reporting clinical outcomes. SKA extracted data from all articles. The extracted data was cross-reviewed by Valerie S. Acuna.
3. Results
3.1. Study selection and characteristics
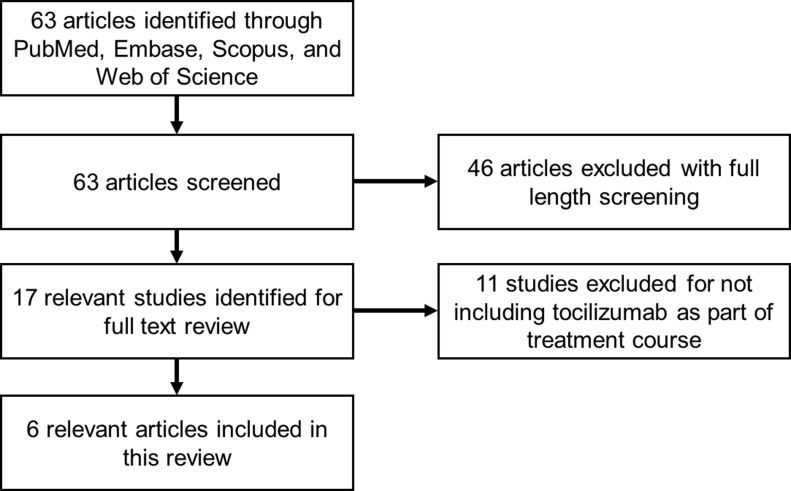
A total of 63 articles met the initial search criteria. Out of the 63 screened articles, 17 were selected for full-text review with 6 articles meeting inclusion criteria with all publications dated in 2020 (Fig. 1 ) [[5], [6], [7], [8], [9], [10]]. The study characteristics for retrospective analyses and case reports are summarized in Table 1, Table 2 , respectively. There were two retrospective analyses and 4 case reports.
Fig. 1.
Search flow diagram.
Table 1.
Retrospective analyses involving the use of tocilizumab in patients with COVID-19.
| Author (year) | Country | Population (n) | Mean age | Male (%) | HTN (%) | DM (%) | Stroke (%) | COPD (%) | Symptoms > 20% | Critically ill (%) | Mean IL-6 level prior to TCZ (pg/mL) | TCZ dosing | Death (%) | Hospitalized (%) | Discharged (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Luo (2020) | China | 15 | 73 | 80 | 60 | 27 | 20 | 0 | NR | 47 | 111.05 | Range of 80-600 mg IV with average of 1.47 doses | 20 | 80 | NR |
| Xu (2020) | China | 21 | 57 | 86 | 43 | 24 | 5 | 5 | Fever (100%), Cough (67%), Phlegm (43%), Fatigue (29%), SOB (29%) | 19 | 132.38 | 400 mg IV x 1 dose | 0 | 10 | 90 |
Abbreviations: COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus; HTN: hypertension; IL-6: interleukin 6; IV: intravenous; NR: not reported; SOB: shortness of breath; TCZ: tocilizumab.
Table 2.
Case reports involving the use of tocilizumab in patients with COVID-19.
| Author (Year) | Country | Age | Sex | Chronic illness | Symptoms | Bilateral patchy groundglass opacities due to COVID-19 | Mechanical ventilation | TCZ dosing | Death | Recovered |
|---|---|---|---|---|---|---|---|---|---|---|
| Ferrey (2020) | United States | 56 | M | ESRD, CAD, CMOP | Cough, fever, N/V, SOB, diarrhea, myalgias, afib, ARDS | Yes | Yes | NR | No | Unknown |
| Michot (2020) | France | 42 | M | mCCRCC | Cough, fever, SOB | Yes | No | 8 mg/kg IV x 2 doses | No | Yes |
| Mihai (2020) | Switzerland | 57 | F | SSc-ILD, T2DM, obesity | Cough, headache, malaise | No | No | 8 mg/kg IV every 4 weeks x 3 years | No | Yes |
| Zhang (2020) | China | 60 | M | MM | Chest tightness, SOB | Yes | No | 8 mg/kg IV x 1 dose | No | Yes |
Abbreviations: afib: atrial fibrillation; ARDS: acute respiratory distress syndrome; CAD: coronary artery disease; CMOP: cardiomyopathy; ESRD: end-stage renal disease; F: female; IV: intravenous; N/V: nausea and vomiting; NR: not reported; M: male; mCCRCC: metastatic clear cell renal cell carcinoma; MM: multiple myeloma; SOB: shortness of breath; SSc-ILD: systemic sclerosis with interstitial lung disease; T2DM: type II diabetes mellitus; TCZ: tocilizumab.
3.2. Observational studies
Luo and colleagues reported the use of TCZ in 15 Chinese patients diagnosed with COVID-19 [5]. The mean age of the cohort was 73 years and 80% of the population was male. The chronic illnesses of the patient population included hypertension, diabetes mellitus (DM), and stroke. The study reported that 47% of patients were critically ill, 40% were seriously ill, and 13% were moderately ill. Mean IL-6 levels prior to TCZ therapy was 111.05 pg/mL (normal IL-6 range: 5-15 pg/mL). Dosing of TCZ ranged from 80-600 mg IV with an average of 1.47 doses per patient. Concurrent administration of methylprednisolone was given to 53% of patients. Five patients received 2 or more doses of TCZ and all five patients remained clinically stable. In 67% of patients, IL-6 levels initially increased sharply after TCZ but decreased thereafter. Death occurred in 20% of the population while the other 80% remained hospitalized at time of publication.
Xu and colleagues reported the use of TCZ in 21 Chinese patients diagnosed with COVID-19 [6]. The mean age of the cohort was 57 years and 86% of the population was male. The chronic illnesses of the patient population included hypertension, DM, stroke, and chronic obstructive pulmonary disease. Symptoms greater than 20% included fever, cough, phlegm, fatigue, and shortness of breath (SOB). The study reported that 19% of the patients were critically ill while 81% were seriously ill. Mean IL-6 levels prior to TCZ therapy were 132.38 pg/mL. All patients received lopinavir and methylprednisolone prior to TCZ therapy. Dosing of TCZ was 400 mg IV for one dose. No deaths were reported, 10% of the patients were hospitalized, and 90% of the patients were discharged at time of publication.
3.3. Case reports
Four patients were identified that received TCZ that were diagnosed with COVID-19 [[7], [8], [9], [10]]. Patient ages ranged from 42-60 years with 75% of the cases being male. Chronic illnesses varied including end-stage renal disease, coronary artery disease, cardiomyopathy, renal cell carcinoma, multiple myeloma, systemic sclerosis, type II DM, and obesity. Common symptoms across cases included cough, fever, and SOB. Three cases reported bilateral patchy groundglass opacities due to COVID-19 and one patient required mechanical ventilation. Regarding TCZ dosing, two of the cases reported giving TCZ at time of diagnosis for COVID-19 [8,10]. One of the cases had been receiving TCZ for three years prior to COVID-19 diagnosis and only had a mild case of COVID-19 [9]. All patients were alive at time of publication with 75% of the cases reporting recovery from COVID-19.
4. Discussion
TCZ is currently not approved by the Food and Drug Administration (FDA) for use in patients with COVID-19. However, TCZ is FDA approved for adults and pediatric patients 2 years of age or older receiving chimeric antigen receptor (CAR) T-cell therapy that experience severe or life-threatening CRS [11]. CRS is a systemic inflammatory response that can be triggered by a multitude of factors such as infections [12]. Commonly associated with CRS are respiratory symptoms ranging from cough and tachypnea in mild cases to acute respiratory distress syndrome (ARDS) that includes SOB, hypoxemia, and bilateral opacities on chest x-ray. Mechanical ventilation may be required in some ARDS cases. Similarly, COVID-19 can progress in the same fashion as the respiratory signs and symptoms associated with CRS [13]. Furthermore, elevated markers such as lactate dehydrogenase, c-reactive protein, and IL-6 are associated with CRS [12]. Interestingly, these same biomarkers have shown marked elevations in patients with severe cases of COVID-19 [3,14]. Considering that Luo and colleagues saw more than half of their patients have an elevation followed by gradual reduction in IL-6 levels following TCZ administration, IL-6 can be an important biomarker to trend regarding how well TCZ therapy is working in a COVID-19 patient [5].
For clinicians considering TCZ therapy, important monitoring parameters must be employed. All patients should obtain a latent tuberculosis (TB) test before TCZ therapy [11]. If the result is positive, TB treatment must be performed prior to administration since TCZ competes with IL-6 at the receptor level leading to reduced macrophage and cytotoxic T-cell differentiation; in turn, TCZ can reduce the antimycobacterial activity of IL-6 [15]. Also, patients should be monitored for TB while receiving treatment with TCZ. However, COVID-19 patients can deteriorate quickly where a latent TB test may not be possible prior to treatment. Data from a large number of clinical trials has shown that the risk for latent TB reactivation is very low or absent where the benefit of administering TCZ may outweigh this risk [15]. Furthermore, patients should be monitored for neutropenia, thrombocytopenia, elevated liver enzymes, and abnormal lipid tests while on TCZ [11]. Patients with increased risk for gastrointestinal perforation should be monitored while on TCZ. Hypersensitivity reactions, including anaphylaxis, can occur while on TCZ and therapy may need to be discontinued. Screening for hepatitis B should be performed prior to TCZ administration since those patients were excluded from clinical trials [11]. We emphasize that the aforementioned monitoring parameters for TCZ were from FDA approved indications and the safety profile for TCZ in COVID-19 patients is yet to be understood.
There are limitations to this systematic review. None of the studies included were randomized, controlled trials thus correlation does not imply causation regarding our findings. Furthermore, the total number of patients included in this review was small and may not be generalizable to the entire population of patients that may be treated with TCZ. Dosing of TCZ was inconsistent across studies and some cases did not state a specific dose. Lastly, the safety profile of TCZ therapy in patients with COVID-19 has not been reported.
Ongoing phase III clinical trials assessing TCZ in patients with COVID-19 and pending results will determine the extent of TCZ’s utility as shown in Table 3 (trial details can be found at www.clinicaltrials.gov). Although inhibition of IL-6 has received significant attention, additional pathways and treatments are actively being investigated (Table 4 ). Important questions that remain are related to whether TCZ can be used to prevent CRS from occurring in patients with COVID-19, identifying eligible patient characteristics that are likely to respond to TCZ, optimal TCZ dosing, and considerations for combination therapy with steroids or other cytokine inhibitors. Current guidance from both the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) make no recommendation for or against the use of tocilizumab in patients with COVID-19; however, both the IDSA and ATS agree that tocilizumab in the context of a clinical trial is worthwhile [16,17].
Table 3.
Ongoing phase III trials assessing tocilizumab monotherapy in patients with COVID-19.
| Trial (NCT identifier) | Population (estimated enrollment) | Intervention | Comparator | Primary outcome | Estimated completion |
|---|---|---|---|---|---|
| COVACTA (NCT04320615) | Hospitalized adults with COVID-19 with SPO2 ≤ 93% or PaO2/FiO2 < 300 mg (n = 330) | TCZ 8 mg/kg IV up to 800 mg x 1 dose (additional dose may be given if symptoms worsen/no improvement shown) | Placebo | Clinical status assessed using a 7-category ordinal scale at day 28 | September 30, 2021 |
Abbreviations: IV: intravenous; PaO2/FiO2: ratio of arterial oxygen pressure to fractional inspired oxygen; SPO2: oxygen saturation; TCZ: tocilizumab.
Table 4.
Alternative targets in the clinical pipeline for supportive care of patients with COVID-19.
| Trial (NCT identifier) | Agent | Target | Clinical trial phase |
|---|---|---|---|
| COV-AID (NCT04330638) | Anakinra | IL-1 | III |
| NCT04340232 | Baricitinib | JAK | II |
| NCT04324021 | Emapalumab | IFN-γ | II |
| NCT04276688 | Interferon beta-1b | IFN-β | II |
| CORIMUNO-VIRO (NCT04341870) | Sarilumab | IL-6 | II |
| COV-AID (NCT04330638) | Siltuximab | IL-6 | III |
Abbreviations: IFN-β: interferon beta; IFN-γ: interferon gamma; IL-1: interleukin-1; IL-6: interleukin-6; JAK: Janus kinase.
5. Conclusion
TCZ is an option for compassionate use in patients with COVID-19. Clinicians should consider enrolling COVID-19 patients in clinical trials evaluating the safety and efficacy of TCZ. If TCZ is to be used in a COVID-19 patient, screening and monitoring parameters, especially latent TB testing, should be performed prior and during TCZ therapy. Current phase III trials will be crucial in understanding the place in therapy of TCZ as a supportive care option in alleviating the severe respiratory symptoms associated with COVID-19.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
References
- 1.Maps & Trends. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/data. (Accessed April 13, 2020).
- 2.Lu C.C., Chen M.Y., Chang Y.L. Potential therapeutic agents against COVID-19: what we know so far. J Chin Med Assoc. 2020 doi: 10.1097/JCMA.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan J., Zou R., Zeng L. The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflamm Res. 2020 doi: 10.1007/s00011-020-01342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020 doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu X., Han M., Li T. Effective treatment of severe COVID-19 patients with tocilizumab. ChinaXiv. 2020;202003(00026):v1. doi: 10.12074/202003.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrey A.J., Choi G., Hanna R.M. A case of novel coronavirus disease 19 in a chronic hemodialysis patient presenting with gastroenteritis and developing severe pulmonary disease. Am J Nephrol. 2020:1–6. doi: 10.1159/000507417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michot J.M., Albiges L., Chaput N. Tocilizumab, an anti-IL6 receptor antibody, to treat Covid-19-related respiratory failure: a case report. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.03.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mihai C., Dobrota R., Schröder M. COVID-19 in a patient with systemic sclerosis treated with tocilizumab for SSc-ILD. Ann Rheum Dis. 2020;79(5):668–669. doi: 10.1136/annrheumdis-2020-217442. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X., Song K., Tong F. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4(7):1307–1310. doi: 10.1182/bloodadvances.2020001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genentech, Inc.; South San Francisco, CA: 2017. Actemra [Package Insert] [Google Scholar]
- 12.Shimabukuro-Vornhagen A., Gödel P., Subklewe M. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantini F., Nannini C., Niccoli L., Petrone L., Ippolito G., Goletti D. Risk of tuberculosis reactivation in patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis receiving non-anti-TNF-targeted biologics. Mediators Inflamm. 2017 doi: 10.1155/2017/8909834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhimraj A., Morgan R.L., Schumaker A.H. Infectious Diseases Society of America; 2011. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19 Infection.https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ Published April 11, 2020. (Accessed April 12, 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson K.C., Chotirmall S.H., Bai C., Rello J. American Thoracic Society. 2020. Interim Guidance on Management Pending Empirical Evidence.https://www.thoracic.org/professionals/clinical-resources/disease-related-resources/covid-19-guidance.pdf Published April 3, 2020. (Accessed 12 April 2020) [Google Scholar]