Graphical abstract

Keywords: Aminoglycosides, Cochlear toxicity, Nephrotoxicity, Antibiotics
Abstract
Aminoglycoside antibiotics, used to treat persistent gram-negative infections, tuberculosis, and life-threatening infections in neonates and patients with cystic fibrosis, can infer acute kidney injury and irreversible hearing loss. The full repertoire of cellular targets and processes leading to the toxicity of aminoglycosides is not fully resolved, making it challenging to devise rational directions to circumvent their adverse effects. As a result, there has been very limited effort to rationally address the issue of aminoglycoside-induced toxicity. Here we provide an overview of the reported effects of aminoglycosides on cells of the inner ear and on kidney tubular epithelial cells. We describe selected examples for structure–toxicity relationships established by evaluation of both natural and semisynthetic aminoglycosides. The various assays and models used to evaluate these antibiotics and recent progress in development of safer aminoglycoside antibiotics are discussed.
Streptomycin was isolated from the soil bacterial actinomycete Streptomyces griseus in 1943.1 Its characterization led to the development of the aminoglycoside (AG) class of antibiotics, which are important for veterinary use as well as for treatment of Gram negative infections in humans.1, 2 Over eight decades since their discovery, AGs continuously attract the interest of the scientific community; a quick search on SciFinder revealed that since the discovery of streptomycin over 46,000 papers have been published on the topic of AGs, more than 900 of them in 2019.
AG antibiotics perturb with the fidelity of bacterial protein synthesis by binding to the decoding A-site region of the bacterial ribosome.3, 4, 5 Despite an alarming increase in the emergence of bacterial pathogens with resistance to at least one of the clinically used AGs, members of this class of antibiotics are still highly effective against a broad spectrum of Gram-negative pathogens.6 AGs are successfully used for treatment of cystic fibrosis patients who suffer from severe and reoccurring pulmonary infections7, 8 and, more generally, for treatment of patients with severe bacterial infections.9 To date, multiple AGs have been isolated and thousands of semi-synthetic derivatives have been synthesized in an attempt to improve their pharmacological properties and to reveal structure–activity relationship.10, 11, 12, 13, 14, 15, 16 Only a handful of natural and semisynthetic AGs are currently in clinical use, however. These include neomycin, tobramycin, gentamicin, and the semisynthetic derivative of the natural AG kanamycin A known as amikacin17.
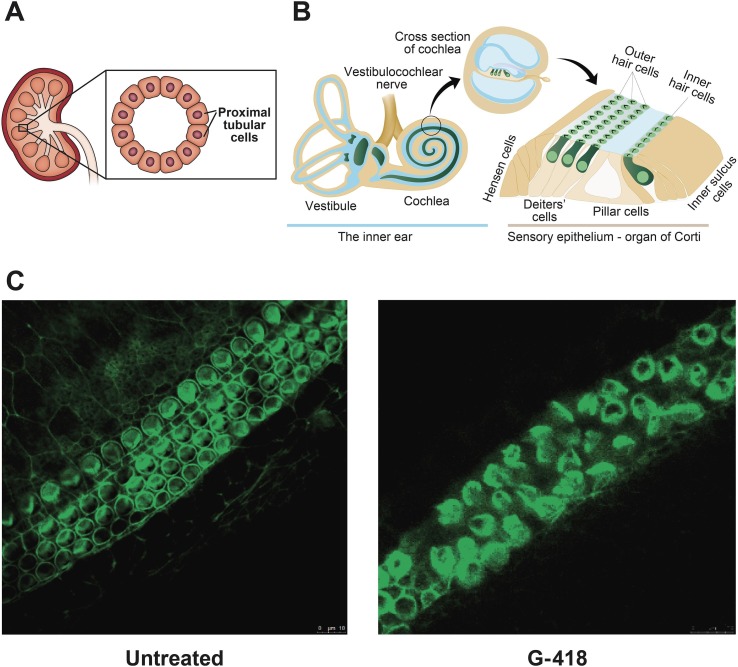
Unfortunately, improvement in the prognosis of patients treated with AGs is overshadowed by frequently occurring toxic side effects that render them last-resort antibiotics. The main side effects caused are ototoxicity and nephrotoxicity.18, 19 In kidney proximal tubular cells (Fig. 1 A), AGs perturb with intracellular processes and induce the death of epithelial cells leading to tubular necrosis. Vestibular toxicity occurs due to damage to the vestibular cranial nerve branch responsible for balance, and cochlear toxicity occurs due to damage to cochlear hair cells and to the cranial nerve branch responsible for hearing (Fig. 1B). An example of the destructive effects of geneticin, also termed G-418, on explants of mouse organ of corti is shown in Fig. 1C. Due to cochlear hair cell death, ototoxicity is mostly irreversible. Ototoxicity is dose dependent, and certain patients are genetically more sensitive to the damaging effects of these antibiotics.20, 21, 22, 23, 24 Notably, when administered through inhalation for treatment of respiratory infections, the toxic side-effects of AGs are mitigated significantly.25, 26, 27
Fig. 1.
Treatment with AGs is frequently accompanied by nephrotoxic and ototoxic side-effects. (A) Schematic illustration of kidney proximal tubular cells. (B) Schematic illustration of the inner ear. (C) Images of organotypic cultures of mouse organ of corti cells cultured with or without 0.02 mM G-418 for 24 h and stained for actin.
The molecular basis for the different mechanisms responsible for resistance to AGs have been extensively studied, and detailed structural information about the enzymes that confer resistance to AGs through chemical modifications, the most frequently encountered bacterial resistance to AGs, is available.6 In contrast, the full scope of cellular targets directly and indirectly affected by AGs leading to nephrotoxic and ototoxic side effects has not yet been resolved, making it challenging to devise rational directions to circumvent these toxicities. Success has been achieved in the development of semi-synthetic AGs that overcome enzyme-mediated deactivation. For example, dibekacin, arbekacin, and amikacin were approved for clinical use in the early 1970s, and positive phase III clinical trial results were reported for plazomicin, a semisynthetic derivative of the AG sisomicin, in 2018.12, 28, 29 In contrast, there has been little effort to address the issue of AG-induced toxicity and, to date, no AGs modified with the intention of reducing ototoxicity and nephrotoxicity are in clinical use. In this digest article we provide a short overview of the current mechanistic understanding of AG-induced toxicity and how structure–toxicity relationships can be studied using various models.
At the phenotypic level, several activity-enhancement effects have been documented in mammalian cells exposed to AGs. These include enhancement of protein cleavage by calcium-dependent cysteine protease, of production of reactive oxygen species, of activation of c-Jun N-terminal kinases, of release of cytochrome c from mitochondria, and of activation of caspases.30, 31, 32, 33 The cellular targets that trigger these effects through binding of AGs are still largely unknown.
Amongst the molecules known to interact with AGs is ferric iron (Fe3+), which binds to AGs to form Fe2+– AG complexes in the cytosol.34 Other molecules that effectively bind AGs include phospholipids and RNA and DNA that are highly negatively charged under physiological conditions. Several specific AG-binding proteins have been discovered in recent years including HSP73, calreticulin and CLIMP-63; these proteins are known to activate caspases and Bcl-2 signaling cascades that are known to be involved in the toxicity of AGs to mammalian cells.35 No structures of these proteins in complex with AGs are available, currently making it challenging to rationally design AGs with potent antibacterial activity yet reduced affinity for these AG-binding proteins.
Evidence suggests that perturbation of eukaryotic translation is a major mechanism for the toxicity of AGs: The relatively high sequence similarity between the decoding A-site of bacterial ribosomes, the main target of AGs in the ribosomal complex, and that of the eukaryotic cytosolic and mitochondrial ribosomes leads to limited selectivity. For example, G-418, a highly toxic AG banned for clinical use, binds both the bacterial and the eukaryotic ribosomes with high affinity. 36, 37 Its high affinity to the eukaryotic decoding region results from high conformational plasticity between the target ribonucleotides and this AG. Convincing evidence for the effects of AGs on the two eukaryotic translation machineries were obtained through isolation of mitochondria from mammalian cells treated with different AGs and by development of in vitro translation assays using bacterial ribosomes engineered to have the A-site sequence of the human mitochondrial and cytosolic ribosomes.38, 39, 40, 41 A collection of X-ray structures of complexes between AGs and sequences of the bacterial and eukaryotic cytosolic and mitochondrial A-sites are available thereby setting the stage for rational design of AGs with higher selectivity for the bacterial ribosome.42, 43, 44, 45
AG-induced perturbation of translation can take place in any eukaryotic cell, however, several unique features in inner ear hair cells and kidney proximal tubular epithelial cells make them more vulnerable to these antibiotics. AG-induced nephrotoxicity results from excessive accumulation of the antibiotic in the proximal tubular cells of the kidneys (Fig. 1A).18 Since AGs are largely unaffected by metabolism, they reach the kidneys intact and in high concentrations, therefore increasing the risk of acute kidney injury and chronic kidney disease. Treatment with AGs can, therefore, result in severe damage to kidney functions, and these antibiotics cannot be prescribed to patients suffering from a chronic kidney disease. Between 5 and 10% of adult patients treated with AGs have significant increases in serum creatinine, indicative of kidney malfunction.8 Fortunately, since proximal tubular cells can regenerate, nephrotoxicity is usually reversible.
One of the unique properties of the cochlear hair cells is high endocochlear potential (+80 mV) that drives positively charged ions into these cells.35 This endocochlear potential results from a high concentration of K+ ions that accumulate in the hair cells through mechanically gated channels located in the sensory hair cell bundles; these channels are non-selective cation carriers and allow positively charged molecules like AGs to enter the hair cell.
We recently provided evidence that for some AGs (e.g., G-418), the dominant toxic effect on auditory cells is likely an increase in cell permeabilization.11 In addition, we showed that stress responses in mammalian cells treated with certain AGs, which had been largely overlooked, may lead to over expression of certain proteins rather than a general inhibition of translation; this suggests that small-molecule based intervention in stress response pathways may help reduce the toxic effects of AGs. Notably, the numerous studies published over the past decades have provided evidence that not all AGs exert the same toxic side effects and that even subtle structural differences fundamentally change the toxicity profile.46, 47 This strongly suggests that chemical modifications can be used as an avenue to reduce the toxicity of AGs without diminishing their antibacterial potency.
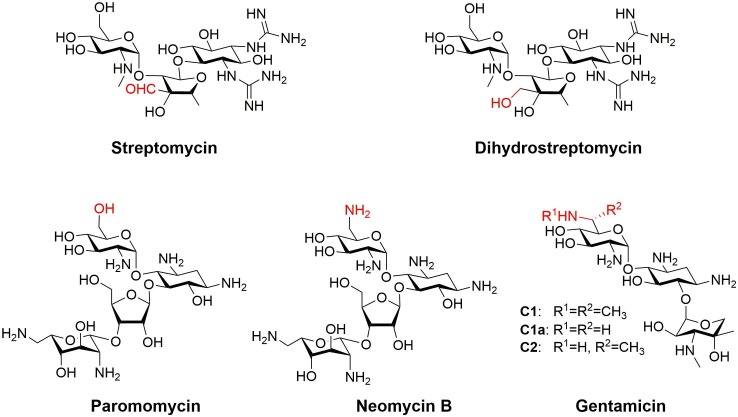
Soon after the discovery of the first AGs, it became evident that structural differences can significantly affect toxicity but not abrogate antibacterial activity. Developed in 1946, dihydrostreptomycin is a semisynthetic derivative of streptomycin (Fig. 2 ).48 Dihydrostreptomycin is generated by a single-step reduction of the aldehyde functionality of the parent AG to the corresponding primary alcohol. Streptomycin and Dihydrostreptomycin have similar antibacterial potencies yet the latter has improved stability.49 Although vestibular damage is less common with dihydrostreptomycin than with streptomycin, dihydrostreptomycin more frequently causes hearing loss and symptoms are more severe than those caused by the parent AG. This resulted in removal of the first semisynthetic AG from the market.49
Fig. 2.
Structures of the AGs streptomycin and its semisynthetic derivative dihydrostreptomycin and of paromomycin, neomycin B and gentamicin.
Durán and co-workers compared the ototoxicity of paromomycin, neomycin, gentamicin (a mixture composed of a number of structurally related AGs), and gentamicin C1a, which is a major component of the gentamicin mixture (Fig. 2).46 Paromomycin and neomycin B differ solely by the chemical group at the 6′ position: an alcohol in the case of paromomycin and a primary amine in the case of neomycin B. Comparison of mouse cochlear hair cell survival in the presence of these two antibiotics revealed that paromomycin reduced hair cell viability by approximately 4% and that neomycin B reduced the viability by approximately 20%. The gentamicin mixture was more toxic than was its component gentamicin C1a; there the AGs reduced survival of hair cells by approximately 66% and 33%, respectively.
Böttger and Crich and co-workers used an in vitro translation model to compare the selectivity of neomycin B and paromomycin for eukaryotic versus bacterial ribosomes.16 They showed that paromomycin inhibited translation of hybrid bacterial ribosomes containing the A-site sequence of mammalian cytosolic ribosomes more potently than neomycin B.16 In contrast, neomycin B inhibited translation by hybrid ribosomes containing the eukaryotic mitochondrial A-site more potently than paromomycin.
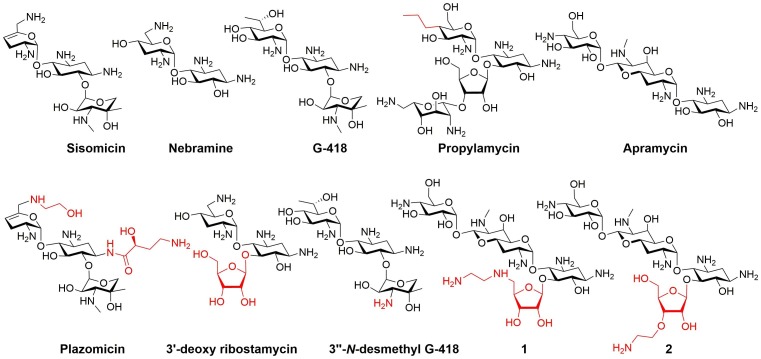
The newest semisynthetic AG plazomicin (also known as ACHN-490), developed by Achaogen (San Francisco, CA, USA), passed phase III clinical trial and was approved for treatment of severe urinary tracts infections in 2018.50 This AG derivative has two additions to the parental sisomicin skeleton at its N-1 and N-6′ positions (Fig. 3 ).29 Although the design of plazomicin was not intended to reduce AG toxicity, plazomicin did not induce significant ototoxicity in a guinea pig model. In a preliminary study in humans an increase in serum creatinine occurred in 7% of the patients and no significant ototoxic effects were reported.50, 51
Fig. 3.
Structures of natural AGs and of semisynthetic analogs.
We previously demonstrated that 5-O-ribosylation of 4,6-disubstituted-2-deoxystreptamine AGs containing a 2′-equatorial amine resulted in improved selectivity for inhibition of prokaryotic over eukaryotic translation by cytosolic ribosomes.10 For example, 5-O-ribosylation of nebramine, which yield 3′-deoxy ribostamycin (Fig. 3) resulted in an increase of about 4-fold in the ratio of the IC50 value for inhibition of eukaryotic translation divided by the IC50 value for inhibition of prokaryotic translation compared to the ratio of the parent nebramine. The improved selectivity resulted from improvement in the inhibition of prokaryotic translation and reduction in the inhibition of cytosolic eukaryotic translation.10 More recently we showed that 3′′-N-demethylation of G-418 to yield 3′′-N-desmethyl G-418 (Fig. 3) markedly improved selectivity for inhibition of E. coli ribosomes compared to eukaryotic ribosomes.11 Molecular dynamics simulations of complexes between these AGs and rRNA fragments corresponding to bacterial, eukaryotic cytosolic, and eukaryotic mitochondrial A-sites indicated that the minor N-demethylation modification to the G-418 scaffold, significantly altered the effect of the AG on the flexibility of the A-site. Furthermore, differences in A-site sequences, such as those that differentiate between the mitochondrial and bacterial A-sites, resulted in significant differences in interactions with AGs.11
As an inner ear cell model immortalized mouse inner ear cells that express markers of auditory sensory cells (HEI-OC1 cells) were used to further assess cell viability in the presence of 3″-N-desmethyl G-418 and the parent AG.52, 53 The effect of the AGs on the viability of HEI-OC1 cells correlated well with their effects on cytosolic translation in vitro: G-418 inhibited eukaryotic cytosolic translation about 17-fold more potently than its 3′′-N-desmethyl derivative, and the parent AG was about an order of magnitude (~9-fold) more toxic to HEI-OC1 cells than its 3′′-N-desmethyl derivative. This result is of particular interest since N-demethylation of G-418 reduced the undesired inhibition of cytosolic eukaryotic translation without significantly affecting its antibacterial activity.11
Propylamycin (Fig. 3), a semisynthetic AG developed by Crich and co-workers, is another example of an AG carrying a simple modification that shows improved selectivity for the prokaryotic ribosome over both mitochondrial and cytosolic hybrid ribosomes.14 The ototoxicity of propylamycin was assessed in the guinea pig model, in which the shift in threshold of auditory brain stem responses is used as a measure of ototoxicity. At all three frequencies tested, the threshold shifts for propylamycin only marginally increased from those of the untreated control animals over the tested dose range. In contrast, the clinically used AG gentamicin caused significant shifts. Microscopic examination of the guinea pigs' cochlear hair cells revealed the reduced ototoxicity of propylamycin compared to gentamicin. After propylamycin treatment, cochlear hair cells were comparable to those of the control, even in the basal turn, the region most sensitive to AGs, although gentamicin treatment resulted in extensive damage.
Another study by the same group of a series of 5-O-(d-ribofuranosyl) apramycin derivatives indicated that certain modifications enhance selectivity for bacterial ribosomes.13 For example, derivative 1 (Fig. 3), with an ethylene diamine modification at the C-5 position of the ribofuranose, was a more effective inhibitor of bacterial ribosome-mediated translation than apramycin without an undesired increase in inhibition of translation mediated by the hybrid ribosomes with eukaryotic A-sites. Moreover, in the mouse cochlear explant model, derivative 1 had a significantly higher LD50 than apramycin. Derivative 2, with an amino ethyl modification at the C-3 position of the ribofuranose (Fig. 3), had lower selectivity for bacterial relative to the mitochondrial hybrid ribosome but higher selectivity for the bacterial compared to the cytosolic hybrid ribosome than the parent.13 This derivative was further tested in a mouse model of infection with E. coli and displayed a significantly better clearance of the infection than apramycin.
Concluding remarks
Despite the high percentage of patients who suffer from ototoxic and/or nephrotoxic side effects following treatment with AGs, the well-established efficacy of these antibiotics, especially against reoccurring Gram-negative infections, makes them an irreplaceable component of the current World Health Organization's arsenal of antibiotics. Although AG-induced toxicity has been poorly addressed by drug designers, in recent years several groups have made progress toward deciphering how AGs influence various processes in eukaryotic cells with the goal of drawing structure–toxicity relationship guidelines that will facilitate the development of safer AG antibiotics. It is well established that the extent of perturbation of the two eukaryotic translation machineries, the ribosomes in the cytosol and those in the mitochondria, correlates well with the toxicity of AGs. Therefore, in vitro translation assays using bacterial or mammalian ribosomes and hybrid bacterial ribosomes containing the A-sites of either the mammalian cytosolic or mitochondrial ribosomes are useful tools to predict the extent of toxicity of most AG derivatives. However, due to the tremendous structural diversity among different members of the AG class of antibiotics, it is not surprising that we and others have provided experimental evidence for additional effects of AGs such as membrane permeabilization and stress response-mediated effects. These effects likely result from the interactions of AGs with targets other than the translation machineries, and their weight in the overall toxicity of the antibiotic depends on the specific AG and on the test model. Fortunately, chemical modification strategies can be applied to moderate the non-ribosomal effects as well.
Selective delivery is another strategy that should be considered and further developed to reduce the toxicity of AGs. The fact that AG administration through inhalation, used for treatment of respiratory bacterial infections, reduces significantly their toxic side-effects, supports this hypothesis.
Ideally, AGs should be resistant to enzyme-mediated deactivation and not toxic to mammalian cells. The recent example of plazomicin, the semisynthetic sisomicin derivative that gained FDA approval in 2018, provides hope that AGs can be modified to both block resistance mechanisms and to moderate toxicity. This AG is not deactivated by most known AG-modifying enzymes and has not shown significant toxic side effects in animal models. While this AG did induce nephrotoxic effects in humans, it caused no significant ototoxic side effects during clinical trials. Moreover, recent examples reported by our group and by Crich and co-workers clearly demonstrate that chemical modifications can be harnessed to significantly improve the selectivity of AGs for the bacterial translation machinery and to reduce off-ribosomal toxicities without a significant loss of antibacterial activity.
Despite their toxicity and, although resistance to AGs is on the rise, in recent decades, the pharmaceutical industry has shown little interest in development of novel AG antibiotics likely due to low profits that result from the relatively rapid decline in efficacy due to emergence of resistance. Hopefully, the current state will change before an international health crisis draws humanity one more step back to the pre-antibiotic era. The current lack of effective drugs for treatment of the current COVID-19 pandemic caused by SARS-CoV-2 is an example for the terrible effects that infectious diseases can inflict on human health and on global economy. In the meantime, it is imperative that researchers continue to study the mechanisms of toxicities caused by AGs and structure–toxicity and structure-resistance relationships to pave the way for development of AGs with improved toxicity profiles and high efficacy against resistant bacteria.
Author contributions
Moriah Jospe-Kaufman and Micha Fridman wrote the manuscript, Liza Siomin evaluated the effect of G-418 on explants of organ of corti and generated the images in Fig. 1C.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgments
We are thankful to Dr. Amiel Dror and Prof. Karen Avraham, Department of Human Molecular Genetics & Biochemistry, Sackler Faculty of Medicine, Tel Aviv University for generating Fig. 1B. This work was supported by Tel Aviv University Breakthrough Innovative Research Grant (Karen Avraham and Micha Fridman). Moriah Jospe-Kaufman thanks the Israeli Ministry of Science, Technology & Space for the Levi Eshkol Scholarship (Scholarship number 315461).
References
- 1.Schatz A., Bugle E., Waksman S.A. Streptomycin, a Substance Exhibiting Antibiotic Activity Against Gram-Positive and Gram-Negative Bacteria. Exp Biol Med. 1944;55(1):66–69. doi: 10.3181/00379727-55-14461. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi Y., Igarashi M. Destination of aminoglycoside antibiotics in the ‘post-antibiotic era’. Nat Publ Gr. 2018;71(1):4–14. doi: 10.1038/ja.2017.117. [DOI] [PubMed] [Google Scholar]
- 3.Berkov-Zrihen Y, Fridman M. Synthesis of aminoglycosides. Mod Synth Methods Carbohydr Chem From Monosaccharides to Complex Glycoconjugates. 2013:161-190. doi:10.1002/9783527658947.ch6.
- 4.François B., Russell R.J., Murray J.B. Crystal structures of complexes between aminoglycosides and decoding A site oligonucleotides: role of the number of rings and positive charges in the specific binding leading to miscoding. Nucleic Acids Res. 2005;33(17):5677–5690. doi: 10.1093/nar/gki862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pape T., Wintermeyer W., Rodnina M.V. Conformational switch in the decoding region of 16S rRNA during aminoacyl-tRNA selection on the ribosome. Nat Struct Biol. 2000;7(2):104–107. doi: 10.1038/72364. [DOI] [PubMed] [Google Scholar]
- 6.Garneau-Tsodikova S., Labby K.J. Mechanisms of resistance to aminoglycoside antibiotics: Overview and perspectives. Medchemcomm. 2016;7(1):11–27. doi: 10.1039/c5md00344j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonsen K.A., Anderson-berry A.L., Delair S.F., Dele H. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014;27(1):21–47. doi: 10.1128/CMR.00031-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard M., Frizzell R.A., Bedwell D.M. Aminoglycoside antibiotics restore CFTR function by overcoming premature stop mutations. Nat Med. 1996;2(4):467–469. doi: 10.1038/nm0496-467. [DOI] [PubMed] [Google Scholar]
- 9.Francis S.P., Katz J., Fanning K.D. A novel role of cytosolic protein synthesis inhibition in aminoglycoside ototoxicity. J Neurosci. 2013;33(7):3079–3093. doi: 10.1523/JNEUROSCI.3430-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herzog I.M., Louzoun Zada S., Fridman M. Effects of 5-O-ribosylation of aminoglycosides on antimicrobial activity and selective perturbation of bacterial translation. J Med Chem. 2016;59(17):8008–8018. doi: 10.1021/acs.jmedchem.6b00793. [DOI] [PubMed] [Google Scholar]
- 11.Zada S.L., Ben Baruch B, Simhaev L., Engel H., Fridman M. Chemical modifications reduce auditory cell damage induced by aminoglycoside antibiotics chemical modifications reduce auditory cell damage induced by aminoglycoside antibiotics. J Am Chem Soc. 2020;142(6):3077–3087. doi: 10.1021/jacs.9b12420. [DOI] [PubMed] [Google Scholar]
- 12.Cox G., Ejim L., Stogios P.J. Plazomicin retains antibiotic activity against most aminoglycoside modifying enzymes. ACS Infect Dis. 2018;4(6):980–987. doi: 10.1021/acsinfecdis.8b00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quirke JCK, Rajasekaran P, Sarpe VA, Sonous A, Osinnii I, Gysin M, Haldimann K, Fang Q, Shcherbakov D, Hobbie SN, Sha S, Schacht J, Vasella A, Böttger EC, Crich D. Apralogs: Apramycin 5 ‑ O ‑ Glycosides and Ethers with Improved Antibacterial Activity and Ribosomal Selectivity and Reduced Susceptibility to the Aminoacyltranserferase (3)-IV Resistance Determinant. 2020;142(1):530-544. doi:10.1021/jacs.9b11601. [DOI] [PMC free article] [PubMed]
- 14.Matsushita T., Sati G.C., Kondasinghe N. Design, multigram synthesis, and in vitro and in vivo evaluation of propylamycin: a semisynthetic 4,5-deoxystreptamine class aminoglycoside for the treatment of drug-resistant enterobacteriaceae and other gram-negative pathogens. J Am Chem Soc. 2019;141(12):5051–5061. doi: 10.1021/jacs.9b01693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fair RJ, Mccoy LS, Hensler ME, Aguilar B, Nizet V. Singly Modified Amikacin and Tobramycin Derivatives Show Increased rRNA A-Site Binding and Higher Potency against Resistant Bacteria. 2014; (9):2164-2171. doi:10.1002/cmdc.201402175. [DOI] [PMC free article] [PubMed]
- 16.Sati G.C., Shcherbakov D., Hobbie S.N., Vasella A., Böttger E.C., Crich D. N6′, N6′′′, and O4′ modifications to neomycin affect ribosomal selectivity without compromising antibacterial activity. ACS Infect Dis. 2017;3(5):368–377. doi: 10.1021/acsinfecdis.6b00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie J., Talaska A.E., Schacht J. New developments in aminoglycoside therapy and ototoxicity. Hear Res. 2011;281(1–2):28–37. doi: 10.1016/j.heares.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rougier F., Claude D., Maurin M., Maire P. Aminoglycoside nephrotoxicity. Curr Drug Targets - Infect Disord. 2004;4(2):153–162. doi: 10.2174/1568005043340858. [DOI] [PubMed] [Google Scholar]
- 19.Selimoglu E. Aminoglycoside-induced ototoxicity. Curr Pharm Des. 2007:119–126. doi: 10.2174/138161207779313731. [DOI] [PubMed] [Google Scholar]
- 20.O’Sullivan M.E., Perez A., Lin R., Sajjadi A., Ricci A.J., Cheng A.G. Towards the prevention of aminoglycoside-related hearing loss. Front Cell Neurosci. 2017;11:325. doi: 10.3389/fncel.2017.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lacy M.K., Nicolau D.P., Nightingale C.H., Quintiliani R. The pharmacodynamics of aminoglycosides. Clin Infect Dis. 1998;27(1):23–27. doi: 10.1086/514620. [DOI] [PubMed] [Google Scholar]
- 22.Igumnova V., Veidemane L., Vīksna A., Capligina V., Zole E., Ranka R. The prevalence of mitochondrial mutations associated with aminoglycoside-induced deafness in ethnic Latvian population: the appraisal of the evidence. J Hum Genet. 2019;64(3):199–206. doi: 10.1038/s10038-018-0544-6. [DOI] [PubMed] [Google Scholar]
- 23.Prezant T.R., Agapian J.V., Bohlman M.C. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat Genet. 1993;4(3):289–294. doi: 10.1038/ng0793-289. [DOI] [PubMed] [Google Scholar]
- 24.Kros C.J., Desmonds T. Drug-induced hearing loss: Infection raises the odds. Sci Transl Med. 2015;7(298):1–4. doi: 10.1126/scitranslmed.aac9811. [DOI] [PubMed] [Google Scholar]
- 25.LoBue P.A. Inhaled Tobramycin. Chest. 2005;127(4):1098–1101. doi: 10.1016/s0012-3692(15)34452-4. [DOI] [PubMed] [Google Scholar]
- 26.Yagi K., Ishii M., Namkoong H. The efficacy, safety, and feasibility of inhaled amikacin for the treatment of difficult-to-treat non-tuberculous mycobacterial lung diseases. BMC Infect Dis. 2017;17(1):1–9. doi: 10.1186/s12879-017-2665-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsey B.W., Pepe M.S., Quan J.M. Intermitted administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med. 1999;340(1):23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 28.Kawaguchi H., Naito T., Nakagawa S., Fujisawa K.-I. BB-K8, A new semisynthetic aminoglycoside antibiotic. J Antibiot (Tokyo) 1972;25(12):695–708. doi: 10.7164/antibiotics.25.695. [DOI] [PubMed] [Google Scholar]
- 29.Aggen J.B., Armstrong E.S., Goldblum A.A. Synthesis and spectrum of the neoglycoside ACHN-490. Antimicrob Agents Chemother. 2010;54(11):4636–4642. doi: 10.1128/AAC.00572-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagai J., Takano M. Molecular aspects of renal handling of aminoglycosides and strategies for preventing the nephrotoxicity. Drug Metab Pharmacokinet. 2004;19(3):159–170. doi: 10.2133/dmpk.19.159. [DOI] [PubMed] [Google Scholar]
- 31.Davis R.J. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103(2):239–252. doi: 10.1016/S0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 32.Matsui J.I., Gale J.E., Warchol M.E. Critical signaling events during the aminoglycoside-induced death of sensory hair cells in vitro. J Neurobiol. 2004;61(2):250–266. doi: 10.1002/neu.20054. [DOI] [PubMed] [Google Scholar]
- 33.Mangiardi D.A., McLaughlin-Williamson K., May K.E., Messana E.P., Mountain D.C., Cotanche D.A. Progression of hair cell ejection and molecular markers of apoptosis in the avian cochlea following gentamicin treatment. J Comp Neurol. 2004;475(1):1–18. doi: 10.1002/cne.20129. [DOI] [PubMed] [Google Scholar]
- 34.Guthrie O.W. Aminoglycoside induced ototoxicity. Toxicology. 2008;249(2–3):91–96. doi: 10.1016/j.tox.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 35.Karasawa T., Steyger P.S. Intracellular mechanisms of aminoglycoside-induced cytotoxicity. Integr Biol. 2011;3(9):879–886. doi: 10.1039/c1ib00034a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prokhorova I., Altman R.B., Djumagulov M. Aminoglycoside interactions and impacts on the eukaryotic ribosome. Proc Natl Acad Sci U S A. 2017;114(51):E10899–E10908. doi: 10.1073/pnas.1715501114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garreau De Loubresse N., Prokhorova I., Holtkamp W., Rodnina M.V., Yusupova G., Yusupov M. Structural basis for the inhibition of the eukaryotic ribosome. Nature. 2014;513(7519):517–522. doi: 10.1038/nature13737. [DOI] [PubMed] [Google Scholar]
- 38.Hobbie S.N., Akshay S., Kalapala S.K., Bruell C.M., Shcherbakov D., Böttger E.C. Genetic analysis of interactions with eukaryotic rRNA identify the mitoribosome as target in aminoglycoside ototoxicity. Proc Natl Acad Sci U S A. 2008;105(52):20888–20893. doi: 10.1073/pnas.0811258106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Böttger E.C., Schacht J. The mitochondrion : A perpetrator of acquired hearing loss. Hear Res. 2013;303:12–19. doi: 10.1016/j.heares.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kandasamy J., Atia-Glikin D., Shulman E. Increased selectivity toward cytoplasmic versus mitochondrial ribosome confers improved efficiency of synthetic aminoglycosides in fixing damaged genes: a strategy for treatment of genetic diseases caused by nonsense mutations. J Med Chem. 2012;55(23):10630–10643. doi: 10.1021/jm3012992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matt T., Ng C.L., Lang K. Dissociation of antibacterial activity and aminoglycoside ototoxicity in the 4-monosubstituted 2-deoxystreptamine apramycin. Proc Natl Acad Sci U S A. 2012;109(27):10984–10989. doi: 10.1073/pnas.1204073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vicens Q., Westhof E. Crystal structure of paromomycin docked into the eubacterial ribosomal decoding A site. Structure. 2001;9(8):647–658. doi: 10.1016/S0969-2126(01)00629-3. [DOI] [PubMed] [Google Scholar]
- 43.Kondo J., Koganei M., Kasahara T. Crystal structure and specific binding mode of sisomicin to the bacterial ribosomal decoding site. ACS Med Chem Lett. 2012;3(9):741–744. doi: 10.1021/ml300145y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hermann T., Tereshko V., Skripkin E., Patel D.J. Apramycin recognition by the human ribosomal decoding site. Blood Cells, Mol Dis. 2007;38(3):193–198. doi: 10.1016/j.bcmd.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Vicens Q., Westhof E. Crystal structure of a complex between the aminoglycoside tobramycin and an oligonucleotide containing the ribosomal decoding a site. Chem Biol. 2002;9(6):747–755. doi: 10.1016/S1074-5521(02)00153-9. [DOI] [PubMed] [Google Scholar]
- 46.Ishikawa M., García-Mateo N., Čusak A. Lower ototoxicity and absence of hidden hearing loss point to gentamicin C1a and apramycin as promising antibiotics for clinical use. Sci Rep. 2019;9(1):1–15. doi: 10.1038/s41598-019-38634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kobayashi M., Sone M., Umemura M., Nabeshima T., Nakashima T., Hellström S. Comparisons of cochleotoxicity among three gentamicin compounds following intratympanic application. Acta Otolaryngol. 2008;128(3):245–249. doi: 10.1080/00016480701558948. [DOI] [PubMed] [Google Scholar]
- 48.Bartz Q.R., Controulis J., Crooks H.M., Rebstock M.C. Dihydrostreptomycin. J Am Chem Soc. 1946;68(11):2163–2166. doi: 10.1021/ja01215a013. [DOI] [PubMed] [Google Scholar]
- 49.Glorig A. The effect of dihydrostreptomycin hydro- chloride and sulfate on the auditory mechanism. Ann Otol Rhinol Larynology. 1951;60(2):327–335. doi: 10.1177/000348945106000204. [DOI] [PubMed] [Google Scholar]
- 50.Shaeer K.M., Zmarlicka M.T., Chahine E.B., Piccicacco N., Cho J.C. Plazomicin: A Next-Generation Aminoglycoside. Pharmacotherapy. 2019;39(1):77–93. doi: 10.1002/phar.2203. [DOI] [PubMed] [Google Scholar]
- 51.Cass R.T., Brooks C.D., Havrilla N.A. Pharmacokinetics and safety of single and multiple doses of ACHN-490 injection administered intravenously in healthy subjects. Antimicrob Agents Chemother. 2011;55(12):5874–5880. doi: 10.1128/AAC.00624-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalinec G., Thein P., Park C., Kalinec F. HEI-OC1 cells as a model for investigating drug cytotoxicity. Hear Res. 2016;335:105–117. doi: 10.1016/j.heares.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 53.Kalinec G.M., Fernandez-Zapico M.E., Urrutia R., Esteban-Cruciani N., Chen S., Kalinec F. Pivotal role of Harakiri in the induction and prevention of gentamicin-induced hearing loss. Proc Natl Acad Sci U S A. 2005;102(44):16019–16024. doi: 10.1073/pnas.0508053102. [DOI] [PMC free article] [PubMed] [Google Scholar]