Graphical abstract
Chemical compounds studied in this article: Diminazene aceturate (PubChem CID: 5284544), Resorcinolnaphthalein (PubChem CID: 337218), Xanthenone (PubChem CID: 7020)
Abbreviations: ACE2, angiotensin-converting enzyme 2; ACEI, ACE inhibitor; Ang, angiotensin; ARB, angiotensin II receptor blocker; ARDS, acute respiratory distress syndrome; AT2, type II apical surface of epithelial cells; COPD, chronic obstructive pulmonary disease; COVID-19, Coronavirus disease 2019; DIC, disseminated intravascular coagulopathy; HIF-1α, hypoxia-inducible factor-1α; hrsACE2, human recombinant soluble ACE2; PAH, pulmonary arterial hypertension; rACE2, recombinant ACE2; RAS, renin-angiotensin system; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Keywords: Angiotensin-converting enzyme 2, Severe acute respiratory syndrome coronavirus 2, Coronavirus disease 2019
Abstract
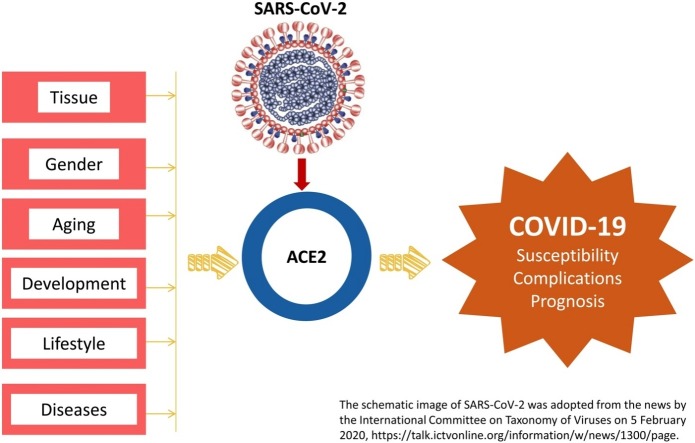
The renin-angiotensin system (RAS) is crucial for the physiology and pathology of all the organs. Angiotensin-converting enzyme 2 (ACE2) maintains the homeostasis of RAS as a negative regulator. Recently, ACE2 was identified as the receptor of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the coronavirus that is causing the pandemic of Coronavirus disease 2019 (COVID-19). Since SARS-CoV-2 must bind with ACE2 before entering the host cells in humans, the distribution and expression of ACE2 may be critical for the target organ of the SARS-CoV-2 infection. Moreover, accumulating evidence has demonstrated the implication of ACE2 in the pathological progression in tissue injury and several chronic diseases, ACE2 may also be essential in the progression and clinical outcomes of COVID-19. Therefore, we summarized the expression and activity of ACE2 in various physiological and pathological conditions, and discussed its potential implication in the susceptibility of SARS-CoV-2 infection and the progression and prognosis of COVID-19 patients in the current review.
1. Introduction
The renin-angiotensin system (RAS) maintains the homeostasis of blood pressure and the balance of fluid and salts [1]. The homeostasis of RAS is critical for the physiological and pathological regulation in various organs, including the heart, kidneys, and lungs [1]. Angiotensin-converting enzyme (ACE) 2 is a potent negative regulator of RAS, which is critical for maintaining the homeostasis of RAS [2,3]. The human ACE2 gene is located on chromosome Xp22 and includes 18 exons. Functioning as a typical zinc metallopeptidase, ACE2 protein, which contains 805 amino acids, is a type I integral membrane glycoprotein containing a single catalytic domain [3]. In RAS, ACE2 degrades Angiotensin (Ang) II, which is potent in vasoconstriction, pro-inflammation, and pro-fibrosis, and converts it into Ang (1-7) which is vasodialatic, anti-proliferative and apoptotic [3]. Besides the systemic effect in blood pressure regulation, ACE2 has local regulatory effects in the pathological changes in several organs, including the heart, kidneys, and lungs [2]. ACE2 also regulates the amino acid absorption in the kidney and the gut and modulates the expression of transporters for amino acid [2]. ACE2 was identified as the receptor for severe acute respiratory syndrome coronavirus (SARS-CoV), which could rapidly cause acute lung failure in humans [4]. Hence ACE2 is vital for the viral entry to the host cells during SARS-CoV infection.
Coronavirus disease 2019 (COVID-19) pandemic as declared by the World Health Organization on 11 March 2020 [5] has accumulated over one million confirmed cases globally as of the early April 2020 [6]. Epidemiological study revealed that COVID-19 infected people of all ages [8]. The elderly and co-morbid patients are at higher risk of having poor clinical outcomes and prognosis [8,9]. High-throughput sequencing identified the pathogen of COVID-19 as a novel betacoronavirus [10], which was named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses [11]. Comparing to SARS-CoV, SARS-CoV-2 is more infective for causing more than 120 times of infections globally (1,051,635 confirmed cases of COVID-19 as of 4 April 2020 [6] vs. 8096 SARS patients [12]) but seems to be less virulent because only parts of the patients showed similar severity as SARS-CoV infection [8]. Also, the global case fatality rate of COVID-19 was 5.4 % (56,985/1,051,635, according to the data on 4 April 2020 [6]), which was lower than that of SARS-CoV infection (9.6 %, 774/8096) [12]. Similar to SARS-CoV, SARS-CoV-2 mainly transmits through respiratory droplets and direct contact according to the reported cases [8,13]. Also, the entry of SARS-CoV-2 to the host cells requires binding with the receptor via the S-spike on the surface of the virus [10]. Computational modeling [10,14] and viral infection experiments using HeLa cells that express ACE2 protein from humans, Chinese horseshoe bats, civets, pigs, and mice [15] provided evidence that ACE2 is the receptor for SARS-CoV-2. Results in the Cryo-EM experiments demonstrated that SARS-CoV-2 had a ten times higher affinity to ACE2 comparing to SARS-CoV, which was consistent with the higher efficiency of infection of SARS-CoV-2 [16]. These findings indicated that ACE2 might be crucial for the human infection of SARS-CoV-2 and for the progression and prognosis of COVID-19. Understanding the expression and activity of ACE2 under different physiological and pathological conditions may help to predict the susceptibility of SARS-CoV-2 in different cohorts of people and the clinical outcomes and prognosis of COVID-19 patients. In the present review, we will summarize the regulation and function of ACE2 in different physiological and pathological states and discuss the potential implication in SARS-CoV-2 infection-induced COVID-19.
2. Expression and activity of ACE2 in physiological condition
2.1. Tissue distribution of ACE2
Organs that express a higher level of ACE2 are potential targets of SARS-CoV-2 infection. Therefore, the distribution and abundance of ACE2 in organs may be closely related to the clinical symptoms of COVID-19. It is known that ACE2 is enriched in the heart, kidneys, and testes, and is also broadly distributed in the lungs, liver, intestine, and brain [2]. The lung is the primary target organ of SARS-CoV-2 infection [8,9,17]. Most of the hospitalized patients infected with SARS-CoV-2 developed pneumonia, which was typically characterized as ground-glass opacity and bilateral patchy shadowing in chest computed tomography [8,17]. Pathological examination on the biopsy samples obtained from a deceased COVID-19 patient revealed diffused alveolar damage with cellular fibromyxoid exudates in both lungs [18]. An analysis of the single-cell RNA sequencing data obtained from 43,134 human lung cells revealed that 0.64 % of cells in lungs expressed ACE2 and 83 % of ACE2 was expressed on type II apical surface of epithelial cells (AT2) [19]. Other types of cells, including type I apical epithelial cells, airway epithelial cells, fibroblasts, endothelial cells, and macrophages, also express ACE2 at lower levels than AT2 [19]. These results indicated that AT2 is likely to be the primary target of SARS-CoV-2 in the lungs [19].
In addition to respiratory symptoms, the common complications found in patients infected with SARS-CoV-2 include acute cardiac injury and acute kidney injury [9,17]. Rectal swabs, stool, urine, and saliva sampled from some COVID-19 patients were positive for the SARS-CoV-2 virus [8]. These findings demonstrated the broad distribution of SARS-CoV-2 in humans, whose pattern was similar to the distribution of ACE2. An analysis on the single-cell RNA sequencing data from human organs revealed that cells that express ACE2 included 7.5 % of cardiomyocyte, 4 % of proximal tubular epithelial cells, 2.4 % of bladder urothelial cells, 30 % of ileum epithelial cells, 1 % of esophageal epithelial cell, and 2 % of respiratory tract epithelial cells [20]. SARS-CoV-2 may attack these cells after binding with ACE2. The tissue damage and inflammation may cause severe complications that may worsen the clinical outcomes. Moreover, positive detection of the RNA of SARS-CoV-2 in the samples of gastrointestinal tissues and stool from COVID-19 patients suggested that fecal-oral transmission might be possible [21]. It is noteworthy that the placental villi and the uterus also express ACE2 [22,23], indicating the possibility of vertical maternal-fetal transmission. Current reports on clinical observations indicated that this transmission route is possible [[24], [25], [26]] but may be low risk [27,28].
2.2. ACE2 expression in different genders
Some of the sex hormones affect the homeostasis of RAS. Although the ACE2 activity showed no difference between the male and female [29], the males have higher expression levels of ACE2 in the lungs compared to the females [19]. Moreover, at least five types of cells in the lungs from the males express ACE2, while only two to four types in the female’s lungs do so [19]. Consistent with these findings, the SARS-CoV-2 infected males were slightly but not statistically significantly higher than the females (58.1 % vs. 41.9 %) in a survey included 1099 patients [8].
The female sex hormones may affect ACE2 [29]. The ACE/ACE2 activity ratio in the male serum is higher than that in the females, which may partially attribute to the activation effect of estrogen on ACE2 activity [30]. During pregnancy, the fluctuation of hormones may drastically change the expression of ACE2 both in the reproductive system and in other organs [23]. The ACE2 mRNA expression in the uterus of pregnant rats was significantly higher than the one in the unpregnant rats [23], indicating the implication of progesterone in the regulation of ACE2 expression [31]. Similar up-regulation of ACE2 expression was also found in the kidneys of pregnant rats [32]. These results suggested that pregnant women infected with SARS-CoV-2 should be watched for severe complications and rapid progression.
2.3. ACE2 expression during aging
According to the data which collected 1099 patients with laboratory-confirmed COVID-19 from 552 hospitals in mainland China, the average age of the severe patients (n = 173) was significantly older than the non-severe ones (n = 926) by seven years [8]. Among the critically ill COVID-19 patients, the non-survivors (64.6 years) were older than the survivors (51.9 years) [9]. These data indicated that aging might be correlated with the pathological progression and poor prognosis of COVID-19. Results obtained from the genetic knockout mice model demonstrated that ACE2 depletion caused physiological early aging independent of Ang (1-7), indicating the regulatory role of ACE2 in aging [33]. ACE2 expression in the lungs may decrease during aging according to the results in aged rats [34]. In humans, the ACE2 activity did not differ from the young and aged males but showed significant difference in the young and aged females [29,30]. This finding indicated that the effect of aging in ACE2 activity might have a gender difference [29,30]. In a study that included 220 healthy Chinese volunteers, young Chinese females showed significantly higher ACE2 activity than aged females [30]. However, the activity of ACE2 in women during aging is controversial, as a Spanish group reported lower ACE2 activity in the young females comparing to the aged females [29]. Such controversy may due to sample number-induced deviation or race, which requires further validation and investigation.
2.4. ACE2 expression during development
Although children of all ages were susceptible to COVID-19, accumulating clinical data indicated lower infection rates and better clinical outcomes in children compared with adults [7,8]. Recently, a retrospective study analyzed clinical data from 2135 pediatric cases with COVID-19 and found that over 90 % of these pediatric patients were asymptomatic, mild, or moderate cases [35]. Although there currently lacks an explanation to this phenomenon, the authors speculated a potential implication of ACE2 [35].
Results in the animal studies implied that ACE2 expression in adults might be higher than that in children. Sheep could be used as an animal model for studying the development of RAS [36]. The expression of ACE2 was significantly lower in the neonatal sheep comparing to that in the adult ones [36]. This finding seems to indicate that lower ACE2 expression might be correlated with the lower susceptibility of children to SARS-CoV-2. However, the expression and activity of ACE2 during the development of human children and teenagers are largely unclear [35].
2.5. Effect of lifestyle on ACE2 expression
2.5.1. Cigarette smoking
According to the latest study on COVID-19, 12.6 % were current smokers (137/1085), and these patients are more severe than never-smokers as indicated by the severe rate (smoker: 21.2 %, 29/137 vs. never-smoker: 14.5 %, 134/927) [8]. These data suggested that cigarette smoking may affect the pathogenesis and progression of COVID-19. The ACE2 content in the blood of healthy male volunteers was decreased by long term smoking experience [37]. The effect of cigarette smoking may attribute to nicotine, which could suppress the activity of the ACE2/Ang (1-7)/Mas receptor axis and upregulate the ACE/Ang II/Ang II type 1 receptor [38]. The decreased ACE2 level is accompanied by the imbalance of ACE/ACE2 and hence the disturbance in RAS homeostasis. Animal experiments have demonstrated that downregulation of ACE2 contributes to the pathogenesis of cigarette smoking-induced pulmonary arterial hypertension (PAH) [39].
2.5.2. Diet
The expression and activity of ACE2 may be promptly modulated by diets, especially the salt, glucose and fat. High salt diet increased activity of ACE2 in the urine in mice, while low salt intake decreased it [40]. The activity of ACE2 in the urine may reflect the ACE2 in the kidneys [40]. Indeed, it has been found that high salt diet increased the ACE/ACE2 ratio in the glomeruli which resulted in renal dysfunction via inducing oxidative stress [41]. ACE2 was also implicated in the hypertension whose pathogenesis was closely correlated with high salt intake. High salt intake increased the prevalence of hypertension in human with ACE2 rs2285666 TT and rs714205 GG genes [42]. In the spontaneous hypertension rat model, high salt diet profoundly worsened the symptoms of hypertension and impaired kidneys via decreasing ACE2 [43]. High glucose diet could also up-regulate ACE and down-regulate ACE2, leading to the imbalance of ACE/ACE2 in the heart [44]. Strikingly, the effect of diet could affect the offspring. Animal experiments using high salt diet-fed pregnant sheep revealed that the suppress effect of high salt diet on ACE2 may affect the embryo and last post natal [45]. Western diet could decrease the methylation of Ace2 gene in the kidneys and brain stem of perinatal mice and resulted in autonomic dysfunctions in the male offspring [46]. These findings collectively demonstrated the impact of diet on ACE2 may be involved in the pathogenesis of several chronic diseases and may even affect the offspring.
3. ACE2 in disease
COVID-19 patients with comorbidities were more likely to progress to critically ill patients compared with those without comorbidities [9,17]. It has been found that patients with coexisting disorders, including chronic obstructive pulmonary disease (COPD), hypertension, coronary heart disease, chronic kidney disease or diabetes were significantly more severe than patients without any existing disorders (Table 1 ) [8]. It is likely that patients with these chronic diseases were usually aged, weak in immunity, or poor in respiratory tract function, including the weakened movement of respiratory mucosal cilia cells, reduced ability to excrete, and cough reflexes. Several complications have been reported in COVID-19 patients. Some of them were closely correlated with the severity of the disease (Table 2 ). Studying the implication and regulation of ACE2 in the diseased status would help in the understanding of the progression of COVID-19 and prognosis.
Table 1.
Coexisting disorders in COVID-19 patients [8].
| Coexisting disorders | Prevalence among 1099 COVID-19 patients | Severity ratio in COVID-19 patients with coexisting disorders |
|---|---|---|
| COPD | 1.1 % (12/1099) | 50 % (6/12) |
| Hypertension | 15 % (165/1099) | 24.8 % (41/165) |
| Coronal heart disease | 2.5 % (27/1099) | 37 % (10/27) |
| Chronic kidney disease | 0.7 % (8/1099) | 37.5 % (3/8) |
| Diabetes | 7.4 % (81/1099) | 34.6 % (28/81) |
| Cerebrovascular disease | 1.4 % (15/1099) | 26.7 % (4/15) |
Table 2.
Complications and severity rate in COVID-19 patients.
| Complications | Prevalence |
Severity rate in COVID-19 patients with complications |
||||
|---|---|---|---|---|---|---|
| [8] | [17] | [47] | [8] | [17] | [47] | |
| Pneumonia | 91.1 % (972/1067) | — | — | 17.7 % (172/972) | — | — |
| ARDS | 3.4 % (37/1099) | 19.6 % (27/138) | 29 % (12/41) | 73 % (27/37) | 81.5 % (22/27) | 91.7 % (11/12) |
| Acute kidney injury | 0.5 % (6/1099) | 3.6 % (5/138) | 7 % (3/41) | 83.3 % (5/6) | 60 % (3/5) | 100 %(3/3) |
| Shock | 1.1 % (12/1099) | 8.7 % (12/138) | 7 % (3/41) | 91.7 % (11/12) | 91.7 % (11/12) | 100 % (3/3) |
| DIC | 0.1 % (1/1099) | — | — | 100 % (1/1) | — | — |
| Rhabdomyolysis | 0.2 % (2/1099) | — | — | 0 %(0/2) | — | — |
| Infection | — | — | 10 % (4/41) | — | — | 100 % (4/4) |
| Arrhythmia | — | 16.7 % (23/138) | — | — | 69.6 % (16/23) | — |
| Acute cardiac injury | — | 7.2 % (10/138) | 12 % (5/41) | — | 80 % (8/10) | 80 % (4/5) |
Abbreviations: ARDS: Acute respiratory distress syndrome; DIC: Disseminated intravascular coagulopathy.
3.1. ACE2 and pulmonary diseases
Since SARS-CoV-2 mainly transmits via respiratory droplets and that ACE2 is mostly distributed on the AT2 cells, the lung is the most preliminary target organ for SARS-CoV-2 infection, and pneumonia was the most common complication seen in COVID-19 patients with the occurrence of 91.1 % [8]. Acute respiratory distress syndrome (ARDS) is one of the most severe complications in COVID-19 patients, with the severity rate ranged from 73 % to 92 % [8,17,47]. ACE2 has well-established protective effects in the lungs [2]. ACE2 polymorphism was correlated with the severity of ARDS [2]. Ace2 knockout mice showed severe ARDS-like injury pathology in the lungs comparing to the wild type controls [48]. These symptoms can be alleviated by treatment of catalytically active, but not enzymatically inactive, recombinant ACE2 (rACE2) protein [48]. Moreover, ACE2 was also protective of SARS-infection induced acute lung disease [49]. The expression of ACE2 in the lungs of mice infected with SARS-CoV was greatly reduced, which aggravated the lung failure [49]. These results convincingly proved that ACE2 is protective against lung injury and may be a therapeutic target for ARDS shown in COVID-19.
ACE2 mRNA expression decreased in the lungs of COPD rats model comparing to controls [50]. The reduction of ACE2 in the COPD lungs revealed the dysregulation of RAS, which may attribute to the up-regulation of ET-1. Because ET-1 increased in the sputum and serum samples of COPD patients [51], and ET-1 dose- and time-dependently decreased the expression and activity of ACE2 in the cell culture of human bronchial epithelial cells [52]. Recent data showed that COVID-19 patients with COPD as coexisting disorder were more likely to progress into severe cases [8], which may partially attribute to the decrease in ACE2.
Many pulmonary diseases, such as COPD, are accompanied by hypoxia [53]. In the early phase of hypoxia, ACE2 expression was profoundly elevated in pulmonary artery smooth muscle cells. Later, with the elevation of the hypoxia-inducible factor-1α (HIF-1α), the expression of ACE, the target gene of HIF-1α, increased accompanied by decrease of ACE2 [54]. Animal experiments demonstrated that similar regulation by HIF-1α on ACE2 level, possibly via miRNA let-7b after hypoxia in rats [55]. These results indicated that hypoxia and the cellular adaptation to hypoxia could profoundly induce the fluctuation of ACE2 in the lungs.
3.2. ACE2 and cardiovascular diseases
3.2.1. Hypertension
RAS dysregulation is primarily implicated in the pathogenesis and progression of hypertension [56]. Being the negative regulator of RAS, ACE2 decreases the blood pressure in animal models of hypertension [2]. A decrease of ACE2 has been reported in the lungs of patients with idiopathic PAH [57]. Similarly, ACE2 was also decreased in the blood vessel, the kidneys, and the brain in the hypertension models [58,59]. Since ACE2 was protective of tissue injuries in the lungs [48] and heart [60], these results suggested that COVID-19 patients with hypertension may be more likely to develop complications and progress into severe cases. Indeed, data in Table 1 showed that COVID-19 patients with hypertension have a high severity rate [8]. Besides, severe cases of COVID-19 have significantly higher systolic pressure comparing to the non-severe cases (145 mmHg vs. 122 mmHg) [47]. This phenomenon may due to the deprivation of ACE2 by the SARS-CoV-2, leading to a drastic decrease of ACE2.
3.2.2. Coronal heart disease
ACE2 is enriched in heart and is known as an essential regulator of heart function [60], suggesting heart may be one of the primary target organs of SARS-CoV-2 infection. Patients infected with SARS-CoV-2 were at higher risk of progressing into severe cases when having coronal heart disease as comorbidity [8,17]. Acute cardiac injury is one of the most common complications found in COVID-19 patients [17,47]. According to a retrospective study published in the Lancet that included 41 COVID-19 patients, 12 % developed acute cardiac injury, 80 % of these cases were diagnosed as severe cases [47]. Therefore, coronal heart disease in the COVID-19 patients was closely watched both as baseline comorbidity and as complications. The pathogenesis and progression of cardiac injury were one of the preliminary factors that determined the clinical outcomes and prognosis of COVID-19 patients [17,47]. The cardiac injury found in COVID-19 patients may be at least partially attributed to the fluctuation of ACE2. In the rat model of myocardial infarction, the infarcted and peri-infarcted regions showed increased ACE2 expression comparing to surviving myocardial tissue after three days of infarction [61]. After 28 days of infarction, the surviving tissue also showed elevated ACE2 expression comparing to normal control [61]. The up-regulation of ACE2 in the heart after myocardial infarction may be triggered by a compensatory protective mechanism [62]. However, in the lungs of patients with PAH, the ACE2 expression and phosphorylation were drastically decreased [57]. Therefore, the pulmonary function of COVID-19 may progressively deteriorate upon the occurrence of cardiac complications.
3.3. ACE2 and renal disease
The kidneys may be a major target organ of SARS-CoV-2 infection because laboratory examinations demonstrated that the urine samples from some COVID-19 patients were found positive for the virus [8]. Acute kidney injury is one of the most severe complications in COVID-19 patients as SARS-CoV-2 may infect the renal intrinsic cells such as proximal tubular epithelial cells and directly induce renal dysfunction. The occurrence of acute kidney injury as a complication of COVID-19 was 7 % in 41 cases reported in the Lancet, and all of them developed into severe cases [47]. In a cohort of 1099 COVID-19 patients, the occurrence of acute kidney injury was 0.5 %, and the severity rate was 83.3 % [8]. In a consecutive cohort study that included 701 COVID-19 patients, renal dysfunction characterized as elevation in serum creatine, blood urea nitrogen, the appearance of proteinuria or hematuria showed a significantly higher risk of mortality compared with normal renal function [63]. Renal dysfunction was an independent risk factor of mortality in hospitalized patients [63]. These studies demonstrated that acute kidney injury was one of the most critical complications that occurred in COVID-19 patients.
ACE2 is enriched in the kidneys [2]. The activity of ACE2 in the renal cortex is even higher than that in the heart [2]. The expression level of ACE2 decreased both in the acute kidney injury [64] and in several models of chronic kidney disease induced by hypertension, diabetes and nephrectomy [65], which disrupted the homeostasis of RAS in the kidneys and worsened the pathological changes in the kidneys [2,65]. Accumulating evidence has also demonstrated that ACE2 played a crucial role in the pathogenesis and progression of renal diseases as reviewed in [65]. ACE2 knockout could worsen renal dysfunction modeled in ischemia/reperfusion [64] and aggravate the fibrotic pathologies in obstructive renal diseases [66]. Further investigation in the implication of ACE2 in the renal dysfunction occurred in the COVID-19 patients, and in the clinical outcome in the COVID-19 patients with chronic kidney diseases as comorbidity is required.
3.4. ACE2 and diabetes
ACE2 modulates the expression of neutral amino acid transporters on the cell surface of epithelial cells, including the insulin secretion pancreatic β-cells and/or growth of pancreatic islet cells [2]. Ace2 knockout mice showed impairments in the islet dysfunction and abnormality in glucose tolerance [67]. The diabetic mice model infected with an adenovirus expressing human ACE2 showed an increase in the production of insulin and a reduction in cellular apoptosis in the pancreatic islet [68]. Moreover, ACE2 could improve the endothelial function in the pancreatic islet microvascular unit [69]. These findings demonstrated that ACE2 prevented pancreatic dysfunction in diabetes. ACE2 was implicated in the diabetic nephrology. In the human kidneys with diabetic nephropathy, ACE2 was profoundly suppressed while ACE was increased [70]. The ratio of ACE/ACE2 was positively correlated with the systolic pressure, the fasting blood glucose level, serum creatine, and proteinuria in the patients [70]. rACE2 showed promising prevention against diabetic nephrology [2]. COVID-19 patients with diabetes as coexisting disorders had a worse clinical outcome. These phenomena may partially attribute to the deprivation of ACE2 by SARS-CoV-2 infection.
4. Therapies that targeting on ACE2 and RAS
4.1. rACE2
SARS-CoV-2 binds with ACE2 to enter the host cells, leading to the systematic deprivation of ACE2. rACE2 has shown a therapeutic effect in pulmonary injury [48], diabetic nephrology and hypertension [2]. Recently, it was found that a human recombinant soluble ACE2 (hrsACE2) which has already been tested in phase 1 and phase 2 clinical trials effectively reduced the recovery of SARS-CoV-2 from Vero cells and prevented the infection of SARS-CoV-2 in engineered human blood vessel organoids and human kidney organoids [71]. This progress suggested the therapeutic potential of hrsACE2 in COVID-19.
4.2. ACE2 activator
Since ACE2 activation has demonstrated a protective effect on pulmonary injury, including ARDS, developing ACE2 activator as a potential therapy may be a promising approach against COVID-19. Diminazene aceturate [72], resorcinolnaphthalein and xanthenone [73] were found to activate ACE2 in a drug screening test, which showed potential as a candidate therapy for COVID-19.
4.3. ACE inhibitor (ACEI) and angiotensin II receptor blocker (ARB)
ACEI and ARB are commonly-used treatments for hypertension. The safety and effect of using these drugs on COVID-19 patients with hypertension are still unclear. Animal experiments revealed that these drugs might decrease the systolic pressure in normal rats and up-regulate ACE2 [74]. Since the ACE2 expression is suppressed in hypertension [56] and may be further deprived by SARS-CoV-2 virus upon infection, the application of ARB may be protective for the pulmonary injury under careful blood pressure management.
5. Conclusion
ACE2 is currently under intensive investigation as it is the receptor of SARS-CoV-2, the novel coronavirus that causes COVID-19 pandemic. We summarized the increasingly emerging findings in the COVID-19 and discussed the potential role of ACE2 in the pathogenesis, progression, and prognosis of COVID-19. The susceptibility of SARS-CoV-2 in different cohorts seemed to correlate with the ACE2 level. The distribution of target organs that are susceptible to the SARS-CoV-2 infection and complications of COVID-19 is similar to the one of ACE2. The three functions of ACE2, which are a peptidase that negatively modulates RAS, a regulator for the amino acid transport in the kidneys, and a receptor for the virus, including SARS-CoV-2 and SARS-CoV, together with the local protective effect on the tissue injury, are implicated in the progression and prognosis of COVID-19. These findings indicated that targeting ACE2 by using hrsACE2 or ACE2 activator may be a potential therapeutic strategy for COVID-19. Prescription of drugs that may affect ACE2, such as ACEI and ARB, in COVID-19 patients requires close observation of the blood pressure and the progression of COVID-19.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China 81800598, the 65th batch of General Program of the China Postdoctoral Science Fund 2019M651906, Jiangsu 2019 Scheme for Innovative talents and Entrepreneurs, 2019 Nanjing Technology Innovation Projects for Overseas Scholars.
Contributor Information
Li Yang, Email: yangly2018njmu@163.com.
Ran You, Email: yourannch@163.com.
References
- 1.Patel S., Rauf A., Khan H., Abu-Izneid T. Renin-angiotensin-aldosterone (RAAS): the ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017;94:317–325. doi: 10.1016/j.biopha.2017.07.091. [DOI] [PubMed] [Google Scholar]
- 2.Kuba K., Imai Y., Ohto-Nakanishi T., Penninger J.M. Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 2010;128:119–128. doi: 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamming I., Cooper M.E., Haagmans B.L., Hooper N.M., Korstanje R., Osterhaus A.D., Timens W., Turner A.J., Navis G., van Goor H. The emerging role of ACE2 in physiology and disease. J. Pathol. 2007;212:1–11. doi: 10.1002/path.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . 2020. Coronavirus Disease 2019 (COVID-19) Situation Report – 51.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10 [Google Scholar]
- 6.World Health Organization . 2020. Coronavirus Disease 2019 (COVID-19) Situation Report – 75.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200404-sitrep-75-covid-19.pdf?sfvrsn=99251b2b_2 [Google Scholar]
- 7.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . 2003. Cumulative Number of Reported Probable Cases of Severe Acute Respiratory Syndrome (SARS)https://www.who.int/csr/sars/country/en/ (Accessed 30 March 2020) [Google Scholar]
- 13.Wang G., Jin X. The progress of 2019 novel coronavirus event in China. J. Med. Virol. 2020;92:468–472. doi: 10.1002/jmv.25705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., Zhong W., Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. bioRxiv. 2020 doi: 10.1101/2020.01.26.919985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. The single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to Wuhan 2019-nCoV infection. Front. Med. 2020 doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valdes G., Neves L.A., Anton L., Corthorn J., Chacon C., Germain A.M., Merrill D.C., Ferrario C.M., Sarao R., Penninger J., Brosnihan K.B. Distribution of angiotensin-(1-7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta. 2006;27:200–207. doi: 10.1016/j.placenta.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Levy A., Yagil Y., Bursztyn M., Barkalifa R., Scharf S., Yagil C. ACE2 expression and activity are enhanced during pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R1953–R1961. doi: 10.1152/ajpregu.90592.2008. [DOI] [PubMed] [Google Scholar]
- 24.Zeng H., Xu C., Fan J., Tang Y., Deng Q., Zhang W., Long X. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020 doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong L., Tian J., He S., Zhu C., Wang J., Liu C., Yang J. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020 doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng L., Xia S., Yuan W., Yan K., Xiao F., Shao J., Zhou W. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., Li J., Zhao D., Xu D., Gong Q., Liao J., Yang H., Hou W., Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan C., Lei D., Fang C., Li C., Wang M., Liu Y., Bao Y., Sun Y., Huang J., Guo Y., Yu Y., Wang S. Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry? Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez-Atucha A., Izagirre A., Fraile-Bermudez A.B., Kortajarena M., Larrinaga G., Martinez-Lage P., Echevarria E., Gil J. Sex differences in the aging pattern of renin-angiotensin system serum peptidases. Biol. Sex Differ. 2017;8:5. doi: 10.1186/s13293-017-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Y., Li X., Wu N., Wang N., Qiu C., Li J. Study on the correlation among sex, age and the activity of ACE, ACE2 and the ratio of ACE/ACE2. J. Qiqihar Med. Univ. 2018;39:884–887. doi: 10.3969/j.issn.1002-1256.2018.08.005. [DOI] [Google Scholar]
- 31.Neves L.A., Stovall K., Joyner J., Valdes G., Gallagher P.E., Ferrario C.M., Merrill D.C., Brosnihan K.B. ACE2 and ANG-(1-7) in the rat uterus during early and late gestation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R151–R161. doi: 10.1152/ajpregu.00514.2007. [DOI] [PubMed] [Google Scholar]
- 32.Brosnihan K.B., Neves L.A., Joyner J., Averill D.B., Chappell M.C., Sarao R., Penninger J., Ferrario C.M. Enhanced renal immunocytochemical expression of ANG-(1-7) and ACE2 during pregnancy. Hypertension. 2003;42:749–753. doi: 10.1161/01.hyp.0000085220.53285.11. [DOI] [PubMed] [Google Scholar]
- 33.Nozato S., Yamamoto K., Takeshita H., Nozato Y., Imaizumi Y., Fujimoto T., Yokoyama S., Nagasawa M., Takeda M., Hongyo K., Akasaka H., Takami Y., Takeya Y., Sugimoto K., Mogi M., Horiuchi M., Rakugi H. Angiotensin 1-7 alleviates aging-associated muscle weakness and bone loss, but is not associated with accelerated aging in ACE2-knockout mice. Clin. Sci. 2019;133:2005–2018. doi: 10.1042/CS20190573. [DOI] [PubMed] [Google Scholar]
- 34.Xie X., Chen J., Wang X., Zhang F., Liu Y. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci. 2006;78:2166–2171. doi: 10.1016/j.lfs.2005.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z., Tong S. Epidemiology of COVID-19 among children in China. Pediatrics. 2020 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 36.Chen K., Bi J., Su Y., Chappell M.C., Rose J.C. Sex-specific changes in renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 gene expression and enzyme activity at birth and over the first year of life. Reprod. Sci. 2016;23:200–210. doi: 10.1177/1933719115597760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y., Zhao L., Zhang Q., Zhang L., Ren G. Effect of long-term smoking on expression of serum ACE and ACE2 as well as its significance. J. Taishan Med. Coll. 2019;40:258–260. doi: 10.3969/j.issn.1004-7115.2019.04.007. [DOI] [Google Scholar]
- 38.Oakes J.M., Fuchs R.M., Gardner J.D., Lazartigues E., Yue X. Nicotine and the renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;315:R895–R906. doi: 10.1152/ajpregu.00099.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan Y.M., Luo L., Guo Z., Yang M., Ye R.S., Luo C. Activation of renin-angiotensin-aldosterone system (RAAS) in the lung of smoking-induced pulmonary arterial hypertension (PAH) rats. J. Renin. Syst. 2015;16:249–253. doi: 10.1177/1470320315576256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wysocki J., Garcia-Halpin L., Ye M., Maier C., Sowers K., Burns K.D., Batlle D. Regulation of urinary ACE2 in diabetic mice. Am. J. Physiol. Renal Physiol. 2013;305:F600–F611. doi: 10.1152/ajprenal.00600.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernardi S., Toffoli B., Zennaro C., Tikellis C., Monticone S., Losurdo P., Bellini G., Thomas M.C., Fallo F., Veglio F., Johnston C.I., Fabris B. High-salt diet increases glomerular ACE/ACE2 ratio leading to oxidative stress and kidney damage. Nephrol. Dial. Transplant. 2012;27:1793–1800. doi: 10.1093/ndt/gfr600. [DOI] [PubMed] [Google Scholar]
- 42.He Y., Yang W., Liu S., Gan L., Zhang F., Mu C., Wang J., Qu L., Wang R., Deng J., Ye Q., Yang X., Dong Y., Wang Q., Wei C., Hou Z., Yang L. Interactions between angiotensin-converting enzyme-2 polymorphisms and high salt intake increase the risk of hypertension in the Chinese Wa population. Int. J. Clin. Exp. Pathol. 2017;10:11159–11168. [PMC free article] [PubMed] [Google Scholar]
- 43.Berger R.C., Vassallo P.F., Crajoinas Rde O., Oliveira M.L., Martins F.L., Nogueira B.V., Motta-Santos D., Araujo I.B., Forechi L., Girardi A.C., Santos R.A., Mill J.G. Renal effects and underlying molecular mechanisms of long-term salt content diets in spontaneously hypertensive rats. PLoS One. 2015;10 doi: 10.1371/journal.pone.0141288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lavrentyev E.N., Malik K.U. High glucose-induced Nox1-derived superoxides downregulate PKC-betaII, which subsequently decreases ACE2 expression and ANG(1-7) formation in rat VSMCs. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H106–H118. doi: 10.1152/ajpheart.00239.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mao C., Liu R., Bo L., Chen N., Li S., Xia S., Chen J., Li D., Zhang L., Xu Z. High-salt diets during pregnancy affected fetal and offspring renal renin-angiotensin system. J. Endocrinol. 2013;218:61–73. doi: 10.1530/JOE-13-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mukerjee S., Zhu Y., Zsombok A., Mauvais-Jarvis F., Zhao J., Lazartigues E. Perinatal exposure to western diet programs autonomic dysfunction in the male offspring. Cell. Mol. Neurobiol. 2018;38:233–242. doi: 10.1007/s10571-017-0502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xue T., Wei N., Xin Z., Qingyu X. Angiotensin-converting enzyme-2 overexpression attenuates inflammation in rat model of chronic obstructive pulmonary disease. Inhal. Toxicol. 2014;26:14–22. doi: 10.3109/08958378.2013.850563. [DOI] [PubMed] [Google Scholar]
- 51.Roland M., Bhowmik A., Sapsford R.J., Seemungal T.A., Jeffries D.J., Warner T.D., Wedzicha J.A. Sputum and plasma endothelin-1 levels in exacerbations of chronic obstructive pulmonary disease. Thorax. 2001;56:30–35. doi: 10.1136/thorax.56.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang H., Li Y., Zeng Y., Wu R., Ou J. Endothelin-1 downregulates angiotensin-converting enzyme-2 expression in human bronchial epithelial cells. Pharmacology. 2013;91:297–304. doi: 10.1159/000350395. [DOI] [PubMed] [Google Scholar]
- 53.Tuder R.M., Yun J.H., Bhunia A., Fijalkowska I. Hypoxia and chronic lung disease. J. Mol. Med. 2007;85:1317–1324. doi: 10.1007/s00109-007-0280-4. [DOI] [PubMed] [Google Scholar]
- 54.Zhang R., Wu Y., Zhao M., Liu C., Zhou L., Shen S., Liao S., Yang K., Li Q., Wan H. Role of HIF-1alpha in the regulation ACE and ACE2 expression in hypoxic human pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;297:L631–L640. doi: 10.1152/ajplung.90415.2008. [DOI] [PubMed] [Google Scholar]
- 55.Zhang R., Su H., Ma X., Xu X., Liang L., Ma G., Shi L. MiRNA let-7b promotes the development of hypoxic pulmonary hypertension by targeting ACE2. Am. J. Physiol. Lung Cell Mol. Physiol. 2019;316:L547–L557. doi: 10.1152/ajplung.00387.2018. [DOI] [PubMed] [Google Scholar]
- 56.Te Riet L., van Esch J.H., Roks A.J., van den Meiracker A.H., Danser A.H. Hypertension: renin-angiotensin-aldosterone system alterations. Circ. Res. 2015;116:960–975. doi: 10.1161/circresaha.116.303587. [DOI] [PubMed] [Google Scholar]
- 57.Zhang J., Dong J., Martin M., He M., Gongol B., Marin T.L., Chen L., Shi X., Yin Y., Shang F., Wu Y., Huang H.Y., Zhang J., Zhang Y., Kang J., Moya E.A., Huang H.D., Powell F.L., Chen Z., Thistlethwaite P.A., Yuan Z.Y., Shyy J.Y. AMP-activated protein kinase phosphorylation of angiotensin-converting enzyme 2 in endothelium mitigates pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2018;198:509–520. doi: 10.1164/rccm.201712-2570OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mendoza-Torres E., Oyarzun A., Mondaca-Ruff D., Azocar A., Castro P.F., Jalil J.E., Chiong M., Lavandero S., Ocaranza M.P. ACE2 and vasoactive peptides: novel players in cardiovascular/renal remodeling and hypertension. Ther. Adv. Cardiovasc. Dis. 2015;9:217–237. doi: 10.1177/1753944715597623. [DOI] [PubMed] [Google Scholar]
- 59.Xia H., Sriramula S., Chhabra K.H., Lazartigues E. Brain angiotensin-converting enzyme type 2 shedding contributes to the development of neurogenic hypertension. Circ. Res. 2013;113:1087–1096. doi: 10.1161/CIRCRESAHA.113.301811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E., Oliveira-dos-Santos A.J., da Costa J., Zhang L., Pei Y., Scholey J., Ferrario C.M., Manoukian A.S., Chappell M.C., Backx P.H., Yagil Y., Penninger J.M. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 61.Burrell L.M., Risvanis J., Kubota E., Dean R.G., MacDonald P.S., Lu S., Tikellis C., Grant S.L., Lew R.A., Smith A.I., Cooper M.E., Johnston C.I. Myocardial infarction increases ACE2 expression in rat and humans. Eur. Heart J. 2005;26:369–375. doi: 10.1093/eurheartj/ehi114. discussion 322-364. [DOI] [PubMed] [Google Scholar]
- 62.Zisman L.S., Keller R.S., Weaver B., Lin Q., Speth R., Bristow M.R., Canver C.C. Increased angiotensin-(1-7)-forming activity in failing human heart ventricles: evidence for upregulation of the angiotensin-converting enzyme Homologue ACE2. Circulation. 2003;108:1707–1712. doi: 10.1161/01.CIR.0000094734.67990.99. [DOI] [PubMed] [Google Scholar]
- 63.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., Li J., Yao Y., Ge S., Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney. Int. 2020;S0085-2538:30255–30256. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fang F., Liu G.C., Zhou X., Yang S., Reich H.N., Williams V., Hu A., Pan J., Konvalinka A., Oudit G.Y., Scholey J.W., John R. Loss of ACE2 exacerbates murine renal ischemia-reperfusion injury. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soler M.J., Wysocki J., Batlle D. ACE2 alterations in kidney disease. Nephrol. Dial. Transplant. 2013;28:2687–2697. doi: 10.1093/ndt/gft320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Z., Huang X.R., Chen H.Y., Penninger J.M., Lan H.Y. Loss of angiotensin-converting enzyme 2 enhances TGF-beta/smad-mediated renal fibrosis and NF-kappaB-driven renal inflammation in a mouse model of obstructive nephropathy. Lab. Invest. 2012;92:650–661. doi: 10.1038/labinvest.2012.2. [DOI] [PubMed] [Google Scholar]
- 67.Niu M.J., Yang J.K., Lin S.S., Ji X.J., Guo L.M. Loss of angiotensin-converting enzyme 2 leads to impaired glucose homeostasis in mice. Endocrine. 2008;34:56–61. doi: 10.1007/s12020-008-9110-x. [DOI] [PubMed] [Google Scholar]
- 68.Bindom S.M., Hans C.P., Xia H., Boulares A.H., Lazartigues E. Angiotensin I-converting enzyme type 2 (ACE2) gene therapy improves glycemic control in diabetic mice. Diabetes. 2010;59:2540–2548. doi: 10.2337/db09-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu C.L., Wang Y., Yuan L., Li Y., Li X.Y. The angiotensin-converting enzyme 2/angiotensin (1-7)/Mas axis protects the function of pancreatic beta cells by improving the function of islet microvascular endothelial cells. Int. J. Mol. Med. 2014;34:1293–1300. doi: 10.3892/ijmm.2014.1917. [DOI] [PubMed] [Google Scholar]
- 70.Mizuiri S., Hemmi H., Arita M., Ohashi Y., Tanaka Y., Miyagi M., Sakai K., Ishikawa Y., Shibuya K., Hase H., Aikawa A. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am. J. Kidney Dis. 2008;51:613–623. doi: 10.1053/j.ajkd.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 71.Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Pozo C.Hd., Prosper F., Romero J.P., Wirnsberger G., Zhang H.B., Slutsky A.S., Conder R., Montserrat N., Mirazimi A., Penninger J.M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020 doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shenoy V., Gjymishka A., Jarajapu Y.P., Qi Y., Afzal A., Rigatto K., Ferreira A.J., Fraga-Silva R.A., Kearns P., Douglas J.Y., Agarwal D., Mubarak K.K., Bradford C., Kennedy W.R., Jun J.Y., Rathinasabapathy A., Bruce E., Gupta D., Cardounel A.J., Mocco J., Patel J.M., Francis J., Grant M.B., Katovich M.J., Raizada M.K. Diminazene attenuates pulmonary hypertension and improves angiogenic progenitor cell functions in experimental models. Am. J. Respir. Crit. Care Med. 2013;187:648–657. doi: 10.1164/rccm.201205-0880OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hernandez Prada J.A., Ferreira A.J., Katovich M.J., Shenoy V., Qi Y., Santos R.A., Castellano R.K., Lampkins A.J., Gubala V., Ostrov D.A., Raizada M.K. Structure-based identification of small-molecule angiotensin-converting enzyme 2 activators as novel antihypertensive agents. Hypertension. 2008;51:1312–1317. doi: 10.1161/HYPERTENSIONAHA.107.108944. [DOI] [PubMed] [Google Scholar]
- 74.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., Diz D.I., Gallagher P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]