Highlights
-
•
Throughout a five years period, HRV, RSV, and influenza viruses were responsible for two-thirds of acute respiratory infections among children in Qatar.
-
•
RSV, influenza, and HMPV circulated in winter, whereas HRV was highly active during other seasons.
-
•
RSV is more prevalent among infants, while influenza circulates more among children above five years of age.
-
•
Influenza–RSV co-infections are significantly associated with age.
-
•
Gender-dependent differences affect infection rates.
Keywords: Epidemiology, Respiratory infections, Influenza, RSV, Pediatrics, Surveillance
Abstract
Background
Studies on the etiology of respiratory infections among children in Qatar and surrounding countries are limited.
Objectives
To describe the prevalence and seasonality of RSV, influenza, and other respiratory pathogens among children in Qatar.
Methods
We retrospectively collected and analyzed data of 33,404 children (<15 years) presented with influenza-like illness from 2012 to 2017.
Results
At least one respiratory pathogen was detected in 26,138 (78%) of patients. Together, human rhinoviruses (HRV), respiratory syncytial virus (RSV), and influenza viruses comprised nearly two-thirds of all cases, affecting 24%, 19.7%, and 18.5%, respectively. A prevalence of 5-10% was recorded for adenovirus, parainfluenza viruses (PIVs), human bocavirus (HboV), and human coronaviruses (HCoVs). Human metapneumovirus (HMPV), enteroviruses, M. pneumonia, and parechovirus had prevalences below 5%. While RSV, influenza, and HMPV exhibited strong seasonal activity in the winter, HRV was active during low RSV and influenza circulation. The burden of RSV exceeds that of influenza among young age groups, whereas influenza correlated positively with age. Further, HRV, adenovirus, influenza, and RSV infection rates varied significantly between male and females.
Conclusion
This comprehensive multi-year study provides insights into the etiology of ILI among children in Qatar, which represents the Gulf region. Our results reinforce the significance of active surveillance of respiratory pathogens to improve infection prevention and control strategies, particularly among children.
1. Introduction
Acute Respiratory infections (ARIs) are the world's most common illnesses, causing a persistent public health concern in both developed and developing countries (Monto, 2002, World Health Organization, 2004). Respiratory infections are easily transmitted among individuals resulting in high morbidity and mortality rates particularly in children and the elderly (Goldstein et al., 2015, Walsh and Falsey, 2012, Bednarska et al., 2015). According to the WHO, respiratory infections were responsible for about a million deaths in children younger than four years old during 2017 (World Health Organization (WHO), 2017).
Children often experience several illness episodes due to respiratory infections throughout the year (Monto, 2002). Many pathogens have been reported to cause respiratory infections; however, viruses are considered the main cause, accounting for 40%–50% of infections in hospitalized children in developing countries (Simoes et al., 2006, Comach et al., 2012, Tregoning and Schwarze, 2010). The most commonly reported virus is respiratory syncytial virus (RSV) (Al-Romaihi et al., 2019, Krilov, 2001). Other common viruses include influenza A (IAV) and B viruses (IBV), parainfluenza viruses (PIVs), adenovirus, and human rhinovirus (HRV) (Monto, 2002, Williams et al., 2002, Pavia, 2011). Additionally, several new viruses have been also found to manifest clinically as influenza-like illnesses (ILIs), including human metapneumovirus (HMPV), human coronaviruses (HCoVs), and human bocavirus (HboV) (Pavia, 2011, Debiaggi et al., 2012, Walker et al., 2013). Importantly, until now, most of these respiratory viruses have no approved vaccines or medications (Tregoning and Schwarze, 2010).
The distribution and prevalence of each virus vary from region to region, mainly due to differences in geographic areas, seasonal timing, and socioeconomic status (Tang et al., 2008). Qatar is a demographically diverse country with high population mobility. Recently, studies investigating the epidemiology of respiratory viruses such as RSV and influenza virus have been reported from Qatar (Al-Romaihi et al., 2019, Alkuwari et al., 2011, Janahi et al., 2017). However, the epidemic characteristics of these two viruses, in addition to other respiratory pathogens, are still not well established. Therefore, a better understanding of viruses’ epidemiology is essential to reduce the burden on health care systems and avoid antibiotics misuse.
In fact, despite the efforts to control the antibiotic resistance, antibiotics are still commonly prescribed for the treatment of ARIs that are mostly caused by viral pathogens. Describing the major role of viral infections in ARIs would highlight the unnecessary overuse of antibiotics among ARIs patients.
In this article, we report on the prevalence, seasonal variations, and age distribution of respiratory viruses in children less than 15 years old over six years in the State of Qatar.
2. Methodology
2.1. Specimen collection and case definition
Throat, nasal or nasopharyngeal swabs, and nasal aspirate were obtained from ILI patients below 15 years old. ILI is defined by fever ≥38 °C and cough or sore throat with onset within the last ten days (WHO, 2014). Samples were collected at Hamad General Hospital, sentinel sites, and primary healthcare centers from January 2012 to December 2017. For the isolation of Middle East Respiratory Syndrome virus (MERS CoV), additional sputum samples were collected.
2.2. Laboratory diagnosis
Samples were tested by multiplex real-time PCR for detection of pathogen genes by TaqMan® technology. Specimens were tested for influenza A/B/H1N1 only (Xpert® Flu assay, Cepheid, U.S.) or a full panel of respiratory pathogens (Respiratory Pathogens 21 kit, Fast-Track Diagnostics, Luxemburg), depending on the physician's request. This kit enables the identification of 20 viruses and one bacteria that cause upper respiratory tract infections. Besides, FTD MERS-CoV (Fast-Track Diagnostics, Luxemburg) was used to test MERS-CoV. Additionally, starting from June 2017, influenza H3 virus detection was introduced using the same platform. DNA/RNA extraction was performed using EziOne, followed by amplification on ABI7500 system, a CE-In vitro diagnostic (IVD) approved.
2.3. Statistical analysis
Datasheets were cleaned by removing errors and duplicates. Subjects who had similar test results within 14 days were considered as duplicates and their second result was omitted. All samples (n = 33,404) were tested for influenza viruses, and therefore, rates of influenza infection were calculated out of the total number. Prevalences of other respiratory pathogens were calculated out of the number of samples subjected to respiratory pathogens panel (n = 30,946). To determine the relation significance between variables, Pearson chi-square was used at p-value <0.05.
3. Results
3.1. Demographics of enrolled patients
Over a period of six years, 33,404 specimens were collected from children with ILIs symptoms. Table 1 summarizes the demographics of the study population. Briefly, patients enrolled in this study were below 15 years of age (average = 2.6 ± 3.2, median = 1.3). Approximately 80% of samples were received from children under five years of age: 42% (13,897) were below one year, while 38% aged between 1 and 4 years. Older age groups (5 to <15 years) represented only 20% of the cases. Additionally, proportion of male participants (42.9%) was higher than females (33.3%)
Table 1.
Demographics of enrolled patients.
| Category | 2012 No. (%) |
2013 No. (%) |
2014 No. (%) |
2015 No. (%) |
2016 No. (%) |
2017 No. (%) |
Total No. (%) |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Male | 0 | 0 | 0 | 2759 (46.3) | 3312 (58.4) | 8246 (55.1) | 14,317 (42.9) |
| Female | 0 | 0 | 0 | 2083 (35) | 2356 (41.6) | 6706 (44.9) | 11,145 (33.3) |
| Missing data | 1846 (100) | 2081 (100) | 2901 (100) | 1111 (18.7) | 2 (0.03) | 1 (0.01) | 7942 (23.8) |
| Total | 1846 (100) | 2081 (100) | 2901 (100) | 5953 (100) | 5670 (100) | 14,953 (100) | 33,404 (100) |
| Age groups | |||||||
| <1 year | 962 (52.1) | 847 (40.7) | 1384 (47.7) | 2011 (33.8) | 2916 (51.4) | 5777 (38.6) | 13,897 (41.6) |
| 1–4 years | 583 (31.6) | 789 (37.9) | 891 (30.7) | 2523 (42.4) | 1574 (27.8) | 6409 (42.9) | 12,767 (38.2) |
| 5–9 years | 210 (11.4) | 290 (13.9) | 374 (12.9) | 1003 (16.8) | 741 (13.1) | 1991 (13.3) | 4609 (13.8) |
| 10–14 years | 91 (4.9) | 155 (7.4) | 252 (8.7) | 416 (7) | 439 (7.7) | 776 (5.2) | 2129 (6.4) |
| Total | 1846 (100) | 2081 (100) | 2901 (100) | 5953 (100) | 5670 (100) | 14,953 (100) | 33,404 (100) |
3.2. Overall prevalence and seasonality of ILIs
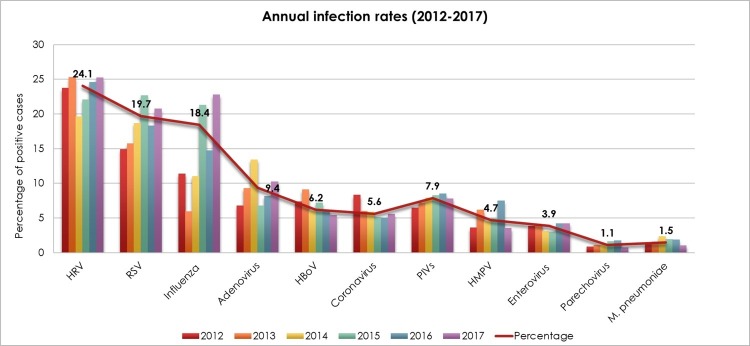
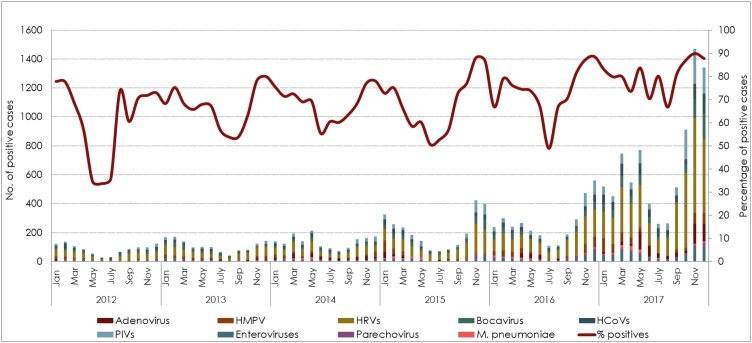
Out of 33,404 samples, 78% (26,138) tested positive for at least one pathogen. Notably, there was gradual increase in ILIs cases over the study years. Both, number of received samples and positivity rates increased annually, ranging from 64% (out of 1846) in 2012 to 84% (out of 14,953) in 2017. Fig. 1 . illustrates the overall prevalence of different respiratory infections. Together, HRV, RSV, and influenza viruses comprised nearly two-thirds of all positive cases, detected in 24%, 19.7%, and 18.4%, respectively. Adenovirus, PIVs, HboV, and HCoVs infections were detected at rates ranging from 5% to 9%. Other pathogens were detected less frequently (<5%), including HMPV, enteroviruses, M. pneumonia, and parechovirus.
Fig. 1.
Annual rate of respiratory infections reported between 2012 and 2017. The rate of infection per year was calculated as the number of positive cases of each specific virus per the total number of tested samples (n = 33,404 tested for influenza viruses only, n = 30,946 tested for influenza and other viruses).
Although HRV, RSV, and influenza were the main contributors to ILIs, their circulation was variable in different years of the study. HRV tended to dominate over other respiratory infections all over the six years except in 2015, where RSV infection was the primary cause of ILIs. Infection with HRV ranged from 19.6% to 25.2%, while RSV was detected in 15.7% to 22.6% in tested cases. Overall, RSV ranked the second leading cause of ILIs from 2012 to 2016. However, the frequency of influenza viruses raised in 2017, affecting almost 23% of the total ILIs cases, and representing the second most frequent infection. In all other surveillance years, influenza viruses or adenovirus were the third leading cause of ILIs, with infection rates ranging from 5.9% to 21% and 6.7% to 13.4%, respectively. The difference in the annual infection rates was significant for most of the infections (p < 0.001). Only PIVs had a frequency that was comparable throughout the study period, affecting more or less than 7% children in each year (p = 0.07) (Fig. 1).
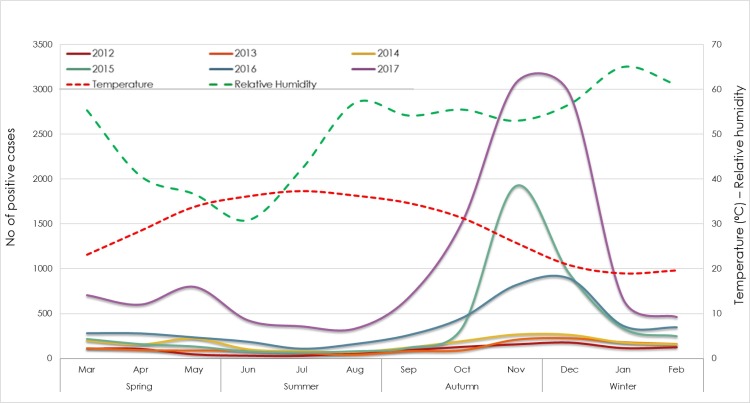
Qatar is characterized by extremely high temperatures during hot months (May–October), while cool months (December–February) exhibit occasional drops in the temperature, intermittent rainfalls, and heavy fog. Considering that the circulation of infections could be affected by weather, we investigated the seasonality of ILIs causing pathogens. Originally, the highest number of received samples was during fall (September–November; 38%) and winter (December–February; 32%), representing almost 70% of all samples. On the other hand, samples received during spring (March–April) and summer (June–August) together represented 30%. Similarly, the epidemic curve of confirmed positive cases with at least one pathogen showed a strong seasonal peak starting from late fall to early winter. The great majority of positive cases was reported in fall (10,535; 40%), and winter (8807; 33.5%), followed by spring (4564; 17.4%), and summer (2384; 9%). Noticeably, these temporal peaks coincided with the decrease in temperature (p < 0.001, r = −0.4) (Fig. 2 ).
Fig. 2.
Monthly distribution of positive cases with at least one respiratory pathogen (2012–2017) in correlation with temperature and relative humidity.
3.3. Influenza viruses
We found that influenza viruses contribute to an average of 18.4% of all respiratory infections. In 2012, influenza viruses were detected in 11% of all ILIs reported cases. This frequency declined to 6% in 2013, before increasing again to the same percentage in 2014 (11%). During the last three years of the surveillance (2015–2017), infection with influenza viruses raised markedly, reaching up to 22.8% (Table 2 ).
Table 2.
Distribution of influenza viruses by year of detection.
| Pathogen | Surveillance year |
||||||
|---|---|---|---|---|---|---|---|
| Total | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | |
| n = 33,404 | n = 1846 | n = 2081 | n = 2901 | n = 5953 | n = 5670 | n = 14,953 | |
| Positive cases |
|||||||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Influenza viruses | 6162 (18.4) | 210 (11.4) | 123 (5.9) | 320 (11) | 1267 (21.3) | 835 (14.7) | 3407 (22.8) |
| Influenza A | 4642 (13.9) | 147 (7.9) | 96 (4.6) | 264 (9.1) | 1145 (19.2) | 354 (6.2) | 2636 (17.6) |
| A/H1N1 | 3236 (9.7) | 85 (0.3) | 33 (0.1) | 188 (0.6) | 967 (2.9) | 75 (0.22) | 1888 (5.7) |
| A/H3N2 | 315 (0.9) | a | a | a | a | a | 315 (0.9) |
| Influenza B | 1520 (4.6) | 63 (3.4) | 27 (1.3) | 56 (1.9) | 122 (2) | 481 (8.5) | 771 (5.2) |
Samples were not tested for influenza A/H3N2.
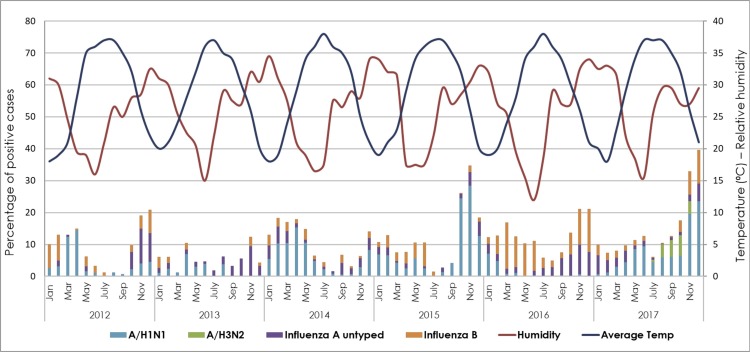
The great majority of influenza-positive cases (75% out of 6162) were infected with IAV, while IBV represented 25% (1520) of the cases. Within IAV-positive cases, 70% (3236) were A/H1N1, and 6.8% (315) were A/H3N2 subtype, while the rest were not subtyped. Interestingly, while IAV was the dominant subtype throughout the study period, IBV was detected at a higher frequency in 2016, reaching to 57% of total influenza-infected subjects (Table 2).
Influenza virus seasonality identified clear major and minor non-overlapping peaks, around November and April, respectively. Except for 2014, the largest proportion of influenza-positive cases was reported between November and December (12%–40% per month). In 2014, a major peak was observed between February and April (18% per month). In fact, the rise in influenza infection rates around April was clearly observed from 2012 to 2014, as well as in 2016, reaching up to 17% positive cases per month. To the contrary, these numbers declined dramatically in other timings of the year (Fig. 3 ).
Fig. 3.
Seasonality of influenza viruses from 2012 to 2017, in correlation with temperature and relative humidity. Percentage of positive samples for each influenza type/subtype was calculated out of the total tested samples for influenza viruses (n = 33,404).
3.4. RSV
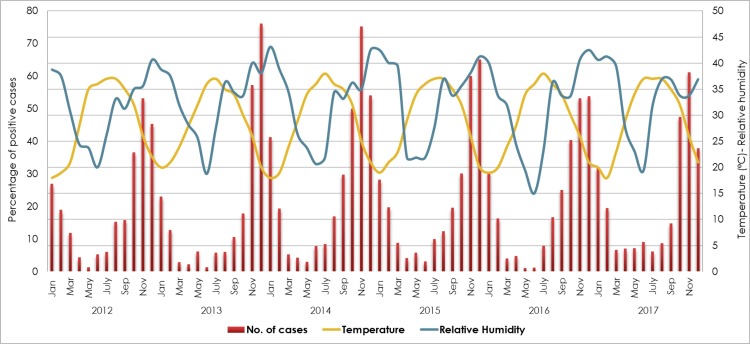
RSV was the second most frequent respiratory infection following HRV, reported from nearly 20% of admitted children (Table 3 ). RSV showed an annual cyclic pattern with a unimodal peak observed consistently between November and December of each year, affecting up to 47% of cases per month. A subsequent significant drop in the virus circulation was observed in the following months, reaching to the lowest frequency between May and June (0.6%–4%). Notably, while the rise in RSV infection rate correlated negatively with the temperature (p < 0.0001; r = −0.5), it correlated positively with the relative humidity peaks (p < 0.0001; r = 0.7) (Fig. 4 ).
Table 3.
Distribution of different respiratory pathogens by year of detection.
| Pathogen | Surveillance year |
||||||
|---|---|---|---|---|---|---|---|
| Total | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | |
| n = 30,946 | n = 1846 | n = 2081 | n = 2901 | n = 4614 | n = 5314 | n = 14,190 | |
| Positive cases |
|||||||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Adenovirus | 2902 (9.4) | 125 (6.8) | 193 (9.3) | 389 (13.4) | 311 (6.7) | 433 (8.1) | 1451 (10.2) |
| HMPV | 1450 (4.7) | 66 (3.6) | 128 (6.2) | 131 (4.5) | 231 (5) | 396 (7.5) | 498 (3.5) |
| HRV | 7443 (24.1) | 438 (23.7) | 526 (25.3) | 569 (19.6) | 1019 (22.1) | 1307 (24.6) | 3584 (25.3) |
| HboV | 1920 (6.2) | 136 (7.4) | 189 (9.1) | 175 (6) | 332 (7.2) | 322 (6.1) | 766 (5.4) |
| HCoV | 1741 (5.6) | 153 (8.3) | 124 (6) | 170 (5.9) | 236 (5.1) | 266 (5) | 792 (5.6) |
| 229E CoV | 188 (0.6) | 19 (1) | 19 (0.9) | 21 (0.7) | 30 (0.7) | 26 (0.5) | 73 (0.5) |
| NL63 CoV | 378 (1.2) | 37 (2) | 23 (1.1) | 45 (1.6) | 40 (0.9) | 62 (1.2) | 171 (1.2) |
| HKU1 CoV | 334 (1.1) | 22 (1.2) | 24 (1.2) | 28 (1) | 58 (1.3) | 66 (1.2) | 136 (1) |
| OC43 CoV | 840 (2.7) | 75 (4.1) | 58 (2.8) | 75 (2.6) | 108 (2.3) | 112 (2.1) | 412 (2.9) |
| MERS-CoV | 1 (0) | 0 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0.0) |
| PIVs | 2436 (7.9) | 119 (6.4) | 150 (7.2) | 230 (7.9) | 381 (8.3) | 450 (8.5) | 1106 (7.8) |
| PIV-1 | 569 (1.8) | 4 (0.2) | 38 (1.8) | 20 (0.7) | 135 (2.9) | 34 (0.6) | 338 (2.4) |
| PIV-2 | 237 (0.8) | 4 (0.2) | 17 (0.8) | 10 (0.3) | 54 (1.2) | 25 (0.5) | 127 (0.9) |
| PIV-3 | 1232 (4) | 86 (4.7) | 71 (3.4) | 148 (5.1) | 150 (3.3) | 291 (5.5) | 486 (3.4) |
| PIV-4 | 398 (1.3) | 25 (1.4) | 24 (1.2) | 52 (1.8) | 42 (0.9) | 100 (1.9) | 155 (1.1) |
| RSV | 6102 (19.7) | 275 (14.9) | 327 (15.7) | 542 (18.7) | 1045 (22.6) | 972 (18.3) | 2941 (20.7) |
| Enterovirus | 1202 (3.9) | 71 (3.8) | 84 (4) | 89 (3.1) | 138 (3) | 222 (4.2) | 598 (4.2) |
| Parechovirus | 341 (1.1) | 16 (0.9) | 23 (1.1) | 33 (1.1) | 74 (1.6) | 91 (1.7) | 104 (0.7) |
| M. pneumoniae | 457 (1.5) | 23 (1.2) | 32 (1.5) | 68 (2.3) | 88 (1.9) | 100 (1.9) | 146 (1) |
Fig. 4.
Seasonality of RSV from 2012 to 2017, in correlation with temperature and relative humidity. Percentage of positive samples for RSV was calculated out of the total tested samples (n = 30,946).
3.5. Influenza–RSV coinfection
Analysis of influenza–RSV co-infectivity revealed that 356 (1%) out of the total number of included children are co-infected with both influenza and RSV (Table 4 ). In other words, 7.3% (249) of children who are infected with influenza are RSV positive. Further, coinfection with RSV was comparable in both influenza A- and B-infected subjects.
Table 4.
Influenza–RSV co-infections.
| Year | Single infection |
Co-infections |
|||||
|---|---|---|---|---|---|---|---|
| Total | Influenza A | Influenza B | RSV | Influenza A–RSV | Influenza B–RSV | Influenza–RSV | |
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| 2012 | 1846 | 147 (7.9) | 63 (3.4) | 275 (14.9) | 22 (1.2) | 2 (0.1) | 24 (1.3) |
| 2013 | 2081 | 96 (4.6) | 27 (1.3) | 327 (15.7) | 4 (0.2) | 2 (0.1) | 6 (0.3) |
| 2014 | 2901 | 264 (9.1) | 56 (1.9) | 542 (18.7) | 5 (0.2) | 4 (0.1) | 9 (0.3) |
| 2015 | 5953 | 1145 (19.3) | 122 (2) | 1045 (17.6) | 31 (0.5) | 5 (0.1) | 36 (0.6) |
| 2016 | 5670 | 354 (6.2) | 481 (8.4) | 972 (17.1) | 17 (0.3) | 15 (0.3) | 32 (0.6) |
| 2017 | 14,953 | 2636 (17.6) | 771 (5.2) | 2941 (19.7) | 196 (1.3) | 53 (0.4) | 249 (1.7) |
| Total | 33,404 | 4642 (13.9) | 1520 (4.6) | 6102 (19.7) | 275 (0.8) | 81 (0.2) | 356 (1) |
3.6. Other respiratory infections
While more than one-third of all ILIs in children was attributed to RSV and influenza viral infections, 64% (16,728) of the cases were positive for other ARIs’ causing agents (Fig. 5 , Table 2). Our data showed that HRV accounted for the highest percentage of respiratory infections in most of the years, with an average infection rate of 24% annually. Although this virus demonstrated a consistent circulation throughout the years, it manifests primarily during fall, reaching 40%. Still, another peak of virus positivity was observed during warmer months around March (29%). Notably, HRV circulation coincided with low influenza and RSV rates.
Fig. 5.
Seasonality of non-RSV and non-influenza ILI pathogens from 2012 to 2017. Percentage of positive samples for each pathogen was calculated out of the total tested samples (n = 30,946).
Adenoviruses, the fourth most common infection, was detected in 9.4% of children. The virus was circulating throughout the year, yet, more active in late spring and early summer (May–June), affecting up to 22% of enrolled children.
PIVs accounted for a substantial proportion of ILIs per year (7.8%). PIV-3 was the most commonly detected type, representing more than 50.1% (1232) of all PIVs-positive cases. This was followed by PIV-4 (23%), PIV-1 (16%), and PIV-2 (9.7%). The general pattern of PIVs circulation was less interpretable than other infections. Nonetheless, in most of the years, a noticeable increase in the virus activity was observed during late spring as well as in late fall to early winter reaching to a positivity rate of 18%. While PIV-1 and 2 circulate without any defined pattern, PIV-3 was more active between fall and early winter in addition to its continuous activity in all other seasons. Lastly, PIV-4 also occurred more commonly in late fall to winter (4%), with few or no detections in the summer season (below 0.2%) (Supplementary Figure 1).
Out of all ILIs cases, 1920 (6.2%) children were positive for HboV. Except in 2014, HboV seemed to increase between February and March of each year, affecting 8% to 20% of children. In 2014, the virus continued to increase from February until July, where it reached it is highest activity, affecting 11% of cases in that month. Besides, a sudden increase in HboV cases was observed in August 2012, where it reached to 20% frequency, compared to 5% recorded in July, and 8% in September of the same year.
HCoVs positive cases accounted for 5.6% (1741) of the total number of samples. OC43 CoV was predominating over other HCoVs, detected in nearly 50% of all HCoV positive cases, followed by NL63 and HKU1 (20% each), and 229E (10%). On the other hand, MERS CoV was reported from only one case in 2014. Remarkably, winter months exhibited higher HCoV circulation.
Other ILIs causing pathogens, such as HMPV, enterovirus, M. pneumonia, and parechovirus showed less than 5% frequency. Out of all tested samples, 1450 (4.7%) were positive for HMPV. This virus displayed a clear seasonality typical of other winter respiratory viruses such as influenza and RSV. Enterovirus, on the other hand, was distributed all over the seasons, with a slight increase in late winter to spring. The overall proportion of enteroviral ARIs was approximately 4%, detected in 1202 of all admitted children. M. pneumonia and parechovirus infections were the least detected respiratory infections (<2%). Unlike parechovirues, M. pneumonia showed a unique temporal circulation between February and May.
3.7. Correlation with demographics
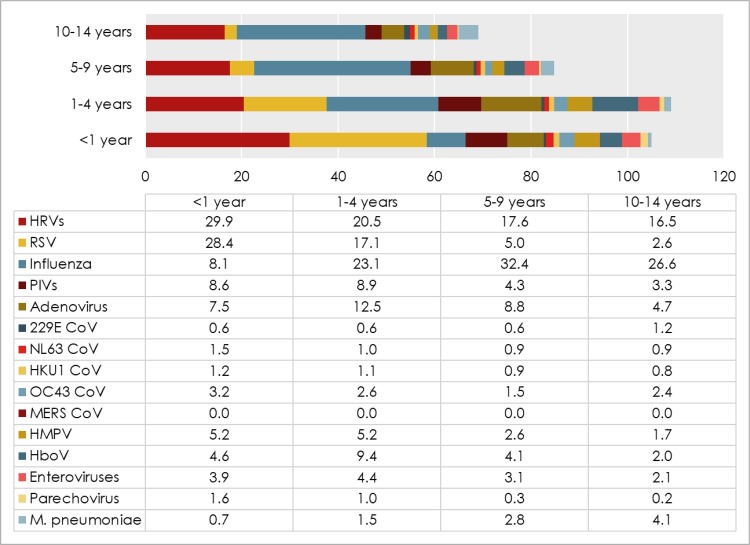
We first analyzed the correlation of each infection and age of participants. A marked variation in the infection rate was observed between different age groups (Fig. 6 ). Influenza infection was significantly lower within infants (<1 year; 8.1%), compared to older age groups (23%–32%). The highest infection rate of both, IAV and IBV was detected among school-age children (5–9 years; 21.4% and 11%, respectively). Contrarily, infants (<1 year) demonstrated the highest proportion of RSV (28.4%), followed by children aged 1–4 years (17%). This frequency decreased significantly with age (p < 0.0001), reaching 2% in 10–14 years age group. Influenza–RSV coinfection was also associated with age. Out of 356 influenza–RSV positive cases, 309 (87%) were children were below five years of age.
Fig. 6.
Infection rates of ILI causing pathogens among different age groups.
The frequency of HRV, HMPV, parechovirus, NL63 and HKU1 CoVs negatively correlated with age (p < 0.0001). On the contrary, M. pneumonia and 229 CoV infections increased significantly with age (p < 0.0001). Children 1–4 years old demonstrated the highest rates of adenovirus, HboV, PIVs, and enterovirus infections, which were detected in 12.5%, 9.4%, 9%, and 4.4% respectively.
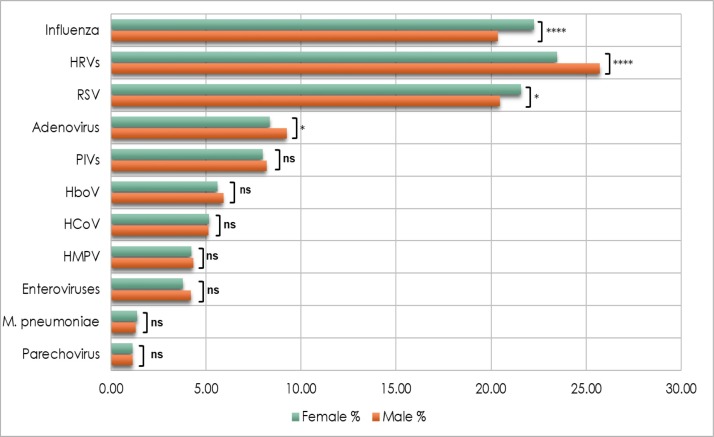
We further analyzed the sex-dependent differences in infection rates. In general, influenza and RSV showed higher circulation among females. For each virus, a detection rate of 22% among females, and 20% of males were recorded. While the difference in influenza infection was statistically significant, RSV infection showed only marginal significance (p = 0.04). Contrarily, males exhibited higher frequencies of HRV (26%) and adenovirus (9%) compared to females (23% and 8%, respectively). On the other hand, rates of HMPV, HboV, HCoV, PIVs, enteroviruses, M. pneumonia, and parechovirus infections were comparable between the two groups (Fig. 7 ).
Fig. 7.
Infection rates of ILI causing pathogens among males and females. The difference in the infection rate between the two groups was calculated using Pearson Chi2 and the Fisher Exact test. p-value less than 0.05 is flagged with one star (*). p-value less than 0.01 is flagged with two stars (**). p-value less than 0.001 is flagged with three stars (***). p-value less than 0.0001 is flagged with four stars (****). Ns: not significant.
4. Discussion
This comprehensive report describes, for the first time, the epidemiology of respiratory infections in children under 15 years in Qatar, spanning a period of six years (2012–2017). The significance of this study comes from the fact that respiratory infections are still causing a serious public health concern particularly among children. Most importantly, the uniqueness of the State of Qatar, which has a rapidly growing population with 87.3% expatriates, imposes unique healthcare challenges including the importation and circulation of respiratory infections.
Our study revealed that respiratory infections are continuously circulating among children, but in a relatively higher prevalence compared to reports from other countries. We found that 78% out of the 33,404 ILIs cases tested positive for at least one respiratory pathogen. Many other similar studies reported lower percentages, including Saudi Arabia (24% of 4611), Iran (59.6% of 156), Cameroon (65% of 365), and Philippines (43% of 1864) (Albogami et al., 2018, Njouom et al., 2012, Halaji et al., 2019, Otomaru et al., 2015). On the other hand, and in accordance with our current findings, a report on respiratory viruses among children (6 months–10 years) in eight countries (Australia, South East Asia, and Latin America) indicated a positivity rate of 79.6% (Taylor et al., 2017). The difference in infection rates could be reflecting the true burden in each region, or it is possibly attributed to differences in the studied population, diagnostic methods, and environmental factors. Of note, the positivity rate among children with ILI exceeds that of ILI in adults considerably, as reported by our group and others (Otomaru et al., 2015, Puzelli et al., 2009, Laguna-Torres et al., 2009, Arango et al., 2015, Ren et al., 2009).
Our data highlighted the wide contribution of HRV, RSV, and influenza in the overall burden of respiratory infections among children in Qatar. These three viruses were the main causing agents of respiratory illnesses in almost two-thirds of admitted children. We found that HRV was positive in nearly a quarter of all cases, and was predominating over other infections throughout the six-year period. Similar to our findings, HRV was reported to cause the highest percentage of ILI in children in different countries, as recently reported by Taylor et al. (Taylor et al., 2017). However, they reported higher prevalence (41.5% out of 6266 cases), probably because they enrolled children aged ten years or younger only, whereas our study included children below 15 years old, noting that HRV is more prevalent among younger age groups. In compliance with our results, a large national surveillance study from Korea (n = 36,915) showed that HRV was predominantly the leading cause of ARIs, with a percentage that decreased from 19% in children below 5 years, to 12% among those aged between 6 and 19 years (Kim et al., 2018). Contrarily, other studies reported lower HRV frequencies, probably due to the smaller sample size (Otomaru et al., 2015).
Infection with RSV was a main etiological agent of ILIs in our study following HRV. RSV is known to be the leading cause of ARIs and hospitalizations in children worldwide. Here, we reported a 20% infection rate, which is close to other reports from Bangkok (15%), US (22%), Russia (23%), Saudi Arabia (23.5%), and India (28%) (Albogami et al., 2018, Meskill et al., 2017, Thongpan et al., 2019, Saxena et al., 2017, Kurskaya et al., 2018). RSV was less frequently detected among children from countries with high influenza and HRV circulation, including Australia, South East Asia and Latin America (9.7%), and East Asia countries, such as Laos (12%), Korea (5%), and China (5.6%) (Kim et al., 2018, Wang et al., 2016, Snoeck et al., 2018). Similar to many other studies, we found that both HRV and RSV prevalence decreased significantly with age, where infants represented the most affected group (Kim et al., 2018, Snoeck et al., 2018, Cui et al., 2016).
Influenza viruses, on the other hand, accounted for approximately 18% of all cases and correlated positively with age in agreement with previous studies (Njouom et al., 2012, Zarychanski et al., 2010). Generally, both IAV and IBV were co-circulating in all seasons, with a noticeable dominance of IAV, except in 2016, where IBV was more prevalent. This high circulation of IBV in the 2016 season has also been reported in other studies (AlIbrahim et al., 2019, Simms et al., 2017). It could be explained by a mismatch between the circulating IBV lineage, and the administered trivalent influenza vaccine used in that season as reported from Lebanon (AlIbrahim et al., 2019). Importantly, despite the availability of quadrivalent vaccines that overcome this issue, young children are typically given a trivalent vaccine, which has only one IBV lineage.
RSV and influenza infections are known to have a fluctuating degree of seasonal overlap. In the current study, 7% of influenza-positive patients were also RSV positive, which is close to a four-years report in Spain (6%) (Reina and Dueñas, 2019). However, several other reports showed higher rates of influenza–RSV coinfections among children reaching 32% (Reina et al., 2014). Furthermore, we found that the majority of coinfections (87%) were among children below five years of age, which confirms previous reports (Nitsch-Osuch et al., 2016).
We also reported significant circulation of other important respiratory pathogens. The prevalence of adenovirus, PIVs, HboV, and HCoVs infections was below 10%, while infection with HMPV, enteroviruses, M. pneumonia, and parechovirus, showed a frequency of <5%. These figures are in line with several epidemiological studies on children (Albogami et al., 2018, Wang et al., 2016). Additionally, except for influenza and M. pneumonia, the prevalence of most respiratory viruses was higher among young age groups, probably due to their underdeveloped immunity.
Numerous studies have shown an association between the transmission of respiratory viruses and climate, particularly humidity and temperature (Lowen et al., 2007). Our data showed a significantly higher positivity rate during cooler months, which coincided with low ambient air temperature and high relative humidity.
Circulation of influenza viruses showed a clear primary peak between November and December, which is consistent with reports from regions with a similar climate (Caini et al., 2018). Additionally, we observed another minor peak around April, which is interestingly similar to what has been reported in Northern Hemisphere countries (Caini et al., 2018). We also found that RSV and HMPV circulate simultaneously with influenza while HRV was primarily active during low RSV and influenza activity, which is similar to previous reports (Njouom et al., 2012, Haynes et al., 2016, Razanajatovo et al., 2011, Nguyen et al., 2016). While many studies failed to determine HRV seasonality (Taylor et al., 2017, Wang et al., 2016), our data indicated a noticeable seasonal activity during early fall at the time of school opening in Qatar, followed by another peak around March. Likewise, infections with adenovirus and M. pneumonia tend to raise at the time of low RSV and influenza circulation. Adenovirus usually does not exhibit a discernible seasonal circulation pattern; however, similar to our results, the increase in virus transmission around May has been previously reported (Gülen et al., 2014). We also found that HboV displayed a consistent increase during late winter/early spring in most of the years, in line with reports from Iran and South Africa (Tabasi et al., 2016, Schildgen et al., 2008). We also found that the overall seasonality of PIVs was ambiguous and lacked any specific circulation trends. However, late fall and early winter exhibited higher PIVs cases, especially with PIV type 3 and 4. Contrarily, according to the CDC, PIV-1 and PIV-2 circulate more commonly during fall, PIV-3 occur frequently in spring and summer, while PIV-4 circulate without any seasonal occurrence (Human Parainfluenza Viruses, 2017). On the other hand, HCoVs were more prevalent in winter, typical of other respiratory viruses. Yet, positive cases were also reported during warm months confirming other reports (Soonnarong et al., 2016, Gaunt et al., 2010). Finally, parechovirus infections were scattered throughout the years, with no temporal patterns, although previous records indicated higher activity between fall and winter (Chiang et al., 2017). This is probably due to the low infection rates reported in our study. In fact, the variation in the reported viruses’ seasonality could possible result from the sampling bias that exists in most retrospective studies, in which more specimens are usually collected during the winter months.
It is recognized that males and females differ in the intensity, prevalence, and pathogenesis of infections (Klein, 2012). Our analysis revealed that influenza and RSV are more prevalent among females, while HRV and adenovirus were higher among male patients. This confirms our previous study on ARIs among adults, as well as other epidemiological studies (Al-Romaihi et al., 2019, Zarychanski et al., 2010, Pouremamal and Pouremamali, 2016).
In summary, this multi-year study systematically investigated the frequency of respiratory pathogens among 33,404 children in Qatar. Given the large number of enrolled patients, this is the largest scale report on ILIs among children in the MENA region to the best of our knowledge. Information obtained from this study shall have important implications on infections’ control and prevention policies, particularly for public health authorities and health systems. For instance, results would help in launching prevention and control programs such as vaccine campaigns according to the country-specific pathogens’ seasonality. Furthermore, the effectiveness of these vaccine and awareness campaigns could be improved by prioritizing high-risk groups with higher infection burden. This epidemiologic study can also improve the diagnosis strategy by providing data about the causes of ILIs in the country, and hence, help in improving the selection of pathogens for regular clinical testing. Nonetheless, future studies that include patients’ vaccination records and clinical symptoms are still needed to understand the clinical significance and the vaccine coverage of respiratory diseases in Qatari population.
Ethics statement
This study was approved by Qatar University, MOPH, as well as HMC-MRC: approval #16335/16.
Conflict of interest
All authors declare no conflict of interest.
Acknowledgment
This study was supported by funds from Hamad Medical Corporation (grant # 16335/16) and Qatar University (grant # QUCG-BRC-2018/2019-1). The publication of this article was funded by Qatar National Library.
Footnotes
Supplementary material related to this article can be found, in the online version, at https://doi.org/10.1016/j.ijid.2020.04.008.
Contributor Information
Hamad E. Al-Romaihi, Email: halromaihi@moph.gov.qa.
Maria K. Smatti, Email: msmatti@qu.edu.qa.
Hebah A. Al-Khatib, Email: halkhatib@qf.org.qa.
Peter V. Coyle, Email: PCoyle@hamad.qa.
Nandakumar Ganesan, Email: nkumar@moph.gov.qa.
Shazia Nadeem, Email: snadeem@moph.gov.qa.
Elmoubasher A. Farag, Email: eabdfarag@moph.gov.qa.
Asmaa A. Al Thani, Email: aaja@qu.edu.qa.
Abdullatif Al Khal, Email: aalkhal@hamad.qa.
Khalid M. Al Ansari, Email: kalansari@sidra.org.
Muna A. Al Maslamani, Email: malmaslamani@hamad.qa.
Hadi M. Yassine, Email: hyassine@qu.edu.qa.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Albogami S.S. Seasonal variations of respiratory viruses detected from children with respiratory tract infections in Riyadh, Saudi Arabia. J Infect Public Health. 2018;11(2):183–186. doi: 10.1016/j.jiph.2017.06.001. [DOI] [PubMed] [Google Scholar]
- AlIbrahim M. Molecular epidemiology and genetic characterization of influenza B virus in Lebanon during 2016–2018. Infect Genet Evol. 2019;75:103969. doi: 10.1016/j.meegid.2019.103969. [DOI] [PubMed] [Google Scholar]
- Alkuwari M.G. Pandemic influenza A/H1N1 vaccination uptake among health care workers in Qatar: motivators and barriers. Vaccine. 2011;29(11):2206–2211. doi: 10.1016/j.vaccine.2010.08.093. [DOI] [PubMed] [Google Scholar]
- Al-Romaihi H.E. Epidemiology of respiratory infections among adults in Qatar (2012–2017) PLoS One. 2019;14(6):e0218097. doi: 10.1371/journal.pone.0218097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango A.E. Influenza-like illness sentinel surveillance in one hospital in Medellin, Colombia 2007–2012. Influenza Other Respir Viruses. 2015;9(1):1–13. doi: 10.1111/irv.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarska K. Evaluation of the activity of influenza and influenza-like viruses in the epidemic season 2013/2014. Adv Exp Med Biol. 2015;857:1–7. doi: 10.1007/5584_2015_116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caini S. Epidemiology of seasonal influenza in the Middle East and North Africa regions, 2010–2016: circulating influenza A and B viruses and spatial timing of epidemics. Influenza Other Respir Viruses. 2018;12(3):344–352. doi: 10.1111/irv.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang G.P.K. Clinical features and seasonality of parechovirus infection in an Asian subtropical city, Hong Kong. PLoS One. 2017;12(9):e0184533. doi: 10.1371/journal.pone.0184533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comach G. Sentinel surveillance of influenza-like illness in two hospitals in Maracay, Venezuela: 2006–2010. PLoS One. 2012;7(9):pe44511. doi: 10.1371/journal.pone.0044511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui D. Clinical and epidemiologic characteristics of hospitalized patients with laboratory-confirmed respiratory syncytial virus infection in Eastern China between 2009 and 2013: a retrospective study. PLoS One. 2016;11(11):e0165437. doi: 10.1371/journal.pone.0165437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiaggi M. The role of infections and coinfections with newly identified and emerging respiratory viruses in children. Virol J. 2012;9:247. doi: 10.1186/1743-422X-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt E.R. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol. 2010;48(8):2940–2947. doi: 10.1128/JCM.00636-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E. Estimating the hospitalization burden associated with influenza and respiratory syncytial virus in New York City, 2003–2011. Influenza Other Respir Viruses. 2015;9(5):225–233. doi: 10.1111/irv.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gülen F. Ten year retrospective evaluation of the seasonal distribution of agent viruses in childhood respiratory tract infections. Turk Pediatri Ars. 2014;49(1):42–46. doi: 10.5152/tpa.2014.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaji M. Viral etiology of acute respiratory infections in children in Southern Iran. Rev Soc Bras Med Trop. 2019;52:e20180249. doi: 10.1590/0037-8682-0249-2018. [DOI] [PubMed] [Google Scholar]
- Haynes A.K. Human metapneumovirus circulation in the United States, 2008 to 2014. Pediatrics. 2016;137(5) doi: 10.1542/peds.2015-2927. [DOI] [PubMed] [Google Scholar]
- Human Parainfluenza Viruses (HPIVs); 2017; Available from: https://www.cdc.gov/parainfluenza/seasons.html
- Janahi I. Viral aetiology of bronchiolitis in hospitalised children in Qatar. BMC Infect Dis. 2017;17(1):139. doi: 10.1186/s12879-017-2225-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.M. Nation-wide surveillance of human acute respiratory virus infections between 2013 and 2015 in Korea. J Med Virol. 2018;90(7):1177–1183. doi: 10.1002/jmv.25069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.L. Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. Bioessays. 2012;34(12):1050–1059. doi: 10.1002/bies.201200099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krilov L.R. Respiratory syncytial virus: update on infection, treatment, and prevention. Curr Infect Dis Rep. 2001;3(3):242–246. doi: 10.1007/s11908-001-0026-3. [DOI] [PubMed] [Google Scholar]
- Kurskaya O. Viral etiology of acute respiratory infections in hospitalized children in Novosibirsk City, Russia (2013-2017) PLoS One. 2018;13(9):pe0200117. doi: 10.1371/journal.pone.0200117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguna-Torres V.A. Influenza-like illness sentinel surveillance in Peru. PLoS One. 2009;4(7):e6118. doi: 10.1371/journal.pone.0006118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowen A.C. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3(10):1470–1476. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meskill S.D. Prevalence of co-infection between respiratory syncytial virus and influenza in children. Am J Emerg Med. 2017;35(3):495–498. doi: 10.1016/j.ajem.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Monto A.S. Epidemiology of viral respiratory infections. Am J Med. 2002;112(Suppl. 6A):4S–12S. doi: 10.1016/s0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- Nguyen D.N.T. Epidemiology and etiology of influenza-like-illness in households in Vietnam; it's not all about the kids! J Clin Virol. 2016;82:126–132. doi: 10.1016/j.jcv.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsch-Osuch A. Incidence and clinical course of respiratory viral coinfections in children aged 0–59 months. Adv Exp Med Biol. 2016;905:17–23. doi: 10.1007/5584_2015_185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njouom R. Viral etiology of influenza-like illnesses in Cameroon, January–December 2009. J Infect Dis. 2012;206(Suppl. 1):S29–S35. doi: 10.1093/infdis/jis573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otomaru H. Influenza and other respiratory viruses detected by influenza-like illness surveillance in Leyte Island, the Philippines, 2010–2013. PLoS One. 2015;10(4):e0123755. doi: 10.1371/journal.pone.0123755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia A.T. Viral infections of the lower respiratory tract: old viruses, new viruses, and the role of diagnosis. Clin Infect Dis. 2011;52(Suppl. 4):S284–S289. doi: 10.1093/cid/cir043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouremamal A., Pouremamali F. The seasonal frequency of human rhinoviruses and enteroviruses in respiratory secretions of patients with respiratory infections in Ahvaz. J Antivir Antiretrovir. 2016 [Google Scholar]
- Puzelli S. Viral causes of influenza-like illness: insight from a study during the winters 2004–2007. J Med Virol. 2009;81(12):2066–2071. doi: 10.1002/jmv.21610. [DOI] [PubMed] [Google Scholar]
- Razanajatovo N.H. Viral etiology of influenza-like illnesses in Antananarivo, Madagascar, July 2008 to June 2009. PLoS One. 2011;6(3):e17579. doi: 10.1371/journal.pone.0017579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reina J., Dueñas J. [Respiratory co-infections between influenza viruses and respiratory syncytial virus (2014–2017)] An Pediatr (Barc) 2019;90(2):118–119. doi: 10.1016/j.anpedi.2018.02.011. [DOI] [PubMed] [Google Scholar]
- Reina J. [Analysis of co-infections between influenza A and influenza B viruses and other respiratory viruses, 2012–2013] Enferm Infecc Microbiol Clin. 2014;32(10):693–695. doi: 10.1016/j.eimc.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L. Prevalence of human respiratory viruses in adults with acute respiratory tract infections in Beijing, 2005–2007. Clin Microbiol Infect. 2009;15(12):1146–1153. doi: 10.1111/j.1469-0691.2009.02746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S. Clinical characterization of influenza A and human respiratory syncytial virus among patients with influenza like illness. J Med Virol. 2017;89(1):49–54. doi: 10.1002/jmv.24607. [DOI] [PubMed] [Google Scholar]
- Schildgen O. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin Microbiol Rev. 2008;21(2):291–304. doi: 10.1128/CMR.00030-07. [table of contents] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms A. Co-circulation of Influenza A and B during the 2016–2017 influenza season at Rush University Medical Center. Open Forum Infect Dis. 2017;4(Suppl. 1):S314–S315. [Google Scholar]
- Simoes E.A.F. 2nd ed. Washington (DC); 2006. Acute respiratory infections in children, in disease control priorities in developing countries. [Google Scholar]
- Snoeck C.J. Etiology of viral respiratory infections in Northern Lao People's Democratic Republic. J Med Virol. 2018;90(10):1553–1558. doi: 10.1002/jmv.25237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soonnarong R. Molecular epidemiology and characterization of human coronavirus in Thailand, 2012–2013. Springerplus. 2016;5(1):1420. doi: 10.1186/s40064-016-3101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabasi M. Human bocavirus infections among children less than two years old in Iran during fall and winter 2012-2013. Iran J Microbiol. 2016;8(1):80–84. [PMC free article] [PubMed] [Google Scholar]
- Tang L.F. Viral pathogens of acute lower respiratory tract infection in China. Indian Pediatr. 2008;45(12):971–975. [PubMed] [Google Scholar]
- Taylor S. Respiratory viruses and influenza-like illness: epidemiology and outcomes in children aged 6 months to 10 years in a multi-country population sample. J Infect. 2017;74(1):29–41. doi: 10.1016/j.jinf.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongpan I. Respiratory syncytial virus, human metapneumovirus, and influenza virus infection in Bangkok, 2016–2017. PeerJ. 2019;7:pe6748. doi: 10.7717/peerj.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregoning J.S., Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010;23(1):74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C.L.F. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381(9875):1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E.E., Falsey A.R. Respiratory syncytial virus infection in adult populations. Infect Disord Drug Targets. 2012;12(2):98–102. doi: 10.2174/187152612800100116. [DOI] [PubMed] [Google Scholar]
- Wang D. Viral etiology of medically attended influenza-like illnesses in children less than five years old in Suzhou, China, 2011–2014. J Med Virol. 2016;88(8):1334–1340. doi: 10.1002/jmv.24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO; 2014. WHO surveillance case definitions for ILI and SARI. [Google Scholar]
- Williams B.G. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002;2(1):25–32. doi: 10.1016/s1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2004. The World Health Report: 2004: changing history. [Google Scholar]
- World Health Organization (WHO) 2017. Number of deaths − acute lower respiratory tract infections (2017) [Google Scholar]
- Zarychanski R. Correlates of severe disease in patients with 2009 pandemic influenza (H1N1) virus infection. CMAJ. 2010;182(3):257–264. doi: 10.1503/cmaj.091884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.