Intrathecal administration of anti-infectives is indicated in central nervous system infections by multiresistant pathogens when drugs that can reach adequate cerebrospinal fluid (CSF) concentrations by systemic therapy are not available. Antibiotics that readily pass the blood-brain and blood-CSF barriers and/or that have low toxicity allowing an increase in the daily dosage should not be used for intrathecal therapy. Intrathecal therapy is accompanied by systemic treatment. Antibacterials indispensable for intrathecal therapy include aminoglycosides, colistin, daptomycin, tigecycline, and vancomycin.
KEYWORDS: antibiotics, antifungal agents, brain abscess, cerebrospinal fluid, intrathecal, intraventricular, meningitis, ventricular shunt, ventriculitis
SUMMARY
Intrathecal administration of anti-infectives is indicated in central nervous system infections by multiresistant pathogens when drugs that can reach adequate cerebrospinal fluid (CSF) concentrations by systemic therapy are not available. Antibiotics that readily pass the blood-brain and blood-CSF barriers and/or that have low toxicity allowing an increase in the daily dosage should not be used for intrathecal therapy. Intrathecal therapy is accompanied by systemic treatment. Antibacterials indispensable for intrathecal therapy include aminoglycosides, colistin, daptomycin, tigecycline, and vancomycin. Limited experience suggests the utility of the antifungals amphotericin B and caspofungin. Intraventricular administration ensures distribution throughout the CSF compartment, whereas intralumbar dosing often fails to attain adequate antibiotic concentrations in the ventricles. The individual dose is determined by the estimated size of the CSF space and by the estimated clearance from CSF. For moderately lipophilic anti-infectives with a molecular weight above approximately 1,000 g/mol, as well as for hydrophilic drugs with a molecular weight above approximately 400 g/mol, one daily dose is normally adequate. The ventricular drain should be clamped for 15 to 120 min to facilitate the distribution of the anti-infective in the CSF space. Therapeutic drug monitoring of the trough levels is necessary only in cases of therapeutic failure.
INTRODUCTION
The blood-brain and blood-cerebrospinal fluid (CSF) barriers protect the central nervous system (CNS) from endogenous and exogenous compounds present in the systemic circulation, thereby ensuring the proper function of the CNS. The barriers, however, present an obstacle for many anti-infective drugs to attain effective concentrations in the central nervous compartments after systemic administration. Intrathecal administration of an anti-infective is indicated in CNS infections caused by multiresistant pathogens when drugs adequate for systemic therapy are not available. In this review, “intrathecal” is used as the generic term, whereas “intraventricular” and “intralumbar” clarify the injection sites “cerebral ventricles” or “lumbar CSF.”
While the incidence of community-acquired meningitis with multiresistant pathogens is still relatively low, particularly external and internal ventricular shunt infections and infections of other devices in the CNS with multiresistant bacteria pose a therapeutic challenge. Typical pathogens of nosocomial meningitis and ventriculitis are methicillin-resistant coagulase-negative staphylococci and Staphylococcus aureus, vancomycin-resistant enterococci, and carbapenemase-producing Gram-negative bacteria (Acinetobacter spp., Klebsiella spp., Escherichia coli, and Pseudomonas aeruginosa) (1–3). Eleven years after the extensive review of intrathecal antibiotic treatment options by Ziai and Lewin (4), 10 years after our review on the pharmacokinetics of anti-infectives in the CNS compartments in this journal (5), and on the background of increasing problems with multiresistant pathogens in nosocomial CNS infections, this review of PubMed-listed publications until January 2020 aims to update the indications, pharmacokinetic principles, and complications of intrathecal antibacterial and antifungal therapy in meningitis and ventriculitis.
ANATOMY AND PATHOPHYSIOLOGY
In simplified terms, the blood-brain and blood-CSF barriers can be viewed as a lipid layer surrounding the CNS, with leaky regions comprising ∼1:5,000 of the entire capillary surface area (6).
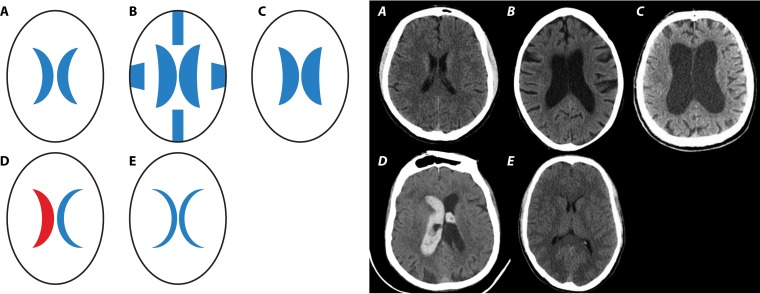
The CSF space consists of the 4 ventricles, the aqueduct, the basal cisterns, and the subarachnoid space over the convexities and in the spinal canal. There is considerable interindividual variation depending on age, the widths of the ventricles and the cerebral subarachnoid space, the length and width of the spinal canal, and the underlying disease (Fig. 1). The volume of the ventricles deduced from postmortem casts ranged from 7.4 to 56.6 ml (mean, 22.4 ml), and the volume of the spinal subarachnoid space was given as approximately 30 ml (7). In vivo estimation of CSF volume using magnetic resonance imaging in healthy volunteers resulted in cranial CSF volumes of approximately 96 ml in young children (19 to 33 months), approximately 250 ml in middle-aged adults (40 to 55 years), and approximately 300 ml in the elderly (71 to 80 years) (8, 9). By three-dimensional whole-body magnetic resonance imaging, mean CSF volumes of 326 ml in healthy adults, 488 ml in patients with communicating hydrocephalus, and 593 ml in patients with noncommunicating hydrocephalus were estimated (10). Blood clots in the ventricle(s) or basal cisterns or a spinal mass lesion can substantially diminish the volume of CSF (Fig. 1).
FIG 1.
Strong variation of the volume of the cerebrospinal fluid (CSF) under normal and pathological conditions. Shown are schematic drawings (bold A to E) and cranial computer tomographic images illustrating real-world conditions (italic A to E). (A) Normal conditions. (B) Communicating hydrocephalus with enlargement of the ventricles and of the subarachnoid space. The volume of the CSF is enlarged. (C) Occlusive hydrocephalus with enlargement of the 1st and 2nd ventricles. Depending on the site of obstruction of the CSF flow, the volume of the CSF can be enlarged or diminished. (D) Intracerebral and intraventricular bleeding. As a consequence of blood clots in the ventricles, basal cisterns, and/or subarachnoid space, the volume of the CSF is diminished. (E) Slit-like ventricles, e.g., as a consequence of brain edema or excessive CSF drainage. Here, the volume of the CSF is diminished. (Panels A, B, C, and E are courtesy of Johannes Gossner, Department of Radiology, Protestant Hospital Göttingen-Weende, and panel D is courtesy of Hans-Heino Rustenbeck, Department of Neuroradiology, University Medicine Gottingen [reproduced with permission].)
Because the compartment is convoluted, the distribution in it cannot be considered homogeneous (11). About two-thirds of the CSF is produced by the choroid plexus, and one-third originates from the extracellular space of the brain and spinal cord. The flow of CSF oscillates depending on heartbeats and respiration. These oscillations facilitate the equilibration of drugs in the CSF space. The net direction of flow is from the ventricles to the cisterna magna and from there to the cerebral convexities and into the spinal canal (7, 12–14).
Unlike the blood-brain and blood-CSF barriers, there is no tight barrier between CSF and extracellular fluid of the nervous tissue. Even large molecules can enter the extracellular space of the brain and spinal cord. The extracellular space of the brain comprises about 15% of the brain volume (7, 12). Drugs can diffuse from the CSF into the extracellular fluid with or without entry into the intracellular space of the different cells of the brain and spinal cord, with or without intracellular metabolism (9). Diffusion into the extracellular space of the brain and spinal cord occurs against the gradient of CSF bulk flow directed from the nervous tissue into the CSF (7). Drugs that are able to cross the lipid layers of the blood-CSF and blood-brain barriers, after intraventricular or intralumbar administration in the absence of adequate plasma concentrations, rapidly disappear from the extracellular fluid of the nervous tissue and from the intracranial compartments (9). Drugs that are almost exclusively eliminated by bulk flow and display no or only minimal intracellular uptake or transcapillary efflux can attain high and long-duration therapeutic concentrations in the CSF (and the adjacent extracellular space of the nervous tissue) (9, 15–18).
The interchange between the CSF, the extracellular fluid of the brain, and other intracranial compartments is studied by using external ventriculostomies and cerebral intraparenchymal microdialysis catheters (19, 20). Due to their invasiveness, both approaches were not used in the same patient to characterize the entry of an antibiotic into the central nervous compartments (19, 20). Positron emission tomography with radiolabeled anti-infectives (21, 22) may in the future provide a means of noninvasive investigation of intercompartmental exchange after intraventricular injection.
PHARMACODYNAMICS
As a consequence of the restricted nutritional supply and the acidic pH of the CSF, pathogens multiply in the CNS less rapidly than in blood or broth. The limited amount of pharmacodynamic data on the CSF compartment suggests that the concept of time- and concentration-dependent antibiotics is also valid for the treatment of CNS infections (23). With β-lactam antibiotics and fluoroquinolones, after intravenous (i.v.) administration, CSF concentrations of ≥10× the minimal bactericidal concentration (MBC) (for most meningeal pathogens, the MBC is very close to the MIC usually determined by clinical routine) are necessary to ensure the rapid killing of bacteria (24, 25). Because CSF concentrations far above the MICs of susceptible bacteria can be reached by this mode of administration, intraventricular or lumbar intrathecal antibiotics can lead to quick CSF sterilization in patients who develop meningitis and ventriculitis after neurosurgery and who require treatment with antibiotics that do not reach adequate CSF concentrations after systemic administration (26).
ANTI-INFECTIVES USED FOR THERAPY OF CNS INFECTIONS
Antibacterials and Antifungals That Should Not Be Administered Intrathecally
Antibiotics that readily pass the blood-brain and blood-CSF barriers and/or have low toxicity and thus allow for an increase in the daily dose should not be used for intrathecal therapy. At very high doses or in the presence of renal failure, even after intravenous infusion, β-lactam antibiotics are neurotoxic and can induce confusion, encephalopathy, myoclonus, and epileptic seizures, including status epilepticus, particularly in patients suffering from various neurological disorders (27, 28). Experimental evidence suggests that intrathecal therapy has a higher risk of seizures than systemic application (29). Among the β-lactam antibiotics frequently used in critically ill patients, the relative potencies to induce epileptic seizures (penicillin G = 1.00) of cefazolin (2.94), cefepime (1.60), and imipenem (0.71) are comparatively high, whereas the epileptogenic potencies of ampicillin (0.21), ceftazidime (0.17), meropenem (0.16), ceftriaxone (0.12), piperacillin (0.11), and cefotaxime (0.088), i.e., the compounds generally used to treat complicated CNS infections, are comparatively low (28). Continuous intravenous infusion instead of discontinuous administration may be useful to avoid high peak concentrations and associated CNS side effects (28).
Either due to low toxicity that makes it possible to increase the daily dose or because they readily penetrate the blood-brain and blood-CSF barriers (5, 19, 20), the intrathecal administration of fluoroquinolones, penicillin G, piperacillin, cefotaxime, ceftriaxone, ceftazidime, meropenem, β-lactamase inhibitors, linezolid, fosfomycin, metronidazole, chloramphenicol, co-trimoxazole, tetracyclines, rifampin, isoniazid, ethambutol, pyrazinamide, fluconazole, posaconazole, voriconazole, and flucytosine is not indicated. Off label, the dose of meropenem has been increased up to 15 g/day (30), and that of cefotaxime has been increased up to 300 mg/kg of body weight/day, with a maximum dose of 24 g/day (31). The maximum intravenous fosfomycin dose used in clinical practice without severe side effects was also 24 g/day (32).
Antibacterials and Antifungals Eligible for Intrathecal Therapy
The following anti-infectives are too large and/or too toxic to achieve high concentrations in the CNS after intravenous administration: vancomycin, teicoplanin, gentamicin, tobramycin, netilmicin, amikacin, streptomycin, colistin, polymyxin B, daptomycin, amphotericin B, and caspofungin (4, 33). Despite its relatively high percentage of CSF penetration, the intrathecal application of tigecycline appears attractive, as CSF concentrations with the usual intravenous dose of 100 mg/day ranged from only 0.035 to 0.048 mg/liter. Increasing the daily dose above 200 mg/day is not well tolerated (34, 35). We systematically searched for pharmacokinetic data on these compounds after intrathecal administration (intraventricular or intralumbar) in PubMed. Physicochemical properties of the anti-infectives studied were retrieved from the PubChem database (https://pubchem.ncbi.nlm.nih.gov), unless otherwise indicated.
EVIDENCE FOR THE EFFECTIVENESS AND SAFETY OF INTRATHECAL ANTI-INFECTIVE THERAPY
In the last decades, intraventricular injection of some antimicrobials has been related to significant toxicity. More recent reports, however, suggested that intrathecal administration of colistin, aminoglycosides, and vancomycin is not associated with severe or irreversible toxicity. Toxicity appeared to be dose related, and early reports, e.g., in the case of streptomycin, may have been associated with inappropriate dosing (4). Conversely, since patients receiving intrathecal therapy usually are very sick, adverse drug effects may have been overlooked or attributed to complications of the underlying disease(s). For the latter reasons, the true incidence of adverse effects of intrathecal therapy might have been underestimated.
Case Reports and Case Series
Numerous reports on the successful treatment of nosocomial and device-associated CNS infections have been published in the last decades; e.g., in a 5-year-old patient suffering from carbapenemase-producing Enterobacter cloacae ventriculitis, 6 days of intravenous colistin failed to cure the ventriculitis. CSF cultures became negative after only 2 days of intraventricular colistin at a dose of 10 mg/day (36). A 17-year-old girl weighing 40 kg developed postsurgery meningitis caused by a highly carbapenem-resistant E. cloacae isolate (MIC of imipenem, ≥16 mg/liter). After high-dose intravenous meropenem plus amikacin had failed, she was cured by the addition of 30 mg amikacin administered once daily via external ventriculostomy (37).
In 14 patients with A. baumannii, Pseudomonas aeruginosa, E. coli, Klebsiella pneumoniae, or Serratia marcescens postsurgical meningitis or ventriculitis who received a sequential combination of intravenous antibiotics and intraventricular therapy (gentamicin, n = 4; amikacin, n = 7; colistin, n = 4), the cure rate was 73.3% (38). In 18 consecutive patients with postneurosurgical ventriculitis/meningitis caused by multidrug-resistant A. baumannii treated with either intravenous colistin alone or intravenous plus intraventricular colistin, the CSF sterilization rates were 33.3% in intravenously treated patients and 100% in those treated both intraventricularly and intravenously (P = 0.009). The 5 patients who died of A. baumannii CNS infection were all in the group that received only intravenous colistin (39). In a two-center, matched-pair study in adults with Gram-negative postoperative meningitis due to carbapenem-resistant bacteria, intralumbar or intraventricular antibiotic treatment in addition to systemic treatment (colistin or amikacin) resulted in a lower mortality rate than with intravenous therapy alone (2/23 [8.7%] versus 9/27 [33.3%]). Serious adverse events with intrathecal or intraventricular therapy, in particular seizures or chemical ventriculitis/radiculitis, were not documented (38–40). Also, for 21 patients with postneurosurgical Gram-negative meningitis/ventriculitis with intraventricular or intralumbar therapy with amikacin (7 patients), polymyxin B (9 patients), and colistin (5 patients), no serious adverse effects were reported (41). A recent multicenter retrospective study of 105 patients receiving intraventricular therapy (52.4% received vancomycin, with an average dose of 12.2 mg/day and a median duration of therapy of 5 days, and 47.5% received aminoglycosides, with an average gentamicin dose of 6.7 mg/day and a median duration of therapy of 6 days) together with systemic antibiotic therapy found CSF culture sterilization in approximately 90% of patients. In 9.5% of the patients, either the infection relapsed or the CSF cultures remained positive. The overall in-hospital mortality rate was 18.1% (42).
Randomized Studies
In a small, randomized, single-center study of 10 patients treated with vancomycin (5 patients given 10 mg intraventricularly every 24 h and 5 patients given 500 mg i.v. every 6 h), bacteria were effectively cleared from the CSF in both groups within 3 to 4 days of vancomycin therapy, with the CSF vancomycin concentrations being much higher after intraventricular than after systemic application. The study was too small to detect differences in outcomes (17). The only randomized multicenter study in 52 infants with Gram-negative bacterial meningitis and ventriculitis comparing either systemic ampicillin and gentamicin or intraventricular gentamicin (2.5 mg every 24 h) plus systemic ampicillin and gentamicin in Gram-negative bacillary meningitis in newborns was prematurely terminated because of the higher mortality rate in the intraventricular therapy group (42.9% versus 12.5%) (43). Therefore, intraventricular antibiotics under the conditions tested in this trial (Gram-negative bacteria, mostly E. coli and Salmonella spp., and probably no multiresistant bacteria) should be avoided. It has been argued that the excessive release of endotoxin after intraventricular gentamicin administration caused this sharp increase in mortality.
Due to the lack of positive randomized studies, intraventricular anti-infectives should not be used as a routine treatment for meningitis and meningoencephalitis. Intrathecal administration of anti-infectives must be considered an individual curative treatment in cases where promising intravenous therapeutic options are not available.
Meta-analysis
A recent meta-analysis on ventriculitis/meningitis caused by Gram-negative pathogens (11 studies with 348 adult patients) found that intraventricular plus intravenous therapy was superior to intravenous therapy alone in the eradication of pathogens (odds ratio [OR], 10.06; 95% confidence interval [CI], 2.62 to 38.65), infectious mortality (OR, 0.1; 95% CI, 0.03 to 0.36), and overall mortality (OR, 0.22; 95% CI, 0.08 to 0.60) in the management of carbapenem-resistant bacteria. For infections with other bacteria, combined intraventricular plus intravenous therapy did not prove superior to standard intravenous therapy (44).
PHARMACOKINETICS OF INTRATHECAL THERAPY: GENERAL ASPECTS
Intraventricular versus Intralumbar Injection
The intrathecal application of anti-infectives represents direct access to the extracellular central nervous compartments, bypassing all barriers. High CSF levels can be reached with comparatively small doses, and the side effects are minimal to moderate when adequate formulations of drugs suitable for intrathecal application are used. Due to the net direction of the CSF bulk flow from the ventricles to the basal cisterns and then to the CSF space surrounding the cerebrum and the myelon, intraventricular injection of an anti-infective ensures its distribution throughout the CSF space (unless there is a complete block of the CSF circulation, e.g., in the cerebral aqueduct or by an obstruction of the spinal canal). In neonates with bacterial meningitis, injection of amikacin into one ventricle resulted in approximately equal drug levels in both ventricles 10 h after dosing. Therapeutic peak lumbar CSF concentrations 2 to 10 times lower than the simultaneous intraventricular levels were reached 2 to 4 h after intraventricular dosing (45). In infants, 2 to 48 h after intraventricular dosing of 5 mg gentamicin or tobramycin, concentrations in the ventricular and lumbar CSF were almost equal (46) (Fig. 2).
FIG 2.
Typical concentration-versus-time curves of gentamicin and tobramycin in ventricular, cisternal, and lumbar CSF after injection of 5 to 10 mg of these antibiotics into the lumbar (A) or 5 mg into the ventricular (B) CSF of infants with Gram-negative bacillary meningitis. (Adapted from reference 46 with permission of the Massachusetts Medical Society.) Note that after intralumbar dosing, therapeutic CSF concentrations are attained in cisternal but not in ventricular CSF. Maximum cisternal concentrations were reached 14 h after drug injection into the lumbar CSF. Conversely, intraventricular application led to high lumbar CSF concentrations, with a maximum 2 h after dosing, suggesting a rapid distribution of the injected antibacterial into the entire CSF space.
After drug injection into the lumbar CSF, the distribution in the CSF space is far less homogeneous: drug concentrations in ventricular CSF are highly variable and may not reach therapeutic levels (45, 46). After lumbar injection of 5 to 10 mg gentamicin or tobramycin in infants, high concentrations in lumbar CSF were reached for at least 6 h. Concentrations of both aminoglycosides in the cisternal CSF were always lower than those in the lumbar CSF and peaked at 14 h. Aminoglycoside concentrations in the ventricular CSF, however, ranged from 0 to 2.1 mg/liter (46). Although clinical studies did not prove a lower efficacy of the administration of antibiotics via lumbar drainage than with the ventricular route in patients with meningitis (26), pharmacokinetic data strongly suggest the intraventricular route. This, however, requires the implantation of an external ventriculostomy or of an Ommaya-Rickham reservoir.
After lumbar administration, the prone position for 60 min increased the ventricular concentrations of methotrexate (47). This approach has not been studied with anti-infectives and therefore at present cannot replace the recommended intraventricular route.
Volume of Distribution
Most pharmacokinetic studies on intraventricular therapy have been performed in patients with an external ventriculostomy. The volume of the CSF space in these patients was found to be variable (for details, see Anatomy and Pathophysiology, above), depending on the size of the ventricles, basal cisterns, and the subarachnoid space over the convexities and in the spinal canal (Fig. 1). Consequently, in neonates, the volume of distribution in the CSF space (VCSF) of amikacin was larger in patients with hydrocephalus or with a large abscess communicating with the ventricles than in those with a normal ventricle size (45). To complicate the conditions, there is often a blockage of the exchange between different parts of the CSF space due to the underlying pathology (e.g., intraventricular hemorrhages or tumors obstructing the natural flow of CSF at different levels) requiring the placement of external ventricular drainage (48). Moreover, as the apparent volume of distribution in the central compartment often does not represent the volume of blood plasma, the apparent VCSF after intraventricular injection does not depend only on the volume of the CSF space. VCSF also depends on the physicochemical properties of the compound studied. In the case of hydrophilic compounds, VCSF is equal to the volume of the CSF plus a fraction of the extracellular space of the brain that readily equilibrates with the CSF (16, 18, 45). Consequently, VCSF, even with large hydrophilic drugs, is often larger than the total CSF volume (Table 1). In rats, the CSF volume is approximately 90 μl (7, 49), and the VCSF of mannitol was estimated to be about 180 μl (50). Because lipophilic drugs in the CSF equilibrate more readily with the adjacent extra- and intracellular spaces and can bind to lipid membranes, the VCSF of lipophilic compounds in general is larger than the VCSF of hydrophilic compounds. In experimental rats, the VCSF of various fluoroquinolones was approximately 1.5 to 3 times larger than the VCSF of mannitol (50). For some drugs (e.g., teicoplanin and amphotericin B, both of which are moderately lipophilic), the CSF kinetics in patients were best described by a two-compartmental model accounting for the slow equilibration between the CSF and extracellular (and intracellular) spaces of the nervous tissue (51, 52).
TABLE 1.
Pharmacokinetics of selected anti-infectives after intraventricular dosinga
| Antibiotic | Age, sex, no. of patients | Dose (mg) | Concomitant intravenous therapy (same anti-infective) (no. of patients) | CmaxCSF (mg/liter) | CLCSF out total (ml/min) | VCSF (liters) | t1/2β CSF (h) | Reference |
|---|---|---|---|---|---|---|---|---|
| Aminoglycosides | ||||||||
| Gentamicin | 57 yr, F, 1 | 4 | + | 400–450 | 0.06b | 0.02b | 3.9b | 70 |
| 2–5 mo, 3 | 5 | + | ~45 | NR | NR | 6.2–6.4 | 46 | |
| Tobramycin | 6–12 mo, 3 | 5 | + | ~45 | NR | NR | 6.2–6.4 | 46 |
| Netilmicin | 1 mo–57 yr, M and F, 19 | 3 | + (17), Ø (2) | ∼100 | NR | NR | 2.5–3 | 62 |
| Amikacin | 0.25–2.5 mo, M and F, 8 | 5 | + (7), Ø (1) | >100 | NR | 0.03–0.41 | 9–18c | 45 |
| 66 yr, F, 1 | 30 | + | >200 | NR | NR | NR | 82 | |
| Colistin | 18–73 yr, M and F, 9 | 2.61–5.22 | + (5), Ø (4) | 6.2–22.1 | 0.55 ± 0.23 | 0.32 ± 0.05 | 7.8 ± 3.2 | 18 |
| Daptomycin | 64 yr, M, 1 | 5 | Ø | 74.8 | NR | NR | 2.6–2.8d | 48 |
| 62 yr, M, 1 | 10 | + | 483 | NR | NR | NR | 102 | |
| 5 | + | 139 | ||||||
| 52 yr, F, 1 | 10 | + | 6.3 | NR | NR | NR | 104 | |
| 23 mo, F, 1 | 2.5 | + | 24.4 | NR | NR | NR | 103 | |
| Glycopeptides | ||||||||
| Vancomycin | 82 yr, 1 | 50 | Ø | 233 | NR | 0.25 | 9.3–20.5 | 16 |
| 20 | Ø | 138–179 | ||||||
| 25 yr, 1 | 7.5 | Ø | 80.6 | 0.20e | 0.06 | 3.52 | 58 | |
| 21–81 yr, M and F, 25 | 10 | + | 256 ± 122 | NR | 0.04 | ∼2f | 60 | |
| 3 adults, age not reported | 10 | Ø | Mean, 293; max in 1 patient, 813 | NR | NR | ∼5g | 59 | |
| Newborns, 13 | 5–20 | + (8), Ø (5) | For 20 mg, 125 ± 30; for 10 mg, 95 and 131; for 5 mg, 39 ± 23 | NR | NR | NR | 110 | |
| Teicoplanin | 6 yr, F, 1 | 10 | + | ∼75 | 0.05 + 0.2 via EVD | 0.07 (2nd compartment, 0.11) | t1/2α, 4.6; t1/2β, 26.6 | 52 |
| Quinupristin-dalfopristin | 44 yr, M, 1 | 0.6/1.4 | + | 27.2/22.0 | 0.89h /0.27h | 0.03h /0.02h | 1.2h /0.25h | 116 |
| Tigecycline | 67 yr, M, 1 | 1 | + | 17.4–26.4 | 0.55 | 0.17 | 3.9 | 127 |
| 2 | + | 44.8 | ||||||
| 38 yr, M, 1 | 5 | Ø | 179–310 | NR | NR | NR | 129 | |
| 67 yr, M, 1 | 1 | + | 33.3 | 0.23i | 0.08i | 4.1i | 123 | |
| 5 | 327 | 0.11i | 0.03i | 3.5i | ||||
| 10 | 243 | 0.29i | 0.04i | 1.5i | ||||
| Antifungal | ||||||||
| Amphotericin B | 1 adult, age not reported | 0.1 | + | 0.4 | NR | 0.14j (2nd compartment, 0.68) | 2.96 | 51 |
| 0.3 | 0.8 | |||||||
Extrapolation of pharmacokinetic parameters in CSF not reported in the original publication was performed by noncompartmental methods. The area under the concentration-versus-time curve in CSF (AUCCSF) was calculated by the linear trapezoidal rule. Clearance out of the CSF space (CLCSF out total) was estimated by intraventricular dose/AUCCSF, the elimination rate constant (kel CSF) was estimated by log-linear regression of CSF concentrations measured, and the elimination half-life in CSF (t1/2β CSF) was estimated as ln2/kel CSF. The volume of distribution in the CSF space (VCSF) was estimated by intraventricular dose/AUCCSF · kel CSF. CmaxCSF, maximum concentration of drug in CSF; F, female; M, male; EVD, external ventricular drainage; NR, not reported; Ø, no concomitant intravenous therapy with the same anti-infective.
Extrapolated from data in Fig 2 of reference 70. Because of the very high CSF concentrations measured, the estimates of VCSF and CLCSF are low.
Extrapolated from data in Fig. 2 of reference 45. Note the large variation of VCSF: “large volumes correlated with the presence of hydrocephalus…or a communicating occipital abscess” (45).
Estimated from data presented in Table 2 of reference 48.
Calculated from data presented in the text and in Fig. 1 of reference 58.
Extrapolated from data in Fig. 2 of reference 60.
Estimated from data in Fig. 1 of reference 59.
Estimated from data presented in Table 1 of reference 116.
Estimated from data presented for the first dose in Tables 1 to 3 of reference 123.
A 2nd compartment equilibrating with the CSF space was noted. At steady state, “this accumulated reservoir of drug served to maintain the CSF amphotericin B concentrations above the MIC for C. neoformans for at least 26 h” (51).
Elimination: Hydrophilic versus Lipophilic Compounds
The total clearance from CSF to blood (CLCSF out total) consists of clearance by CSF bulk flow (CLCSF out bulk) plus clearance by retrograde diffusion across the blood-CSF and blood-brain barriers (CLCSF out diff) plus, in the presence of an outward transport system, clearance by active transport (CLCSF out active) (7, 53). Large hydrophilic molecules are mainly eliminated by CSF bulk flow. After intraventricular dosing, their elimination is slower in hydrocephalic patients than in patients with a normal ventricle size (45). Small and/or lipophilic molecules and/or molecules with a high affinity for efflux pumps are eliminated by bulk flow, by retrograde diffusion across the blood-brain and blood-CSF barriers, and/or by active transport. In experimental rats, the CLCSF out total of different fluoroquinolones was 3- to 8-fold higher than the sum of CLCSF out bulk plus CLCSF out active. Moreover, CLCSF out total did not depend on the dose injected and was inhibited neither by the coadministration of another fluoroquinolone nor by probenecid (50). This indicates that active transport appears to be negligible for most anti-infective drugs and that passive elimination by retrograde diffusion across the blood-CSF and blood-brain barriers (CLCSF out diff) in the case of small, moderately lipophilic molecules represents the major contribution to CLCSF out total (50).
Pharmacokinetic principles derived from anti-infectives are supported by data gathered after intraventricular administration of cytostatic drugs in oncology patients and rhesus monkeys. After intraventricular injection, the hydrophilic compounds methotrexate (molecular weight [MW], 454 g/mol; log octanol-water partition coefficient [log P], −1.85) and cytarabine (MW, 243 g/mol; log P, −2.8) behaved similarly to vancomycin and colistin. Conversely, for the moderately lipophilic compound thiotepa (triethylenethiophosphoramide) (MW, 189 g/mol; log P, 0.53) in monkey CLCSF out total exceeded the CSF bulk flow by about 10-fold (CLCSF out thiotepa, 0.36 ml/min [54]; CSF secretion, 0.035 ml/min [7]) and, in one patient, by about 5-fold (CLCSF out thiotepa, 1.8 ml/min [54]; CSF secretion, 0.36 ml/min [7]). This indicates that after intraventricular administration, the lipophilic compound thiotepa rapidly disappeared from the CSF, mainly because of diffusion across the blood-brain and blood-CSF barriers, leading to short elimination half-lives of approximately 30 min in ventricular CSF and lumbar CSF in monkeys and one patient with refractory meningeal leukemia (data extrapolated from Fig. 2 and 4 of reference 54). After intraventricular application, the peak concentrations of thiotepa in lumbar CSF were only about 1/10 of the ventricular concentrations, suggesting ineffective concentrations in parts of the CSF compartment after intraventricular administration. Thepa (triethylenephosphoramide), the active metabolite of thiotepa, did not appear in CSF after intraventricular injection of thiotepa. Conversely, after intravenous infusion of thiotepa, the concentration-versus-time curves of tepa (MW, 173 g/mol; log P, −0.62) ran almost in parallel in plasma and CSF, ensuring effective concentrations in ventricular and lumbar CSF (9, 55).
In healthy humans, the CSF production rate (i.e., the CSF bulk flow) is not constant. In six normal volunteers, a circadian variation was observed, with a minimum production of 30% of maximum values (12 ± 7 ml/h) at about 6:00 p.m. and nightly peak production of 42 ± 2 ml/h at about 2:00 a.m. (56). Inter- and intraindividual variations were also observed in patients with external ventriculostomies. In 12 of these patients, aged 30 to 69 years, the mean CSF flow out of the drain (± standard deviation [SD]) was 7.5 ± 3.4 ml/h (range, 1.6 to 12.1 ml/h) (57).
After intraventricular injection, the elimination half-life (t1/2β CSF) of large hydrophilic molecules in humans is similar: the t1/2β CSF of vancomycin (molecular mass, 1,486 g/mol) ranges from approximately 2 to 20.5 h (16, 58–60). In mice, which have a higher CSF turnover rate than humans (mouse, approximately 55%/h; humans, approximately 23%/h) (7), the t1/2β CSF of vancomycin after intraventricular injection was shorter (0.72 h) (61). The t1/2β CSF of colistin (molecular mass, 1,155 g/mol) after intraventricular administration in humans (7.8 ± 3.2 h) (18) was similar to that of vancomycin. Gentamicin, which is also hydrophilic but has a slightly lower molecular mass (478 g/mol), had a CSF elimination half-life of 6.2 to 6.4 h (46). This underlines that for hydrophilic compounds above a molecular mass of approximately 400 g/mol, bulk flow is the principal component of CLCSF out total. The high variation of t1/2β CSF in the above-mentioned studies originates from several variables: (i) inter- and intraindividual variations of the CSF volume, (ii) inter- and intraindividual variations of the CSF flow via natural pathways, and (iii) the rate of CSF drained by external or internal ventriculostomy (18, 60).
Therapeutic Drug Monitoring
With the usual recommended doses (Table 2), volumes of distribution, and clearance rates (Table 1), high peak CSF concentrations are reached, and trough concentrations after 24 h generally exceed the MIC of fully or moderately susceptible organisms (15, 16, 18, 59). Low doses in conjunction with once-daily dosing, however, increase the risk of subtherapeutic antimicrobial CSF concentrations and should be avoided (e.g., netilmicin at 2 mg in adults) (62). Since CSF sampling frequency appears to be a risk factor for ventriculostomy-related infections, sampling of CSF several times daily on a routine basis is not advisable. Therapeutic drug monitoring of the trough levels immediately before the next intraventricular dose in conjunction with quantitative assessment of the MIC of the pathogen responsible by microdilution or Etest provides valuable data on the relationship between the trough concentration and MIC but at present is not mandatory. In a recent retrospective multicenter study in 105 patients, antimicrobial CSF concentrations were measured in 63% of surviving and 37% of nonsurviving patients (P = 0.02) (42). We are not aware of any other study demonstrating that therapeutic drug monitoring after intraventricular dosing improved outcomes. When therapy has failed or when a patient presents with a high volume of CSF drained through a ventriculostomy catheter, measurement of CSF trough concentrations is helpful to document effective antibiotic concentrations at the end of the dosing interval, i.e., usually 24 h after dosing, or to adjust the dose or dosing intervals. We advise against the continuous intraventricular infusion of antibiotics since it impedes the measurement of intracranial pressure, which frequently is performed via external ventriculostomy, and may be an additional risk factor for catheter-related infections.
TABLE 2.
Intraventricular application of antibiotics to reach effective concentrations within the CNSa
| Antibiotic | Dose(s) in adultsb | Reported side effect(s) | Reference(s) |
|---|---|---|---|
| Aminoglycosides | |||
| Gentamicin | 4–10 mg (1–20 mg) every 24 h | Rare reports of (temporary) hearing loss, epileptic seizures, aseptic meningitis, and CSF eosinophilia; painful radiculitis | 4, 46, 68, 70 |
| Tobramycin | 5–10 mg (5–50 mg) every 24 h | Similar to those of gentamicin | 4, 46, 68 |
| Netilmicin | 7.5–15 mg every 24 h (3–150 mg every 12–24 h) | 62, 79 | |
| Amikacin | 30 mg every 24 h (5–100 mg every 24–48 h) | Similar to those of gentamicin, transient vomiting | 4, 80, 81, 83 |
| Streptomycin | 1 mg every 12–48 h | (Temporary) hearing loss, epileptic seizures, radiculitis, transverse myelitis, arachnoiditis, paraplegia | 88 |
| Polymyxins | |||
| Colistin (polymyxin E) methanesulfonate (12,500 IU = 1 mg) | 10 mg (1.6–40 mg) every 24 h | Meningeal inflammation; with high doses, epileptic seizures, loss of appetite, agitation, eosinophilia, edema, pain, albuminuria, intraventricular hemorrhage | 18, 90, 96, 97 |
| Polymyxin B | 5 mg every 24 h | Similar to those of colistin | 90, 96 |
| Daptomycin | 5–10 mg every 24 h (2.5–10 mg every 12–72 h) | Fever | 48, 100 |
| Glycopeptides | |||
| Vancomycin | 10–20 mg (5–50 mg) every 24 h | Increased CSF leukocyte count, headache, nausea, red man syndrome, possible (temporary) hearing loss and ataxia | 4, 15–17, 59, 60, 108, 109 |
| Teicoplanin | 5–20 mg every 24 h | Headache, rash, transient rise in CSF leukocyte count | 52, 114 |
| Quinupristin-dalfopristin | 1–5 mg every 24 h | Mental obtundation, hydrocephalus, cerebral infarctions | 116–120 |
| Tigecycline | 1–10 mg every 24 h, 2–4 mg every 12 h | Well tolerated | 124–127 |
| Antifungals | |||
| AmB | 0.1–0.5 mg every 24 h (every 24–48 h) | Tinnitus, fever, shivering + fever, nausea, vomiting, photophobia, diplopia, encephalopathy, Parkinson syndrome, arachnoiditis | 51, 132, 133, 136, 137 |
| Liposomal AmB, 1 mg every 24 h | In a 4-yr-old boy with Candida ventriculitis, liposomal AmB was administered intraventricularly without severe side effects | 132 | |
| Caspofungin | 5–10 mg (1–10 mg) every 24 h | Nausea, headache | 72, 138, 139 |
Since the entry of a drug into the ventricles after intralumbar administration is often poor, it appears logical to increase the lumbar dose. However, toxicity, in particular of aminoglycosides and colistin, after intralumbar application appears higher than after intraventricular administration, probably as a consequence of the long presence of high antibiotic concentrations in the spinal canal. For this reason, an increase in the dose for administration into the lumbar CSF is not recommended. AmB, amphotericin B.
The doses and dosing intervals with the greatest clinical experience are given in boldface type. Values in parentheses indicate less-common doses and dosing intervals.
Simultaneous Intrathecal and Intravenous Therapy
Since antibiotics administered intrathecally do not fully equilibrate with the extracellular space of the brain, are drained into the bloodstream, and there reach low concentrations, simultaneous intraventricular and intravenous administration probably helps to prevent compartments with subinhibitory antibiotic concentrations, thus reducing the probability of the selection of resistant bacteria and relapse (33). Combined intrathecal plus intravenous treatment likely achieves slightly higher antibiotic levels in CSF than intraventricular therapy alone. This may be crucial in controlling multidrug-resistant CNS infections (63) but complicates the pharmacokinetic analysis of intrathecal drug therapy. Numerous studies reporting the successful intrathecal administration of antibiotics used concomitant intravenous therapy (18, 42, 60, 63–65). For these reasons, concomitant systemic antibiotic therapy with either the same anti-infective or another compound active against the infective agent is strongly recommended. By using a three-compartmental model (central, peripheral, and CSF compartments) to describe the kinetics of vancomycin after intravenous (990 mg) and intraventricular (10 mg) infusions, it was concluded that (i) the impact of the limited amount of vancomycin from CSF on the pharmacokinetics in the central compartment was negligible, (ii) the main source of drug presence in the CSF was intraventricular injection, and (iii) the effect of the state of the blood-CSF barrier and indicators of inflammation on the vancomycin CSF concentrations in the setting of intraventricular plus intravenous therapy was small (60, 64). When analyzing CSF concentrations after intrathecal administration, most authors were of the opinion that the influence of the plasma concentrations on the kinetics in CSF can be ignored. This is a simplification that we must live with since studies in humans treated with intraventricular anti-infectives alone are scarce.
GENERAL RECOMMENDATIONS FOR INTRATHECAL THERAPY
Indications for Intrathecal Administration of Anti-infectives and Duration of Treatment
According to Infectious Diseases Society of America (IDSA) practice guidelines (66), intrathecal administration of anti-infectives “should be considered for patients with healthcare-associated ventriculitis and meningitis in which the infection responds poorly to systemic antimicrobial therapy alone.” The decision to start intrathecal antimicrobial therapy depends on the results of quantitative susceptibility testing and the lack of anti-infectives that reach the required CSF concentrations for a rapid cidal effect (ideally 10× the MIC) with low toxicity to the host (53).
Although infections complicating external ventriculostomy are frequent, the present evidence does not support the prophylactic intrathecal administration of antibiotics or routine change of catheters after defined intervals (67). Intravenous periprocedural antimicrobial prophylaxis, impregnated catheters, subcutaneous tunneling of external catheters, and standardized protocols with strict adherence to sterile techniques are recommended for CSF shunt insertion or the placement of external ventricular drains (66, 67).
The duration of treatment is highly individualized depending on the clinical conditions (e.g., infected CSF shunt or another intracranial device versus no foreign body) and the pathogen (e.g., long treatment is necessary for Scedosporium spp., and short treatment is often sufficient after the removal of a foreign body infected with coagulase-negative staphylococci). In uncomplicated cases, intrathecal anti-infective therapy is stopped 48 to 72 h after the CSF culture has become sterile (4). Conversely, in patients with repeated positive CSF cultures despite adequate anti-infective therapy, IDSA guidelines recommend continuing therapy for 10 to 14 days after the last positive CSF culture (66).
Dosing of Anti-infectives
Since randomized studies are not available to guide the majority of decisions, recommendations are generally based on expert consensus (66, 68). Based on pharmacokinetic data, intraventricular dosing should be preferred over injections into the lumbar CSF whenever possible (45, 46). The very detailed and pragmatic IDSA guidelines (66) suggest that (i) an intraventricular drain “should be clamped for 15–60 min to allow the agent to equilibrate throughout the CSF” and (ii) “dosages and intervals…should be adjusted based on CSF antimicrobial concentrations to 10–20 times the MIC…, ventricular size…and daily output from the ventricular drain.”
A recent comment noted that CSF “pharmacodynamics may not fit into the 10–20 times the MIC posited by the authors with some pathogens, and toxicity risk should be considered when CSF concentrations suggest clinicians give higher doses than previously studied…The proposed dose adjustment strategies are lower grade recommendations than many of the other important recommendations in the guideline” (69).
An in-depth review of intraventricular therapy with aminoglycosides (68) points out that (i) preservative-free solutions should be used for intrathecal therapy, (ii) intraventricular antibiotics should be used with intravenous antimicrobial agents to treat the suspected pathogens, and (iii) routine therapeutic drug monitoring of intraventricular antibiotic therapy does not appear warranted, “as it is unclear when concentrations should be sampled and what concentrations should be targeted.” Therapeutic drug monitoring “may be considered in selected cases,” e.g., when “CSF cultures remain persistently positive.”
At present, it is unclear in which volume anti-infectives should be dissolved, whether they should be diluted to a certain concentration, whether they should be injected by the push-pull method or by slow injection over several minutes, and whether or not the intrathecal catheter should be rinsed with 0.9% saline after the injection of the anti-infective (46, 68, 70). An approach accounting for the dead space of the proximal ventricular catheter is the extraction of 5 ml CSF followed by the administration of the anti-infective in a 5-ml solution through the most proximal tap of the ventriculostomy tubing (71). We do not advise rinsing the catheter with saline because this may increase the risk of infection. Based on pharmacokinetic data, we suggest that in general, the full dose of hydrophilic anti-infectives should be injected once daily. To slow elimination and increase t1/2β CSF, the external ventricular or lumbar catheter should be clamped after the administration of the anti-infective. The intracranial pressure and the tolerance of clamping by the patient determine the duration of clamping. Clamping intervals of up to 6 h have been reported (72).
Pharmaceutical Prerequisites for Intraventricular and Intrathecal Injection of Drugs
Drugs suitable for injections into the intraventricular or intralumbar CSF must meet the following pharmacopeial requirements: they must be sterile, pyrogen free, endotoxin free, and essentially free of particles of foreign matter and must not contain other contaminants. The drug must be dissolved in water for injection or a sterile sodium chloride solution (concentration, ≤0.9%). The solution must not contain any added coloring agents (U.S. Pharmacopeia 2011) (73). The European Pharmacopoeia (Ph. Eur., 10th ed., 2019) (74) additionally states that solutions for intrathecal injection must be free of antimicrobial preservatives and must be filled in single-dose containers. In the United States and the European Union, at present, only colistin methanesulfonate is licensed for intrathecal application. Compounds present in low quantities as inactive ingredients in nonantibiotic drug preparations licensed for intraventricular or intrathecal use in the European Union are trometamol, Na-Ca-EDTA, HCl, NaCl, NaOH, Na lactate, and glucose.
Adjunctive Treatments
In 50 consecutive children treated for CSF infection with hydrocephalus, 23 patients received neuroendoscopic lavage for the removal of intraventricular debris, whereas 27 patients were treated with antibiotics alone. Within 24 months after shunt implantation, the incidence of shunt revision was higher in conventionally treated than in lavage patients (P < 0.001), and reinfection was observed more frequently in conventionally treated children (P < 0.001) (75). After the placement of two external ventriculostomies in the 1st and 2nd ventricles, intraventricular lavage by extensive irrigation with a colistin solution was carried out until the output was free of pus. Intraventricular lavage was performed after adding 10 mg colistin per 500 ml normal saline (3, 71).
In the presence of an intraventricular blood clot, which may serve as a biological surface for bacteria to form a biofilm and may cause treatment failure, intraventricular fibrinolysis as a therapy of last resort may be necessary to dissolve the clot and to optimize the action of antibiotics (65).
PHARMACOKINETICS OF INDIVIDUAL COMPOUNDS
When pharmacokinetic data were presented in the original publications, we report these results in the text or in Table 1. Extrapolation of pharmacokinetic parameters in CSF from original data was performed by using noncompartmental methods (53). The area under the concentration-versus-time curve in CSF (AUCCSF) was calculated by the linear trapezoidal rule. Clearance out of the CSF space (CLCSF out total) was estimated by intrathecal dose/AUCCSF. The elimination rate constant (kel CSF) was estimated by log-linear regression of CSF concentrations measured, and the elimination half-life in CSF (t1/2β CSF) was determined as ln2/kel CSF. The volume of distribution in the CSF space (VCSF) was estimated by intraventricular dose/AUCCSF · kel CSF.
Aminoglycosides
As a consequence of their narrow therapeutic window in blood and their poor penetration into the CSF, aminoglycosides have been used for several decades for intrathecal (both intralumbar and intraventricular) therapy. Aminoglycosides are hydrophilic compounds with a molecular weight of approximately 500 g/mol (gentamicin, MW of 478 g/mol and log P of −3.1). Gentamicin, tobramycin, netilmicin, and amikacin are suitable for intrathecal therapy. Because of a diverse genetic background, resistance to one aminoglycoside does not imply reduced susceptibility to all aminoglycosides. The lowest resistance rates were observed for amikacin (76). The literature until 2016 has been extensively reviewed (68). Adverse effects reported in single cases include (temporary) hearing loss, epileptic seizures, aseptic meningitis, painful radiculitis, and CSF eosinophilia (4, 46, 70). Adverse effects did not appear to correlate with CSF aminoglycoside concentrations (70). Painful radiculitis appeared to occur after intralumbar but not after intraventricular therapy (46). In the 18 studies reviewed by LeBras and coworkers, no serious toxicities were noted. Adverse effects, however, were not reported in all studies (68).
Gentamicin.
The usual dose of gentamicin administered once daily ranges from 4 to 10 mg (1 to 20 mg) (68, 77). Gentamicin trough concentrations of up to 20 mg/liter 24 h after dosing were well tolerated (70). In infants, after intraventricular injection of 5 mg, peak ventricular CSF concentrations of approximately 45 mg/liter and peak lumbar CSF concentrations 2 h after dosing of approximately 20 mg/liter were attained. VCSF was not reported, and the t1/2β CSF was 6.2 to 6.4 h (46). After injection of 5 to 10 mg by lumbar puncture, the t1/2β CSF in lumbar CSF was 6 h (46). In another study in adults, after injection of a relatively low dose of 4 mg (3 times) or 12 mg (1 time) of gentamicin, the t1/2β CSF ranged from 3.8 to 8.0 h (median, 5.7 h). t1/2β CSF did not depend on the dose administered (78).
Tobramycin.
As with gentamicin, after intraventricular injection of 5 mg of tobramycin in infants, peak ventricular CSF concentrations were approximately 45 mg/liter, and peak lumbar CSF concentrations 2 h after dosing of approximately 20 mg/liter were attained. VCSF was not reported, and the t1/2β CSF was 6.2 to 6.4 h (46). After injection of 5 to 10 mg into the lumbar CSF, cisternal maximum concentrations were approximately 15 mg/liter, peaking 14 h after dosing. The t1/2β CSF in lumbar CSF was 6 h (46).
Netilmicin.
Doses of netilmicin of up to 150 mg twice daily were given intrathecally, without reported side effects, in a 75-year-old female patient with multiresistant A. baumannii ventriculitis. Netilmicin at this dose sterilized CSF rapidly. However, on the 10th day of intrathecal therapy, the patient’s status deteriorated as a consequence of subsequent Enterococcus faecium ventriculitis; she developed a fever and died soon thereafter (79). After a single intraventricular injection of 3 mg, bactericidal concentrations lasted only 8 h. For this reason, a total daily dose of 9 mg divided into 3 doses (3 doses of 3 mg) was recommended in adults by the authors (62). In adults, doses of up to 15 mg/day (1 dose) or 10 mg/day (2 doses) were administered without severe side effects (62).
Amikacin.
In neonates, after intraventricular injection of 5 mg amikacin, the VCSF was estimated to be 0.027 to 0.41 liters depending on the size of the ventricles and on an abscess cavity in contact with the CSF (45). Amikacin at doses of 50 to 100 mg/day was successfully administered intrathecally in 3 cases of postoperative refractory meningitis caused by gentamicin-resistant bacteria (K. pneumoniae and Staphylococcus epidermidis). Observed adverse effects were high-tone hearing impairment and transient vomiting (80). In a 66-year-old woman and a 32-year-old man, a daily dose of 30 mg was administered without severe side effects (81, 82). Amikacin concentrations in CSF were not reported in the latter two patients. In 3 children (11 to 90 months old), doses of 10 to 15 mg were given once daily. CSF amikacin concentrations were also not reported (83).
Streptomycin.
In the absence of therapeutic alternatives, high-dose streptomycin was given intrathecally to treat tuberculous meningitis (15 to 50 mg daily to twice weekly) (84). Another typical scheme was 0.02 g per lb body weight/24 h but not exceeding 1 g daily until the disappearance of fever and then every 72 h (85). Because of severe side effects, e.g., deafness and epileptic seizures, intrathecal doses were reduced in the next decades. At present, neither parenteral nor intrathecal streptomycin is part of the standard therapy of drug-sensitive CNS tuberculosis (86). Even when the mycobacteria are resistant to isoniazid and rifampin, standard therapy does not comprise intrathecal streptomycin (87). A 64-year-old man was treated for vancomycin-resistant Enterococcus faecalis ventriculitis with 1 to 2 mg streptomycin intrathecally every 12 h plus 3 to 9 mg every 12 h through a drainage catheter placed in a purulent fluid-containing cavity in the right frontotemporal region without signs of hearing loss (88). No pharmacokinetic data are available on streptomycin after intrathecal injection.
Colistin and Polymyxin B
Colistin (polymyxin E) is a cationic hydrophilic antimicrobial peptide (MW, 1,155 g/mol; log P, −2.4) introduced into clinical medicine in 1959. Because of the relatively high level of toxicity associated with the parenteral administration of colistin sulfate, a less toxic inactive sulfomethyl prodrug, colistin methanesulfonate (molecular mass, 1,750 g/mol), was developed (89, 90). Colistin methanesulfonate can be administered intravenously and intraventricularly. The prodrug is converted into colistin by hydrolysis both in vitro and in vivo. Colistin represents the cornerstone of treatment of CNS infections with carbapenemase-producing bacteria (1).
Following intravenous infusion of colistin methanesulfonate, the pharmacokinetics of formed colistin is determined by its elimination rather than by its formation: formed colistin has a substantially longer terminal half-life in plasma than colistin methanesulfonate. Colistin is almost exclusively nonrenally cleared, whereas colistin methanesulfonate is predominantly renally cleared (approximately 60% of the dose) (89). In vitro, at high concentrations, colistin methanesulfonate (and colistin) form self-assembling colloids of approximately 2 nm. Rapid conversion of colistin methanesulfonate to colistin occurred below the critical micelle concentration (3.5 mM = 4,380 mg/liter for colistin methanesulfonate), whereas conversion above this concentration was less than 1% (91).
The penetration of colistin methanesulfonate and colistin into the CSF is poor. At steady state in the absence of meningeal inflammation, CSF colistin concentrations reach 5 to 7% of the corresponding plasma levels (63, 92). After intravenous dosing, mean AUCCSF/AUCserum ratios were found to be about 60% higher in patients with ventriculitis than in control patients (0.110 versus 0.070) (63). In human CSF in vitro, the conversion of colistin methanesulfonate to colistin depended on the concentration. After 16 h at 10 mg/liter, conversion was 100%; at 20 mg/liter, it was 96.2%; at 50 mg/liter, it was 88.1%; at 100 mg/liter, it was 62.8%; and at 200 mg/liter, it was 35.9% (18). In vivo, as a sign of the inhibition of conversion to colistin by high colistin methanesulfonate concentrations, the AUCCSF of colistin did not increase substantially when the daily dose of colistin methanesulfonate was increased from 5.22 to 10.44 mg. This implies that further increasing the daily dose of colistin methanesulfonate may not lead to higher CSF concentrations of active colistin (18). Nevertheless, the application of an intraventricular loading dose of 500,000 IU (40 mg) of colistin methanesulfonate followed by a dose of 125,000 to 250,000 IU (10 to 20 mg) every 24 to 48 h plus parenteral colistin has been advocated. With this regime, all 6 patients studied were cured, but 1 patient presented with chemical meningitis, and 1 presented with chemical ventriculitis (93). Since the in vivo conversion of colistin methanesulfonate to colistin in individual patients was not known, in their seminal study on colistin pharmacokinetics after intraventricular dosing of colistin methanesulfonate, Imberti et al. (18) divided the pharmacokinetic parameters measured by Fm, the unknown fraction of colistin methanesulfonate converted to colistin. In vivo, colistin CSF concentrations were maximal after 2.4 to 5.5 h (mean ± SD, 3.7 ± 0.9 h). VCSF/Fm was slightly higher than the usual CSF volume, suggesting some drug exchange with the extracellular fluid of the brain. When colistin methanesulfonate doses of ≥5.22 mg/day were administered, CSF trough levels of colistin were continuously above 2 mg/liter, the usual breakpoint for Gram-negative bacteria. The terminal half-life of the formed colistin ranged from 4.1 to 12.9 h, ensuring trough concentrations above the MIC of susceptible pathogens after once-daily dosing. Approximately one-half of the interindividual variation of the colistin clearance out of the CSF was explained by the amount of CSF drained via external ventriculostomy during the period of pharmacokinetic sampling (R2 = 0.45) (18). In another recent study, a single dose of 10 mg/day intraventricularly together with systemic colistin treatment ensured trough concentrations determined immediately before the next dose of >5 mg/liter (65).
The use of intraventricular polymyxin B, a close relative of polymyxin E (difference of one amino acid only), is less common than the use of intrathecal colistin. The antimicrobial spectra of both compounds are similar. Polymyxin B at 50,000 U/day administered intraventricularly plus 2 doses of 450,000 U/day intravenously has also been used to treat nosocomial meningitis caused by multiresistant Gram-negative bacteria (94). In Acinetobacter baumannii infections, the intrathecal plus intravenous group achieved a higher microbiological clearance rate (91.30% [21/23] versus 18.42% [7/38]; P < 0.01) and reduced CSF inflammatory parameters more efficiently than the group administered intravenous polymyxin B alone. Compared with the intravenous group, the intrathecal/intracerebral group had a low 28-day mortality rate (8.7% versus 55.3%; P = 0.01). In this study, polymyxin B was not nephrotoxic and appeared not to have other adverse effects (94). We are not aware of pharmacokinetic data after intrathecal administration of polymyxin B.
In experimental mice, polymyxins possess substantial proinflammatory and neurotoxic properties (95). While Imberti et al. (18) and Pan et al. (94) did not observe symptoms of CNS toxicity, toxicity related to the intrathecal administration of polymyxins was reported by Falagas and coworkers in 17 of 60 patients, most commonly meningeal irritation (12 cases) (96). Adverse effects were dose dependent. Discontinuation of treatment was necessary for 4 patients, and dose reduction was necessary for another 4 patients. No irreversible toxicity was noted (96). Recently, however, an event of intracranial hemorrhage in a hypertensive patient was considered to be related to the intraventricular administration of colistin (97).
Daptomycin
The entry of daptomycin (MW, 1,621 g/mol; XlogP3-AA [estimation of log P by an atom-additive method adding up contributions of each atom in the molecule {140}], −5.1; plasma protein binding, 90 to 95%) from blood to CSF is poor in both the absence and presence of meningeal inflammation (98). In patients with suspected health care-associated ventriculitis and meningitis, the mean AUCCSF/AUCserum ratios were 0.008 (mean AUCCSF/AUCserum fraction unbound = 0.115) and 0.0045 (98, 99). Several case reports in adults with multiresistant E. faecium and S. epidermidis ventriculitis described the safe and effective use of intraventricular daptomycin at doses of 5 to 10 mg once daily (48, 100). In a 2.5-month-old boy, daptomycin at 2.5 mg/day administered intraventricularly together with intravenous daptomycin and linezolid cured E. faecium ventriculitis without side effects. Concentrations of daptomycin were not measured (101). When daptomycin was injected into the right ventricle of a hydrocephalic 64-year-old male patient, daptomycin concentrations measured in the CSF from the right and left ventricles were discordant. The peak and trough daptomycin concentrations 1 and 18 h after the first dose were 112.2 and 1.34 mg/liter in CSF from the right external ventriculostomy and 37.4 and 0.37 mg/liter in CSF from the left ventriculostomy. Daptomycin accumulation was evident after 3 days of therapy (trough concentrations 16.5 h after dosing of 11.4 and 0.51 mg/liter) (48). The t1/2β CSF estimated from CSF concentrations measured in CSF from the right external ventriculostomy was 2.8 h, and that from the left ventriculostomy was 2.6 h (Table 2). Despite the relatively short t1/2β CSF in all case reports that reported CSF trough concentrations, these values were above the MICs of sensitive pathogens (48, 102–104).
Glycopeptides
Vancomycin.
Together with the aminoglycosides and colistin, vancomycin is the antibiotic used most frequently for intrathecal injections. Because of its hydrophilicity and high molecular mass (1,449 g/mol; log P, −3.1), it tends to penetrate the blood-CSF and blood-brain barriers poorly, particularly when the barrier function is only mildly to moderately impaired and in cases of concomitant dexamethasone administration (105–108). The literature until 2014 has been reviewed extensively (108). Very few side effects of intrathecal vancomycin have been reported, and no contraindications with the exception of hypersensitivity have been noted (64, 108). Indeed, vancomycin CSF concentrations of up to 812.6 mg/liter were documented without apparent adverse effects (17). The reported VCSF ranged from 39 to 250 ml. The t1/2β CSF differed in the individual studies and varied from 2 to 20.5 h. Intraventricular doses of 10 to 20 mg (depending on the ventricle size) of vancomycin every 24 h ensure concentrations above the MIC of susceptible pathogens for the whole dosing interval. CSF concentrations were influenced by the amount of CSF drained by external ventriculostomy, whereas no difference in CSF vancomycin concentrations was noted between patients who received concomitant intravenous vancomycin and those who did not (60, 64). Doses of 10 to 20 mg were well tolerated (17, 59, 109, 110). In neonates, therapeutic CSF concentrations over 24 h were attained with 5 mg vancomycin injected once daily (110).
Teicoplanin.
Teicoplanin is a large, moderately lipophilic, highly protein-bound drug (MW, 1,880 g/mol; XlogP3-AA, 0.5; plasma protein binding, approximately 95%) that enters the CSF poorly after intravenous administration. The intraventricular use of teicoplanin is uncommon because the antibiotic spectra of teicoplanin and vancomycin are similar. In enterococci, however, there are several different types of glycopeptide resistance; e.g., VanA-expressing vancomycin-resistant enterococci are resistant to both vancomycin and teicoplanin, whereas VanB-expressing enterococci are resistant to vancomycin but susceptible to teicoplanin (111). Unlike vancomycin, at high intraventricular doses (0.5 mg), teicoplanin in experimental mice possessed proconvulsive properties (112). It therefore should only be administered intrathecally for the therapy of rare infections with vancomycin-resistant, teicoplanin-susceptible bacteria (113). Depending on the age and ventricular volume, the applied doses were 5 to 20 mg daily. In a 6-year-old girl, once-daily administration of 10 mg teicoplanin intraventricularly produced peak levels of approximately 75 mg/liter and maintained CSF trough levels of 2.5 to 4.5 mg/liter, i.e., above the MIC of susceptible bacteria (52). In other studies using intraventricular teicoplanin at doses of 5 to 20 mg/day, CSF trough levels after 24 h were also above 2 mg/liter (114, 115).
Quinupristin-Dalfopristin
Quinupristin-dalfopristin (molecular mass, 1,713 g/mol; water-soluble derivatives of pristinamycin IA and pristinamycin IIB) is a combination of two streptogramins in a 30:70 ratio administered intravenously. The combination is metabolized nonenzymatically to 3 (quinupristin, 2; dalfopristin, 1) antimicrobially active metabolites (116). It poorly penetrates the CSF. A 44-year-old man with vancomycin-resistant E. faecium ventriculitis successfully received 1 mg, 2 mg, and 4 mg quinupristin-dalfopristin intraventricularly once daily (116). Immediately after dosing, therapeutic CSF concentrations were measured. Estimation of the t1/2β CSF by log-linear regression of the measurements after injection of 2 mg (equal to 0.6 mg quinupristin and 1.4 mg dalfopristin) (Table 1) yielded 1.2 h for quinupristin and 0.25 h for dalfopristin.
In several cases, quinupristin-dalfopristin was well tolerated (116–118). However, one 67-year-old man receiving 5 mg every 24 h deteriorated after three dosages (119). Another 23-year-old male patient developed hydrocephalus and anterior and posterior cerebral artery infarctions after 2 and 7 days of intraventricular (2 mg/24 h) plus systemic (7.5 mg/kg three times a day) therapy (120). In both cases, bacteria were cleared from the CSF. Therefore, intrathecal therapy with these compounds at the doses administered appears effective but may be associated with a high frequency of adverse effects.
Tigecycline
Tigecycline exhibits nonlinear binding to plasma proteins (with the unbound fraction decreasing with increasing tigecycline concentrations [29% at 0.1 mg/liter versus 11% at 1.0 mg/liter]) and has a large volume of distribution of approximately 7 to 10 liters/kg (121). After intravenous administration and in the absence of meningeal inflammation, the AUCCSF/AUCplasma ratio, as the most reliable measure of CSF penetration, was estimated to be 0.11 (122); i.e., its CSF penetration appeared to be lower than that of doxycycline. Tigecycline has been administered with success intrathecally in doses of up to 10 mg twice daily (123), mostly in addition to intravenous therapy, as reported by several investigators. Because it is a relatively small, moderately lipophilic compound (molecular mass, 586 Da; XlogP3-AA, 1.1) allowing retrograde diffusion across the blood-CSF and blood-brain barriers, in addition to elimination by bulk flow, its clearance from CSF to blood is expected to be greater than that of vancomycin or colistin. Therefore, doses of 2 mg were administered at 12-h intervals in an adult (124). In a 5-month-old female infant, tigecycline was administered successfully at a dose of 4 mg once daily for 14 days together with i.v. tigecycline without apparent side effects. CSF concentrations were not determined (125). Intraventricular administration was well tolerated (124–129). However, tigecycline pharmacokinetics after intrathecal and systemic applications have been studied only in one 67-year-old man with pneumonia and meningitis (intraventricular administration of 1 mg and intravenous infusion of 49 mg tigecycline every 12 h) (127). In this patient, the apparent volume of distribution of the CSF space was estimated to be 173 ml, and the elimination half-life in CSF estimated from the mean residence time was indeed only slightly longer than the elimination half-life in plasma (127). In a 38-year-old man who received 5 mg tigecycline every 24 h plus clamping of the drain for 2 h, tigecycline levels ranged from 178.9 to 310.1 mg/liter 2 h after dosing. After 6 h, CSF concentrations of 35.4 to 41.3 mg/liter were noted, and 24 h after dosing, tigecycline CSF concentrations were not detectable (129), pointing to a relatively short t1/2β CSF in the absence of intravenous coadministration.
Antifungals
Amphotericin B.
Amphotericin B is a relatively large, moderately lipophilic compound (924 g/mol; log P, 0.8) with plasma binding of >95% (130). After intravenous injection, CSF concentrations of approximately 0.05 mg/liter, i.e., 10 times lower than necessary for reliable antifungal activity, were observed. Amphotericin B possesses substantial neurotoxicity (131). Daily intrathecal injection of 0.3 mg caused mild arachnoiditis. Arachnoiditis was avoided when the drug at this daily dose was administered as a 1-h intrathecal infusion rather than as an injection (51). Another adverse effect observed after intrathecal amphotericin B therapy was Parkinson’s syndrome (132). Amphotericin B has been administered both as an aqueous solution and in a liposome-encapsulated formula (51, 133, 134). Intrathecal application of liposome-encapsulated amphotericin appears to have fewer and milder side effects, and the daily dose could be increased from 0.5 to 1 mg (134).
In cryptococcal meningitis, intraventricular amphotericin B as an adjunct to systemic therapy was effective: death occurred in 1/6 patients with intraventricular and systemic therapy, compared with 6/7 patients with intravenous administration alone (P = 0.025). The CSF was sterilized in 6/6 patients treated systemically and intraventricularly, compared with 3/7 patients receiving systemic therapy alone (P = 0.049) (135). In a 16-year-old diabetic man with rhinocerebral mucormycosis treated intravenously, intralesionally, and intraventricularly, intraventricular doses were limited to a total of 2.0 mg (average dose, 0.66 mg every other day) because of nausea and vomiting. Conversely, the total amount of 14 mg of intralesional amphotericin B (average dose, 0.5 mg every other day) was administered during 80 days, and eventually, the patient was cured (136). Fever and/or nausea was present in 7 of 10 adults with cryptococcal meningitis unresponsive to conventional therapy receiving intraventricular infusions of 0.25 to 0.5 mg of amphotericin B deoxycholate once daily. Seven patients recovered completely or partially, and three patients died (137).
The exchange between the CSF space and the interstitial space of the brain has been studied in one patient receiving amphotericin B intraventricularly: amphotericin B equilibrated with a second compartment that probably represented the extracellular space and partially the intracellular space of the brain. Amphotericin B rapidly disappeared from CSF after the first injection. After prolonged treatment, the half-life of amphotericin B was increased, because the second compartment served as a reservoir, and amphotericin B accumulated there. The CSF space was estimated to be 139 ml, whereas the volume of distribution in the nervous tissue was estimated to be 677 ml, and the transfer constant between both spaces was 0.78/h, corresponding to a half-life of 0.9 h for exchange between both compartments (51).
The development of azole antifungals, in particular fluconazole and voriconazole, which penetrate the blood-CSF and blood-brain barriers well, has reduced the necessity of the intrathecal and intralesional administration of amphotericin B.
Caspofungin.
Caspofungin acetate is a large water-soluble lipopeptide (molecular mass, 1,213 g/mol; log P, −3.88) that is highly protein bound in plasma (approximately 96%). The use of intravenous caspofungin in CNS infections is limited because of its properties, impeding its entry into the CNS. During conventional intravenous therapy, no therapeutic CSF levels are attained. The pharmacokinetics of caspofungin after intraventricular therapy has not been studied. Clinical experience is confined to case reports: a 2-year-old boy with a cerebral infection by a Scedosporium apiospermum complex isolate after nearly drowning greatly improved by treatment with intraventricular caspofungin (1 mg/day, and later 2 mg/day, via bilateral intraventricular catheters for 19 days) in addition to systemic terbinafine and voriconazole. Treatment with voriconazole and terbinafine was then continued, and the patient survived (138). A 71-year-old woman with Scedosporium apiospermum complex meningoencephalitis received caspofungin at 5 mg once daily via external ventriculostomy for 14 days. Intraventricular caspofungin was well tolerated. The patient experienced some nausea and headaches temporally associated with intraventricular drug administration and clamping of the external ventriculostomy for 30 min. She also received systemic voriconazole and terbinafine, which resulted in CSF sterilization. However, 6 weeks after discontinuation of therapy, the patient deteriorated (139). In a 58-year-old man suffering from a Candida auris shunt infection, caspofungin administered as a single daily dose of 10 mg through the external ventriculostomy followed by clamping of the tube for 6 h over 10 days, together with oral voriconazole and intravenous caspofungin and flucytosine, was able to cure the patient. The treatment was tolerated without apparent side effects (72).
LIMITATIONS OF THIS REVIEW
Most pharmacokinetic data after intrathecal administration are derived from patients who received the same antibiotic intravenously and intrathecally. Since during simultaneous intrathecal and intravenous therapy, the CSF concentrations usually by far exceed the corresponding plasma levels, the influence of the plasma concentrations on the concentration-time curves of the anti-infective in the CSF is considered to be small (18, 42, 60, 63–65). The inclusion of only studies using intrathecal administration alone would have ended in a very sparse set of data. For ethical reasons, this limitation of the study cannot be overcome, since intrathecal in addition to intravenous therapy is necessary for an optimum therapeutic effect in difficult-to-treat CNS infections. Therefore, we do not expect many studies on intrathecal therapy alone in patients with CNS infections in the future, and pharmacokinetic studies after intrathecal administration of anti-infectives without a clinical benefit in critically ill patients without a CNS infection are considered unethical in most countries.
Moreover, as a consequence of the plethora of case reports and small case series on intrathecal treatment of CNS infections, we were unable to present all published data.
CONCLUSIONS
In CNS infections caused by multiresistant pathogens, intraventricular combined with systemic antimicrobial therapy can be life-saving. Intraventricular therapy with drugs used widely (aminoglycosides, colistin methanesulfonate, daptomycin, tigecycline, and vancomycin) at doses without documented severe side effects appears to be safe. Because patients receiving intrathecal therapy usually are very sick, adverse drug effects may be overlooked, and the true incidence of adverse effects of intrathecal therapy might be underestimated. Only colistin is licensed for intrathecal administration in the United States and the European Union. For all other compounds, intrathecal therapy constitutes an off-label use, i.e., use for an unapproved indication. Depending on the estimated CSF volume and, if applicable, the flow rate via external ventriculostomy, the treating physicians should choose a dose within the established dosage range. Generally, the recommended dosing interval is 24 h, and therapeutic drug monitoring is required only in the case of therapeutic failure.
ACKNOWLEDGMENTS
We thank Cynthia Bunker for carefully editing the manuscript.
R. Nau and H. Eiffert received a grant from the B. Braun Foundation, Melsungen, Germany. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We have no financial interests in the drugs discussed in this review. We did not receive support from institutions or companies mentioned in the manuscript.
Biographies

Roland Nau, M.D., M.Sc., studied Medicine, Sociology, and Philosophy and then specialized in Neurology. He was trained in basic sciences under the supervision of Otto Creutzfeldt and Michael Conlon, Max Planck Institutes for Biophysical Chemistry and Experimental Medicine, Göttingen, Germany, and Martin Täuber, University of California, San Francisco. For over 30 years, he has been studying the entry of anti-infectives and other drugs into the cerebrospinal fluid (CSF) in patients and experimental animals. Research interests are focused on infections of the central nervous system, including experimental approaches to improve treatment. He has approximately 250 papers published in PubMed-listed journals. From 2005 to 2007, he headed the Neurochemical Laboratory, Department of Neurology, University of Göttingen, specializing in CSF analysis. Since 2008, he has served as Chief of the Department of Geriatrics, Protestant Hospital Göttingen-Weende, and is Associate Professor at the Department of Neuropathology, School of Medicine, Georg August University of Göttingen, Germany.

Claudia Blei, Pharm.D., studied Economical Engineering in Jena, Germany, and Pharmacy at the Friedrich Alexander University, Erlangen-Nuremberg, Germany. She worked as a Research Assistant at the Department of Pharmaceutical Technology, Université de Montréal, Canada. After receiving her degree in Pharmacy, from 2006 to 2010, she worked at the Hospital Pharmacy of HELIOS Albert Schweitzer Klinik Northeim. Since 2010, she has joined the Hospital Pharmacy of the Georg August University of Göttingen. Her main interest is Antibiotic Stewardship.

Helmut Eiffert, M.D., Ph.D., studied Medicine and Chemistry and then specialized in Infectious Diseases. He received his degrees from the Georg August University of Göttingen (Germany). He conducted research at the Institute of Organic Chemistry concerning the molecular structure and function of new antibiotics from natural sources. He then joined Norbert Hilschmann, Department of Immunochemistry, Max Planck Institute of Experimental Medicine, Göttingen, Germany, as a research fellow and worked in the field of secretory IgA in the human immune system. He spent residencies at the Departments of Internal Medicine, Neuropediatrics, Clinical Chemistry, and Medical Microbiology. He is Infectiologist and Associate Professor and served as Vice Director of the Department of Medical Microbiology, University Hospital, Göttingen. His main research interests are pathogenesis and therapy of infectious diseases of the nervous system. He now serves as Attending Physician at Amedes MVZ for Laboratory Medicine, Medical Microbiology and Infectiology, Göttingen.
This work is dedicated to Hilmar Prange on the occasion of his 75th birthday.
REFERENCES
- 1.Bonomo RA, Burd EM, Conly J, Limbago BM, Poirel L, Segre JA, Westblade LF. 2018. Carbapenemase-producing organisms: a global scourge. Clin Infect Dis 66:1290–1297. doi: 10.1093/cid/cix893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Busl KM. 2019. Healthcare-associated infections in the neurocritical care unit. Curr Neurol Neurosci Rep 19:76. doi: 10.1007/s11910-019-0987-y. [DOI] [PubMed] [Google Scholar]
- 3.Pandey S, Li L, Deng XY, Cui DM, Gao L. 2019. Outcome following the treatment of ventriculitis caused by multi/extensive drug resistance Gram negative bacilli; Acinetobacter baumannii and Klebsiella pneumoniae. Front Neurol 9:1174. doi: 10.3389/fneur.2018.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziai WC, Lewin JJ III. 2009. Improving the role of intraventricular antimicrobial agents in the management of meningitis. Curr Opin Neurol 22:277–282. doi: 10.1097/wco.0b013e32832c1396. [DOI] [PubMed] [Google Scholar]
- 5.Nau R, Sörgel F, Eiffert H. 2010. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev 23:858–883. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crone C. 1971. The blood-brain barrier—facts and questions, p 52–62. In Siesjö BK, Sörensen SC (ed), Ion homeostasis of the brain. Munksgaard, Copenhagen, Denmark. [Google Scholar]
- 7.Davson H, Welch K, Segal MB. 1987. Physiology and pathophysiology of the cerebrospinal fluid. Churchill Livingstone, London, United Kingdom. [Google Scholar]
- 8.Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. 2000. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology 216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- 9.Fleischhack G, Jaehde U, Bode U. 2005. Pharmacokinetics following intraventricular administration of chemotherapy in patients with neoplastic meningitis. Clin Pharmacokinet 44:1–31. doi: 10.2165/00003088-200544010-00001. [DOI] [PubMed] [Google Scholar]
- 10.Lebret A, Hodel J, Rahmouni A, Decq P, Petit E. 2013. Cerebrospinal fluid volume analysis for hydrocephalus diagnosis and clinical research. Comput Med Imaging Graph 37:224–233. doi: 10.1016/j.compmedimag.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Gerber J, Tumani H, Kolenda H, Nau R. 1998. Lumbar and ventricular CSF protein, leukocytes, and lactate in suspected bacterial CNS infections. Neurology 51:1710–1714. doi: 10.1212/WNL.51.6.1710. [DOI] [PubMed] [Google Scholar]
- 12.Spector R, Snodgrass SR, Johanson CE. 2015. A balanced view of the cerebrospinal fluid composition and functions: focus on adult humans. Exp Neurol 273:57–68. doi: 10.1016/j.expneurol.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 13.Dreha-Kulaczewski S, Joseph AA, Merboldt K-D, Ludwig H-C, Gärtner J, Frahm J. 2017. Identification of the upward movement of human CSF in vivo and its relation to the brain venous system. J Neurosci 37:2395–2402. doi: 10.1523/JNEUROSCI.2754-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takizawa K, Matsumae M, Sunohara S, Yatsushiro S, Kuroda K. 2017. Characterization of cardiac- and respiratory-driven cerebrospinal fluid motion based on asynchronous phase-contrast magnetic resonance imaging in volunteers. Fluids Barriers CNS 14:25. doi: 10.1186/s12987-017-0074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayston R, Hart CA, Barnicoat M. 1987. Intraventricular vancomycin in the treatment of ventriculitis associated with cerebrospinal fluid shunting and drainage. J Neurol Neurosurg Psychiatry 50:1419–1423. doi: 10.1136/jnnp.50.11.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reesor C, Chow AW, Kureishi A, Jewesson PJ. 1988. Kinetics of intraventricular vancomycin in infections of cerebrospinal fluid shunts. J Infect Dis 158:1142–1143. doi: 10.1093/infdis/158.5.1142. [DOI] [PubMed] [Google Scholar]
- 17.Pfausler B, Spiss H, Beer R, Kampl A, Engelhardt K, Schober M, Schmutzhard E. 2003. Treatment of staphylococcal ventriculitis associated with external cerebrospinal fluid drains: a prospective randomized trial of intravenous compared with intraventricular vancomycin therapy. J Neurosurg 98:1040–1044. doi: 10.3171/jns.2003.98.5.1040. [DOI] [PubMed] [Google Scholar]
- 18.Imberti R, Cusato M, Accetta G, Marinò V, Procaccio F, Del Gaudio A, Iotti GA, Regazzi M. 2012. Pharmacokinetics of colistin in cerebrospinal fluid after intraventricular administration of colistin methanesulfonate. Antimicrob Agents Chemother 56:4416–4421. doi: 10.1128/AAC.00231-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frasca D, Dahyot-Fizelier C, Adier C, Mimoz O, Debaene B, Couet W, Marchand S. 2014. Metronidazole and hydroxymetronidazole central nervous system distribution: 1. Microdialysis assessment of brain extracellular fluid concentrations in patients with acute brain injury. Antimicrob Agents Chemother 58:1019–1023. doi: 10.1128/AAC.01760-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frasca D, Dahyot-Fizelier C, Adier C, Mimoz O, Debaene B, Couet W, Marchand S. 2014. Metronidazole and hydroxymetronidazole central nervous system distribution: 2. Cerebrospinal fluid concentration measurements in patients with external ventricular drain. Antimicrob Agents Chemother 58:1024–1027. doi: 10.1128/AAC.01762-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salmanoglu E, Kim S, Thakur ML. 2018. Currently available radiopharmaceuticals for imaging infection and the Holy Grail. Semin Nucl Med 48:86–99. doi: 10.1053/j.semnuclmed.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Northrup JD, Mach RH, Sellmyer MA. 2019. Radiochemical approaches to imaging bacterial infections: intracellular versus extracellular targets. Int J Mol Sci 20:5808. doi: 10.3390/ijms20225808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lutsar I, McCracken GH Jr, Friedland IR. 1998. Antibiotic pharmacodynamics in cerebrospinal fluid. Clin Infect Dis 27:1117–1127. doi: 10.1086/515003. [DOI] [PubMed] [Google Scholar]
- 24.Täuber MG, Doroshow CA, Hackbarth CJ, Rusnak MG, Drake TA, Sande MA. 1984. Antibacterial activity of beta-lactam antibiotics in experimental meningitis due to Streptococcus pneumoniae. J Infect Dis 149:568–574. doi: 10.1093/infdis/149.4.568. [DOI] [PubMed] [Google Scholar]
- 25.Nau R, Schmidt T, Kaye K, Froula JL, Täuber MG. 1995. Quinolone antibiotics in therapy of experimental pneumococcal meningitis in rabbits. Antimicrob Agents Chemother 39:593–597. doi: 10.1128/AAC.39.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Remeš F, Tomáš R, Jindrák V, Vaniš V, Setlík M. 2013. Intraventricular and lumbar intrathecal administration of antibiotics in postneurosurgical patients with meningitis and/or ventriculitis in a serious clinical state. J Neurosurg 119:1596–1602. doi: 10.3171/2013.6.JNS122126. [DOI] [PubMed] [Google Scholar]
- 27.Deshayes S, Coquerel A, Verdon R. 2017. Neurological adverse effects attributable to β-lactam antibiotics: a literature review. Drug Saf 40:1171–1198. doi: 10.1007/s40264-017-0578-2. [DOI] [PubMed] [Google Scholar]
- 28.Guilhaumou R, Benaboud S, Bennis Y, Dahyot-Fizelier C, Dailly E, Gandia P, Goutelle S, Lefeuvre S, Mongardon N, Roger C, Scala-Bertola J, Lemaitre F, Garnier M. 2019. Optimization of the treatment with beta-lactam antibiotics in critically ill patients—guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation-SFAR). Crit Care 23:104. doi: 10.1186/s13054-019-2378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Sarro A, Ammendola D, Zappala M, Grasso S, De Sarro GB. 1995. Relationship between structure and convulsant properties of some beta-lactam antibiotics following intracerebroventricular microinjection in rats. Antimicrob Agents Chemother 39:232–237. doi: 10.1128/AAC.39.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerz T, von Loewenich FD, Roberts J, Neulen A, Ringel F. 2018. Cerebrospinal fluid penetration of very high-dose meropenem: a case report. Ann Clin Microbiol Antimicrob 17:47. doi: 10.1186/s12941-018-0299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viladrich PF, Cabellos C, Pallares R, Tubau F, Martínez-Lacasa J, Liñares J, Gudiol F. 1996. High doses of cefotaxime in treatment of adult meningitis due to Streptococcus pneumoniae with decreased susceptibilities to broad-spectrum cephalosporins. Antimicrob Agents Chemother 40:218–220. doi: 10.1128/AAC.40.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dimopoulos G, Koulenti D, Parker SL, Roberts JA, Arvaniti K, Poulakou G. 2019. Intravenous fosfomycin for the treatment of multidrug-resistant pathogens: what is the evidence on dosing regimens? Expert Rev Anti Infect Ther 17:201–210. doi: 10.1080/14787210.2019.1573669. [DOI] [PubMed] [Google Scholar]
- 33.Nau R, Seele J, Djukic M, Eiffert H. 2018. Pharmacokinetics and pharmacodynamics of antibiotics in central nervous system infections. Curr Opin Infect Dis 31:57–68. doi: 10.1097/QCO.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 34.Ray L, Levasseur K, Nicolau DP, Scheetz MH. 2010. Cerebral spinal fluid penetration of tigecycline in a patient with Acinetobacter baumannii cerebritis. Ann Pharmacother 44:582–586. doi: 10.1345/aph.1M480. [DOI] [PubMed] [Google Scholar]
- 35.Falagas ME, Vardakas KZ, Tsiveriotis KP, Triarides NA, Tansarli GS. 2014. Effectiveness and safety of high-dose tigecycline-containing regimens for the treatment of severe bacterial infections. Int J Antimicrob Agents 44:1–7. doi: 10.1016/j.ijantimicag.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Cascio A, Mezzatesta ML, Odierna A, Di Bernardo F, Barberi G, Iaria C, Stefani S, Giordano S. 2014. Extended-spectrum beta-lactamase-producing and carbapenemase-producing Enterobacter cloacae ventriculitis successfully treated with intraventricular colistin. Int J Infect Dis 20:66–67. doi: 10.1016/j.ijid.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 37.He Z, Wang C, Liu B, Feng M, Wang Z. 2019. Successful treatment of serious meningitis caused by extremely carbapenem-resistant Enterobacter cloacae (MIC≥16mg/L) with i.v. meropenem and i.v. amikacin plus intraventricular amikacin. Infect Drug Resist 12:3765–3770. doi: 10.2147/IDR.S224509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang JH, Lin PC, Chou CH, Ho CM, Lin KH, Tsai CT, Wang JH, Chi CY, Ho MW. 2014. Intraventricular antimicrobial therapy in postneurosurgical Gram-negative bacillary meningitis or ventriculitis: a hospital-based retrospective study. J Microbiol Immunol Infect 47:204–210. doi: 10.1016/j.jmii.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 39.De Bonis P, Lofrese G, Scoppettuolo G, Spanu T, Cultrera R, Labonia M, Cavallo MA, Mangiola A, Anile C, Pompucci A. 2016. Intraventricular versus intravenous colistin for the treatment of extensively drug resistant Acinetobacter baumannii meningitis. Eur J Neurol 23:68–75. doi: 10.1111/ene.12789. [DOI] [PubMed] [Google Scholar]
- 40.Shofty B, Neuberger A, Naffaa ME, Binawi T, Babitch T, Rappaport ZH, Zaaroor M, Sviri G, Paul M. 2016. Intrathecal or intraventricular therapy for post-neurosurgical Gram-negative meningitis: matched cohort study. Clin Microbiol Infect 22:66–70. doi: 10.1016/j.cmi.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 41.Khan SA, Waqas M, Siddiqui UT, Shamim MS, Nathani KR, Jooma R, Mehmood F. 2017. Intrathecal and intraventricular antibiotics for postoperative Gram-negative meningitis and ventriculitis. Surg Neurol Int 8:226. doi: 10.4103/sni.sni_81_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewin JJ III, Cook AM, Gonzales C, Merola D, Neyens R, Peppard WJ, Brophy GM, Kurczewski L, Giarratano M, Makii J, Rowe AS, Tesoro EP, Zaniewski A, Clark S, Ziai WC. 2019. Current practices of intraventricular antibiotic therapy in the treatment of meningitis and ventriculitis: results from a multicenter retrospective cohort study. Neurocrit Care 30:609–616. doi: 10.1007/s12028-018-0647-0. [DOI] [PubMed] [Google Scholar]
- 43.McCracken GH Jr, Mize SG, Threlkeld N. 1980. Intraventricular gentamicin therapy in gram-negative bacillary meningitis of infancy. Report of the Second Neonatal Meningitis Cooperative Study Group. Lancet i:787–791. [PubMed] [Google Scholar]
- 44.Karvouniaris M, Brotis AG, Tsiamalou P, Fountas KN. 2018. The role of intraventricular antibiotics in the treatment of nosocomial ventriculitis/meningitis from Gram-negative pathogens: a systematic review and meta-analysis. World Neurosurg 120:e637–e650. doi: 10.1016/j.wneu.2018.08.138. [DOI] [PubMed] [Google Scholar]
- 45.Wright PF, Kaiser AB, Bowman CM, McKee KT Jr, Trujillo H, McGee ZA. 1981. The pharmacokinetics and efficacy of an aminoglycoside administered into the cerebral ventricles in neonates: implications for further evaluation of this route of therapy in meningitis. J Infect Dis 143:141–147. doi: 10.1093/infdis/143.2.141. [DOI] [PubMed] [Google Scholar]
- 46.Kaiser AB, McGee ZA. 1975. Aminoglycoside therapy of gram-negative bacillary meningitis. N Engl J Med 293:1215–1220. doi: 10.1056/NEJM197512112932401. [DOI] [PubMed] [Google Scholar]
- 47.Blaney SM, Poplack DG, Godwin K, McCully CL, Murphy R, Balis FM. 1995. Effect of body position on ventricular CSF methotrexate concentration following intralumbar administration. J Clin Oncol 13:177–179. doi: 10.1200/JCO.1995.13.1.177. [DOI] [PubMed] [Google Scholar]
- 48.Mueller SW, Kiser TH, Anderson TA, Neumann RT. 2012. Intraventricular daptomycin and intravenous linezolid for the treatment of external ventricular-drain-associated ventriculitis due to vancomycin-resistant Enterococcus faecium. Ann Pharmacother 46:e35. doi: 10.1345/aph.1R412. [DOI] [PubMed] [Google Scholar]
- 49.Pardridge WM. 2011. Drug transport in brain via the cerebrospinal fluid. Fluids Barriers CNS 8:7. doi: 10.1186/2045-8118-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ooie T, Suzuki H, Terasaki T, Sugiyama Y. 1996. Kinetics of quinolone antibiotics in rats: efflux from cerebrospinal fluid to the circulation. Pharm Res 13:1065–1068. doi: 10.1023/a:1016014909431. [DOI] [PubMed] [Google Scholar]
- 51.Atkinson AJ Jr, Bindschadler DD. 1969. Pharmacokinetics of intrathecally administered amphotericin B. Am Rev Respir Dis 99:917–924. https://www.atsjournals.org/doi/abs/10.1164/arrd.1969.99.6.917. [DOI] [PubMed] [Google Scholar]
- 52.Beenen LF, Touw DJ, Hekker TA, Haring DA. 2000. Pharmacokinetics of intraventricularly administered teicoplanin in staphylococci ventriculitis. Pharm World Sci 22:127–129. doi: 10.1023/A:1008719806949. [DOI] [PubMed] [Google Scholar]
- 53.Nau R, Sörgel F, Prange HW. 1998. Pharmacokinetic optimisation of the treatment of bacterial central nervous system infections. Clin Pharmacokinet 35:223–246. doi: 10.2165/00003088-199835030-00005. [DOI] [PubMed] [Google Scholar]
- 54.Strong JM, Collins JM, Lester C, Poplack DG. 1986. Pharmacokinetics of intraventricular and intravenous N,N′,N″-triethylenethiophosphoramide (thiotepa) in rhesus monkeys and humans. Cancer Res 46:6101–6104. [PubMed] [Google Scholar]
- 55.Heideman RL, Cole DE, Balis F, Sato J, Reaman GH, Packer RJ, Singher LJ, Ettinger LJ, Gillespie A, Sam J, Poplack DG. 1989. Phase I and pharmacokinetic evaluation of thiotepa in the cerebrospinal fluid and plasma of pediatric patients: evidence for dose-dependent plasma clearance of thiotepa. Cancer Res 49:736–741. [PubMed] [Google Scholar]
- 56.Nilsson C, Ståhlberg F, Thomsen C, Henriksen O, Herning M, Owman C. 1992. Circadian variation in human cerebrospinal fluid production measured by magnetic resonance imaging. Am J Physiol 262:R20–R24. doi: 10.1152/ajpregu.1992.262.1.R20. [DOI] [PubMed] [Google Scholar]
- 57.Klein O, Demoulin B, Jean Auque RT, Audibert G, Sainte-Rose C, Marchal J-C, Marchal F. 2010. Cerebrospinal fluid outflow and intracranial pressure in hydrocephalic patients with external ventricular drainage. Acta Neurol Scand 122:140–147. doi: 10.1111/j.1600-0404.2009.01281.x. [DOI] [PubMed] [Google Scholar]
- 58.Hirsch BE, Amodio M, Einzig AI, Halevy R, Soeiro R. 1991. Instillation of vancomycin into a cerebrospinal fluid reservoir to clear infection: pharmacokinetic considerations. J Infect Dis 163:197–200. doi: 10.1093/infdis/163.1.197. [DOI] [PubMed] [Google Scholar]
- 59.Pfausler B, Haring HP, Kampfl A, Wissel J, Schober M, Schmutzhard E. 1997. Cerebrospinal fluid (CSF) pharmacokinetics of intraventricular vancomycin in patients with staphylococcal ventriculitis associated with external CSF drainage. Clin Infect Dis 25:733–735. doi: 10.1086/513756. [DOI] [PubMed] [Google Scholar]
- 60.Li X, Sun S, Ling X, Chen K, Wang Q, Zhao Z. 2017. Plasma and cerebrospinal fluid population pharmacokinetics of vancomycin in postoperative neurosurgical patients after combined intravenous and intraventricular administration. Eur J Clin Pharmacol 73:1599–1607. doi: 10.1007/s00228-017-2313-4. [DOI] [PubMed] [Google Scholar]
- 61.Miura Y, Fuchigami Y, Nomura S, Nishimura K, Hagimori M, Kawakami S. 2018. Brain microdialysis study of vancomycin in the cerebrospinal fluid after intracerebroventricular administration in mice. AAPS PharmSciTech 20:5. doi: 10.1208/s12249-018-1232-8. [DOI] [PubMed] [Google Scholar]
- 62.Donauer E, Drumm G, Moringlane J, Ostertag C, Kivelitz R. 1987. Intrathecal administration of netilmicin in gentamicin-resistant ventriculitis. Acta Neurochir (Wien) 86:83–88. doi: 10.1007/BF01402289. [DOI] [PubMed] [Google Scholar]
- 63.Ziaka M, Markantonis SL, Fousteri M, Zygoulis P, Panidis D, Karvouniaris M, Makris D, Zakynthinos E. 2013. Combined intravenous and intraventricular administration of colistin methanesulfonate in critically ill patients with central nervous system infection. Antimicrob Agents Chemother 57:1938–1940. doi: 10.1128/AAC.01461-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Popa D, Loewenstein L, Lam SW, Neuner EA, Ahrens CL, Bhimraj A. 2016. Therapeutic drug monitoring of cerebrospinal fluid vancomycin concentration during intraventricular administration. J Hosp Infect 92:199–202. doi: 10.1016/j.jhin.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 65.Perier F, Couffin S, Martin M, Bardon J, Cook F, Mounier R. 2019. Multidrug-resistant Acinetobacter baumannii ventriculostomy-related infection, treated by colistin, tigecycline, and intraventricular fibrinolysis. World Neurosurg 121:111–116. doi: 10.1016/j.wneu.2018.09.218. [DOI] [PubMed] [Google Scholar]
- 66.Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Scheld WM, van de Beek D, Bleck TP, Garton HJ, Zunt JR. 2017. Infectious Diseases Society of America’s clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis 64:e34–e65. doi: 10.1093/cid/ciw861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nag DS, Sahu S, Swain A, Kant S. 2019. Intracranial pressure monitoring: gold standard and recent innovations. World J Clin Cases 7:1535–1553. doi: 10.12998/wjcc.v7.i13.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.LeBras M, Chow I, Mabasa VH, Ensom MH. 2016. Systematic review of efficacy, pharmacokinetics, and administration of intraventricular aminoglycosides in adults. Neurocrit Care 25:492–507. doi: 10.1007/s12028-016-0269-3. [DOI] [PubMed] [Google Scholar]
- 69.Smetana KS, Cook AM. 2018. Cerebrospinal fluid vancomycin dosing and monitoring: the quandary posed by guideline recommendations. Clin Infect Dis 67:980–981. doi: 10.1093/cid/ciy189. [DOI] [PubMed] [Google Scholar]
- 70.Kourtopoulos H, Holm SE. 1976. Intraventricular treatment of Serratia marcescens meningitis with gentamicin. Pharmacokinetic studies of gentamicin concentration in one case. Scand J Infect Dis 8:57–60. [PubMed] [Google Scholar]
- 71.Chen F, Deng X, Wang Z, Wang L, Wang K, Gao L. 2019. Treatment of severe ventriculitis caused by extensively drug-resistant Acinetobacter baumannii by intraventricular lavage and administration of colistin. Infect Drug Resist 12:241–247. doi: 10.2147/IDR.S186646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singhal T, Kumar A, Borade P, Shah S, Soman R. 2018. Successful treatment of C. auris shunt infection with intraventricular caspofungin. Med Mycol Case Rep 22:35–37. doi: 10.1016/j.mmcr.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.US Pharmacopeial Convention. 2010. The United States Pharmacopeia 2011: USP 34. US Pharmacopeial Convention, Rockville, MD. [Google Scholar]
- 74.Council of Europe. 2019. European Pharmacopoeia, 10th ed Council of Europe, Strasbourg, France: https://www.edqm.eu/en/european_pharmacopoeia_10th_edition. [Google Scholar]
- 75.Gaderer C, Schaumann A, Schulz M, Thomale UW. 2018. Neuroendoscopic lavage for the treatment of CSF infection with hydrocephalus in children. Childs Nerv Syst 34:1893–1903. doi: 10.1007/s00381-018-3894-7. [DOI] [PubMed] [Google Scholar]
- 76.Fernández-Martínez M, Ruiz del Castillo B, Lecea-Cuello MJ, Rodríguez-Baño J, Pascual Á, Martínez-Martínez L, Spanish Network for the Research in Infectious Diseases, Spanish Group for Nosocomial Infections . 2018. Prevalence of aminoglycoside-modifying enzymes in Escherichia coli and Klebsiella pneumoniae producing extended spectrum β-lactamases collected in two multicenter studies in Spain. Microb Drug Resist 24:367–376. doi: 10.1089/mdr.2017.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mombelli G, Klastersky J, Coppens L, Daneau D, Nubourgh Y. 1983. Gram-negative bacillary meningitis in neurosurgical patients. J Neurosurg 59:634–641. doi: 10.3171/jns.1983.59.4.0634. [DOI] [PubMed] [Google Scholar]
- 78.Rahal JJ Jr, Hyams PJ, Simberkoff MS, Rubinstein E. 1974. Combined intrathecal and intramuscular gentamicin for gram-negative meningitis. Pharmacologic study of 21 patients. N Engl J Med 290:1394–1398. doi: 10.1056/NEJM197406202902502. [DOI] [PubMed] [Google Scholar]
- 79.Buke C, Sipahi OR, Yurtseven T, Zileli M. 2005. High dose of intrathecal netilmicin in the treatment of nosocomial Acinetobacter baumannii meningitis. J Infect 51:420–422. doi: 10.1016/j.jinf.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 80.Yamashima T, Shoin K, Someya S, Kogure Y, Kubota T, Yamamoto S. 1983. Intrathecal administration of amikacin in postoperative refractory meningitis. Jpn J Antibiot 36:522–528. [PubMed] [Google Scholar]
- 81.Gofman N, To K, Whitman M, Garcia-Morales E. 2018. Successful treatment of ventriculitis caused by Pseudomonas aeruginosa and carbapenem-resistant Klebsiella pneumoniae with i.v. ceftazidime-avibactam and intrathecal amikacin. Am J Health Syst Pharm 75:953–957. doi: 10.2146/ajhp170632. [DOI] [PubMed] [Google Scholar]
- 82.Molinaro M, Morelli P, De Gregori M, De Gregori S, Giardini I, Tordato F, Monzillo V, Pocaterra D, Casari E. 2018. Efficacy of intraventricular amikacin treatment in pan-resistant Pseudomonas aeruginosa postsurgical meningitis. Infect Drug Resist 11:1369–1372. doi: 10.2147/IDR.S169271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiao J, Zhang C, Ye S. 2019. Acinetobacter baumannii meningitis in children: a case series and literature review. Infection 47:643–649. doi: 10.1007/s15010-018-1234-1. [DOI] [PubMed] [Google Scholar]
- 84.Stone RB. 1955. A therapeutic regimen for tuberculous meningitis. Calif Med 82:176–178. [PMC free article] [PubMed] [Google Scholar]
- 85.Bulkeley WC. 1953. Tuberculous meningitis treated with ACTH and isoniazid; a comparison with intrathecal streptomycin. Br Med J ii:1127–1129. doi: 10.1136/bmj.2.4846.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sotgiu G, Nahid P, Loddenkemper R, Abubakar I, Miravitlles M, Migliori GB. 2016. The ERS-endorsed official ATS/CDC/IDSA clinical practice guidelines on treatment of drug-susceptible tuberculosis. Eur Respir J 48:963–971. doi: 10.1183/13993003.01356-2016. [DOI] [PubMed] [Google Scholar]
- 87.Marx GE, Chan ED. 2011. Tuberculous meningitis: diagnosis and treatment overview. Tuberc Res Treat 2011:798764. doi: 10.1155/2011/798764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Varelas PN, Rehman M, Pierce W, Wellwood J, Chua T, Revankar S. 2008. Vancomycin-resistant enterococcal meningitis treated with intrathecal streptomycin. Clin Neurol Neurosurg 110:376–380. doi: 10.1016/j.clineuro.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 89.Bergen PJ, Li J, Nation RL. 2011. Dosing of colistin—back to basic PK/PD. Curr Opin Pharmacol 11:464–469. doi: 10.1016/j.coph.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Horcajada JP, Montero M, Oliver A, Sorlí L, Luque S, Gómez-Zorrilla S, Benito N, Grau S. 2019. Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin Microbiol Rev 32:e00031-19. doi: 10.1128/CMR.00031-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wallace SJ, Li J, Nation RL, Prankerd RJ, Velkov T, Boyd BJ. 2010. Self-assembly behavior of colistin and its prodrug colistin methanesulfonate: implications for solution stability and solubilization. J Phys Chem B 114:4836–4840. doi: 10.1021/jp100458x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Markantonis SL, Markou N, Fousteri M, Sakellaridis N, Karatzas S, Alamanos I, Dimopoulou E, Baltopoulos G. 2009. Penetration of colistin into cerebrospinal fluid. Antimicrob Agents Chemother 53:4907–4910. doi: 10.1128/AAC.00345-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Karaiskos I, Galani L, Baziaka F, Katsouda E, Ioannidis I, Andreou A, Paskalis H, Giamarellou H. 2013. Successful treatment of extensively drug-resistant Acinetobacter baumannii ventriculitis and meningitis with intraventricular colistin after application of a loading dose: a case series. Int J Antimicrob Agents 41:480–483. doi: 10.1016/j.ijantimicag.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 94.Pan S, Huang X, Wang Y, Li L, Zhao C, Yao Z, Cui W, Zhang G. 2018. Efficacy of intravenous plus intrathecal/intracerebral ventricle injection of polymyxin B for post-neurosurgical intracranial infections due to MDR/XDR Acinectobacter [sic] baumannii: a retrospective cohort study. Antimicrob Resist Infect Control 7:8. doi: 10.1186/s13756-018-0305-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Velkov T, Dai C, Ciccotosto GD, Cappai R, Hoyer D, Li J. 2018. Polymyxins for CNS infections: pharmacology and neurotoxicity. Pharmacol Ther 181:85–90. doi: 10.1016/j.pharmthera.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 96.Falagas ME, Bliziotis IA, Tam VH. 2007. Intraventricular or intrathecal use of polymyxins in patients with Gram-negative meningitis: a systematic review of the available evidence. Int J Antimicrob Agents 29:9–25. doi: 10.1016/j.ijantimicag.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 97.Sultana N, Reddy KS, Alam MI. 2019. Intraventricular bleed secondary to intraventricular antibiotics: a case report. Indian J Crit Care Med 23:484–485. doi: 10.5005/jp-journals-10071-23268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kullar R, Chin JN, Edwards DJ, Parker D, Coplin WM, Rybak MJ. 2011. Pharmacokinetics of single-dose daptomycin in patients with suspected or confirmed neurological infections. Antimicrob Agents Chemother 55:3505–3509. doi: 10.1128/AAC.01741-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Piva S, Di Paolo A, Galeotti L, Ceccherini F, Cordoni F, Signorini L, Togni T, De Nicolò A, Rasulo FA, Fagoni N, Latronico N, D’Avolio A. 2019. Daptomycin plasma and CSF levels in patients with healthcare-associated meningitis. Neurocrit Care 31:116–124. doi: 10.1007/s12028-018-0657-y. [DOI] [PubMed] [Google Scholar]
- 100.Denetclaw TH, Suehiro I, Wang PK, Tolliver GL. 2014. Successful treatment of ventriculostomy-associated meningitis caused by multidrug resistant coagulase-negative Staphylococcus epidermidis using low-volume intrathecal daptomycin and loading strategy. Ann Pharmacother 48:1376–1379. doi: 10.1177/1060028014542634. [DOI] [PubMed] [Google Scholar]
- 101.Şahin A, Dalgic N. 2019. Intravenous and intraventricular daptomycin plus intravenous linezolid treatment of an infant with vancomycin-resistant enterococci-induced ventriculoperitoneal shunt infection. World Neurosurg 124:328–330. doi: 10.1016/j.wneu.2019.01.065. [DOI] [PubMed] [Google Scholar]
- 102.Elvy J, Porter D, Brown E. 2008. Treatment of external ventricular drain-associated ventriculitis caused by Enterococcus faecalis with intraventricular daptomycin. J Antimicrob Chemother 61:461–462. doi: 10.1093/jac/dkm501. [DOI] [PubMed] [Google Scholar]
- 103.Jaspan HB, Brothers AW, Campbell AJ, McGuire JK, Browd SR, Manley TJ, Pak D, Weissman SJ. 2010. Multidrug-resistant Enterococcus faecium meningitis in a toddler: characterization of the organism and successful treatment with intraventricular daptomycin and intravenous tigecycline. Pediatr Infect Dis J 29:379–381. doi: 10.1097/INF.0b013e3181c806d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Erritouni M, Ktaich N, Rahal JJ, Figueroa D, Nieto J, Urban C, Mariano N, Eisinger F, Abayev J, Nicolau D, Rubin D, Raifu M, Segal-Maurer S. 2012. Use of daptomycin for the treatment of methicillin-resistant coagulase-negative staphylococcal ventriculitis. Case Rep Med 2012:593578. doi: 10.1155/2012/593578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Viladrich PF, Gudiol F, Liñares J, Pallarés R, Sabaté I, Rufí G, Ariza J. 1991. Evaluation of vancomycin for therapy of adult pneumococcal meningitis. Antimicrob Agents Chemother 35:2467–2472. doi: 10.1128/AAC.35.12.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.París MM, Hickey SM, Uscher MI, Shelton S, Olsen KD, McCracken GH Jr.. 1994. Effect of dexamethasone on therapy of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother 38:1320–1324. doi: 10.1128/AAC.38.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cabellos C, Martinez-Lacasa J, Martos A, Tubau F, Fernández A, Viladrich PF, Gudiol F. 1995. Influence of dexamethasone on efficacy of ceftriaxone and vancomycin therapy in experimental pneumococcal meningitis. Antimicrob Agents Chemother 39:2158–2160. doi: 10.1128/AAC.39.9.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ng K, Mabasa VH, Chow I, Ensom MH. 2014. Systematic review of efficacy, pharmacokinetics, and administration of intraventricular vancomycin in adults. Neurocrit Care 20:158–171. doi: 10.1007/s12028-012-9784-z. [DOI] [PubMed] [Google Scholar]
- 109.Zhang Q, Chen H, Zhu C, Chen F, Sun S, Liang N, Zheng W. 2019. Efficacy and safety of intrathecal meropenem and vancomycin in the treatment of postoperative intracranial infection in patients with severe traumatic brain injury. Exp Ther Med 17:4605–4609. doi: 10.3892/etm.2019.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Matsunaga N, Hisata K, Shimizu T. 2015. An investigation into the vancomycin concentration in the cerebrospinal fluid due to vancomycin intraventricular administration in newborns: a study of 13 cases. Medicine (Baltimore) 94:e922. doi: 10.1097/MD.0000000000000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yadav G, Thakuria B, Madan M, Agwan V, Pandey A. 2017. Linezolid and vancomycin resistant enterococci: a therapeutic problem. J Clin Diagn Res 11:GC07–GC11. doi: 10.7860/JCDR/2017/27260.10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Takechi K, Ishikawa T, Kamei C. 2008. Epileptogenic activity induced by teicoplanin and effects of some antiepileptics in mice. J Pharmacol Sci 107:428–433. doi: 10.1254/jphs.08111FP. [DOI] [PubMed] [Google Scholar]
- 113.Mendes RE, Farrell DJ, Flamm RK, Sader HS, Jones RN. 2015. Analysis of vancomycin susceptibility testing results for presumptive categorization of telavancin. J Clin Microbiol 53:2727–2730. doi: 10.1128/JCM.00611-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cruciani M, Navarra A, Di Perri G, Andreoni M, Danzi MC, Concia E, Bassetti D. 1992. Evaluation of intraventricular teicoplanin for the treatment of neurosurgical shunt infections. Clin Infect Dis 15:285–291. doi: 10.1093/clinids/15.2.285. [DOI] [PubMed] [Google Scholar]
- 115.Fernández Guerrero ML, de Górgolas M, Fernández Roblas R, Campos JM. 1994. Treatment of cerebrospinal fluid shunt infections with teicoplanin. Eur J Clin Microbiol Infect Dis 13:1056–1058. doi: 10.1007/BF02111827. [DOI] [PubMed] [Google Scholar]
- 116.Garey KW, Tesoro E, Muggia V, Pasquier O, Rodvold KA. 2001. Cerebrospinal fluid concentrations of quinupristin-dalfopristin in a patient with vancomycin-resistant Enterococcus faecium [correction of faecalis] ventriculitis. Pharmacotherapy 21:748–750. doi: 10.1592/phco.21.7.748.34573. [DOI] [PubMed] [Google Scholar]
- 117.Tan TY, Pitman I, Penrose-Stevens A, Simpson BA, Flanagan PG. 2000. Treatment of a vancomycin-resistant Enterococcus faecium ventricular drain infection with quinupristin/dalfopristin and review of the literature. J Infect 41:95–97. doi: 10.1053/jinf.2000.0665. [DOI] [PubMed] [Google Scholar]
- 118.Williamson JC, Glazier SS, Peacock JE Jr.. 2002. Successful treatment of ventriculostomy-related meningitis caused by vancomycin-resistant Enterococcus with intravenous and intraventricular quinupristin/dalfopristin. Clin Neurol Neurosurg 104:54–56. doi: 10.1016/S0303-8467(01)00180-9. [DOI] [PubMed] [Google Scholar]
- 119.Kanchanapoom T, Koirala J, Goodrich J, Agamah E, Khardori N. 2003. Treatment of central nervous system infection by vancomycin-resistant Enterococcus faecium. Diagn Microbiol Infect Dis 45:213–215. doi: 10.1016/S0732-8893(02)00523-0. [DOI] [PubMed] [Google Scholar]
- 120.Tush GM, Huneycutt S, Phillips A, Ward JD. 1998. Intraventricular quinupristin/dalfopristin for the treatment of vancomycin-resistant Enterococcus faecium shunt infection. Clin Infect Dis 26:1460–1461. doi: 10.1086/517663. [DOI] [PubMed] [Google Scholar]
- 121.Estes KS, Derendorf H. 2010. Comparison of the pharmacokinetic properties of vancomycin, linezolid, tigecyclin, and daptomycin. Eur J Med Res 15:533–543. doi: 10.1186/2047-783X-15-12-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rodvold KA, Gotfried MH, Cwik M, Korth-Bradley JM, Dukart G, Ellis-Grosse EJ. 2006. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J Antimicrob Chemother 58:1221–1229. doi: 10.1093/jac/dkl403. [DOI] [PubMed] [Google Scholar]
- 123.Wu Y, Chen K, Zhao J, Wang Q, Zhou J. 2018. Intraventricular administration of tigecycline for the treatment of multidrug-resistant bacterial meningitis after craniotomy: a case report. J Chemother 30:49–52. doi: 10.1080/1120009X.2017.1338846. [DOI] [PubMed] [Google Scholar]
- 124.Lauretti L, D’Alessandris QG, Fantoni M, D’Inzeo T, Fernandez E, Pallini R, Scoppettuolo G. 2017. First reported case of intraventricular tigecycline for meningitis from extremely drug-resistant Acinetobacter baumannii. J Neurosurg 127:370–373. doi: 10.3171/2016.6.JNS16352. [DOI] [PubMed] [Google Scholar]
- 125.Şahin A, Dalgic N. 2019. Intraventricular plus intravenous tigecycline for the treatment of daptomycin nonsusceptible vancomycin-resistant enterococci in an infant with ventriculoperitoneal shunt infection. World Neurosurg 130:470–473. doi: 10.1016/j.wneu.2019.07.045. [DOI] [PubMed] [Google Scholar]
- 126.Fang YQ, Zhan RC, Jia W, Zhang BQ, Wang JJ. 2017. A case report of intraventricular tigecycline therapy for intracranial infection with extremely drug resistant Acinetobacter baumannii. Medicine (Baltimore) 96:e7703. doi: 10.1097/MD.0000000000007703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mei S, Luo X, Li X, Li Q, Huo J, Yang L, Zhu L, Feng W, Zhou J, Shi G, Zhao Z. 2016. Development and validation of an LC-MS/MS method for the determination of tigecycline in human plasma and cerebrospinal fluid and its application to a pharmacokinetic study. Biomed Chromatogr 30:1992–2002. doi: 10.1002/bmc.3776. [DOI] [PubMed] [Google Scholar]
- 128.Long W, Yuan J, Liu J, Liu J, Wu M, Chen X, Peng G, Wu C, Zhang C, Wang X, Zhao W, Liu Q. 2018. Multidrug resistant brain abscess due to Acinetobacter baumannii ventriculitis cleared by intraventricular and intravenous tigecycline therapy: a case report and review of literature. Front Neurol 9:518. doi: 10.3389/fneur.2018.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Soto Hernández JL, Soto Ramírez A, Pérez Neri I, Angeles Morales V, Cárdenas G, Barradas VA. 2020. Multidrug-resistant Klebsiella oxytoca ventriculitis, successfully treated with intraventricular tigecycline: a case report. Clin Neurol Neurosurg 188:105592. doi: 10.1016/j.clineuro.2019.105592. [DOI] [PubMed] [Google Scholar]
- 130.Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ. 2002. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob Agents Chemother 46:834–840. doi: 10.1128/AAC.46.3.834-840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Reuhl KR, Vapiwala M, Ryzlak MT, Schaffner CP. 1993. Comparative neurotoxicities of amphotericin B and its mono-methyl ester derivative in rats. Antimicrob Agents Chemother 37:419–428. doi: 10.1128/AAC.37.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fisher JF, Dewald J. 1983. Parkinsonism associated with intraventricular amphotericin B. J Antimicrob Chemother 12:97–99. doi: 10.1093/jac/12.1.97. [DOI] [PubMed] [Google Scholar]
- 133.Tsung LL, Zhu XL, Chu WC, Sun DT, Cheung KL, Leung TF. 2010. Intraventricular amphotericin for absidiomycosis in an immunocompetent child. Hong Kong Med J 16:137–140. [PubMed] [Google Scholar]
- 134.Toprak D, Öcal Demir S, Kadayifci EK, Türel Ö, Soysal A, Bakir M. 2015. Recurrent Candida albicans ventriculitis treated with intraventricular liposomal amphotericin B. Case Rep Infect Dis 2015:340725. doi: 10.1155/2015/340725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Polsky B, Depman MR, Gold JW, Galicich JH, Armstrong D. 1986. Intraventricular therapy of cryptococcal meningitis via a subcutaneous reservoir. Am J Med 81:24–28. doi: 10.1016/0002-9343(86)90177-4. [DOI] [PubMed] [Google Scholar]
- 136.Adler DE, Milhorat TH, Miller JI. 1998. Treatment of rhinocerebral mucormycosis with intravenous interstitial, and cerebrospinal fluid administration of amphotericin B: case report. Neurosurgery 42:644–648. doi: 10.1097/00006123-199803000-00037. [DOI] [PubMed] [Google Scholar]
- 137.Nakama T, Yamashita S, Hirahara T, Okamoto S, Honda S, Watanabe M, Kimura E, Uchino M, Yano S, Kuratsu J, Ando Y. 2015. Usefulness of intraventricular infusion of antifungal drugs through Ommaya reservoirs for cryptococcal meningitis treatment. J Neurol Sci 358:259–262. doi: 10.1016/j.jns.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 138.Mursch K, Trnovec S, Ratz H, Hammer D, Horré R, Klinghammer A, de Hoog S, Behnke-Mursch J. 2006. Successful treatment of multiple Pseudallescheria boydii brain abscesses and ventriculitis/ependymitis in a 2-year-old child after a near-drowning episode. Childs Nerv Syst 22:189–192. doi: 10.1007/s00381-005-1151-3. [DOI] [PubMed] [Google Scholar]
- 139.Williams JR, Tenforwde MW, Chan JD, Ko A, Graham SM. 2016. Safety and clinical response of intraventricular caspofungin for Scedosporium apiospermum complex central nervous system infection. Med Mycol Case Rep 13:1–4. doi: 10.1016/j.mmcr.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cheng T, Zhao Y, Li X, Lin F, Xu Y, Zhang X, Li Y, Wang R, Lai L. 2007. Computation of octanol-water partition coefficients by guiding an additive model with knowledge. J Chem Inf Model 47:2140–2148. [DOI] [PubMed] [Google Scholar]