Abstract
The coronavirus disease 2019 (COVID-19) emerged in Wuhan city, China, in late 2019 and has rapidly spread throughout the world. The major route of transmission of SARS-CoV-2 is in contention, with the airborne route a likely transmission pathway for carrying the virus within indoor environments. Until now, there has been no evidence for detection of airborne severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and this may have implication for the potential spread of the COVID-19. We investigated the air of patient rooms with confirmed COVID-19 in the largest hospital in Iran, on March 17, 2020. To collect the SARS-CoV-2 particles, ten air samples were collected into the sterile standard midget impingers containing 20 mL DMEM with 100 μg/mL streptomycin, 100 U/mL penicillin and 1% antifoam reagent for 1 h. Besides, indoor particle number concentrations, CO2, relative humidity and temperature were recorded throughout the sampling duration. Viral RNA was extracted from samples taken from the impingers and Reverse-Transcription PCR (RT-PCR) was applied to confirm the positivity of collected samples based on the virus genome sequence. Fortunately, in this study all air samples which were collected 2 to 5 m from the patients' beds with confirmed COVID-19 were negative. Despite we indicated that all air samples were negative, however, we suggest further in vivo experiments should be conducted using actual patient cough, sneeze and breath aerosols in order to show the possibility of generation of the airborne size carrier aerosols and the viability fraction of the embedded virus in those carrier aerosols.
Keywords: SARS-CoV-2, COVID-19, Outbreak, Airborne, Tehran, Iran
Graphical abstract
1. Introduction
A novel human coronavirus, named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first detected in Wuhan, China, in late 2019 (Holshue et al., 2020; Sohrabi et al., 2020). COVID-19 is spreading rapidly throughout the world; approximately all developed and developing countries and nations (Amirhossein Takian and Kazempour-Ardebili, 2020; Holshue et al., 2020; Sohrabi et al., 2020; van Doremalen et al., 2020a). In Iran, the first official announcement of death due to COVID-19 was reported by the Ministry of Health and Medical Education on February 19, 2020 (Amirhossein Takian and Kazempour-Ardebili, 2020). On March 31, 2020, Iran with 44,605 COVID-19 cases and 2898 its deaths was the 7th and 6th highest ranking, among 203 countries and territories throughout the world which have experienced COVID-19 outbreak (https://www.worldometers.info/coronavirus/#countries). Given the rapid spread of COVID-19 globally, investigations are under way to better understand the potential of airborne transmission mode of the virus as well as the clinical characteristics and severity of the disease (Ghinai et al., 2020; Guan et al., 2020; Holshue et al., 2020; Liu et al., 2020; van Doremalen et al., 2020a). Although previously conducted investigations have mainly discussed on the person-to-person transmission of SARS-CoV-2, its transmission airborne mode is controversial (Ghinai et al., 2020; Holshue et al., 2020; van Doremalen et al., 2020a; Wilder-Smith et al., 2020). It was reported that airborne transmission plays an important role in the epidemiology of the two zoonotic coronaviruses that emerged over last two decades, SARS-CoV-1 and MERS-CoV (van Doremalen et al., 2020a). To date, the overall evidence regarding the airborne transmission of SARS-CoV-2 remains incomplete and inconclusive (Ghinai et al., 2020; Ong et al., 2020; van Doremalen et al., 2020b). To better manage the risks of COVID-19, particularly for health-care workers (Cheung et al., 2020), it is extremely crucial to address the importance of airborne transmission as one of the most important challenge/question of international COVID-19 outbreak. Hereunder, we investigated the air samples of patient rooms with severe and critical symptoms to detect the SARS-CoV-2.
2. Materials and methods
2.1. Study area
We conducted the present study on March 17, 2020 in hospital wards with confirmed COVID-19 at Imam Khomeini Hospital Complex in Tehran, Iran. Tehran, with about 9 million residents and the day time population of near 13 million people who commute into the city from other cities, is the capital and most populous city of Iran (Faridi et al., 2018; Yousefian et al., 2020). On March 31, 2020, Tehran province with the highest confirmed COVID-19 cases ranked the first place among all provinces in Iran. In the city there are 8 and 13 referral and emergency hospitals that admit patients with severe and critical symptoms. We investigated the indoor air of the wards of intensive care unit (ICU)-Thorax, Internal, ICU-General and ICU-Heart surgery to detect SARS-CoV-2 at Imam Khomeini Hospital complex as the largest hospital in Iran.
2.2. Approach to environmental sampling
As a highly efficient approach to sample the airborne viruses, the impinger technique was used in order to collect the air sample of patient rooms with confirmed COVID-19 (Dart and Thornburg, 2008; Verreault et al., 2008). We have developed four specific experimental setups that consists of a vacuum pump with a flow rate equal to 1.5 L·min−1, the standard midget impinger (from SKC. Inc), rechargeable battery and connecting tubes. Prior to each sampling campaign, all experimental setups were autoclaved (impingers) and disinfected (connecting tubes) using alcohol solution 70% based on the protocol of Centers for Disease Control and Prevention (https://www.cdc.gov/coronavirus/2019-ncov/prepare/cleaning-disinfection.html). We installed our experimental setups in the above-mentioned wards at the height of about 1.5 to 1.8 m from the floor and approximately 2 to 5 m away from the patients' beds with severe and critical symptoms (Fig. 1 ). Some of the patients coughed during the samples collection. The indoor air of each of wards (ten air samples) was pumped into the sterile impinger containing 20 mL of DMEM (Dulbecco's Modified Eagle's Medium) with 100 μg/mL streptomycin, 100 U/mL penicillin and 1% antifoam reagent (isoamyl alcohol) for 1 h and the collected samples immediately transferred to a clinical virology laboratory on ice for detection of the novel coronavirus. A real-time instrument at a flow rate of 1.2 L·min−1 (GRIMM Aerosol Spectrometer, model 11E, Grimm Aerosol Technik GmbH, Ainring, Germany) was used to monitor the indoor particle number concentrations (PNC) in thirty-two various size fractions between 0.25 and 32 μm in time intervals of six-second. Additionally, we recorded indoor CO2 concentration, relative humidity and temperature using AQ110 and HD 110 KIMO (Sauermann Group), respectively.
Fig. 1.
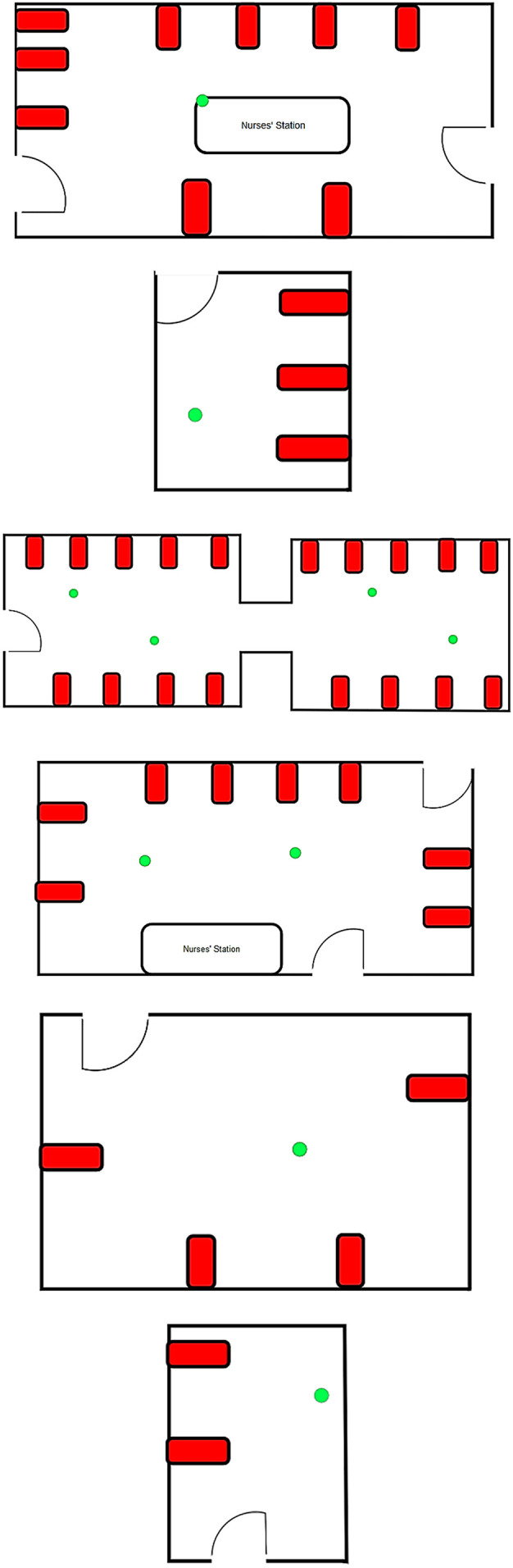
The location of samples (green) and COVID-19 patients' beds (red).
2.3. SARS-CoV-2 detection
All samples after sampling were transferred to the Virology Lab at National Influenza Centre (NIC) under cool condition. The samples then were ultra-centrifuged at 110,000 ×g for 1.5 h at 4 °C, followed by pellet suspending in the sampling medium. To detect the presence of SARS-CoV-2 in the air samples, specific primer and probe real-time reverse transcriptase–polymerase chain reaction (RT-PCR) targeting RNA-dependent RNA polymerase (RdRp) and Envelop (E) genes was used to determine viral genomes. Viral RNA was extracted from sample material and collected in elution buffer, using a Vazyme Viral RNA/DNA Mini Kit (Vazyme, China) prior to RT-PCR. PCR amplification were performed using The SuperScript™ III One-Step RT-PCR System with Platinum™ Taq DNA Polymerase (Invitrogen, USA). Each reaction contained 12.5 μL Invitrogen 2X Master Mix, 0.5 μl of SARS-CoV-2 specific primer and probe sets suggested by WHO (ModularDx Kit, Wuhan Cov RdRP and E gens (Life Science. Berlin, Germany)), 2 L of nuclease free water. Finally, 5 L of RNA sample were prepared for real-time PCR (PCR). Prepared reactions were run with initial conditions of 50 °C for 30 min, 95 °C for 2 min, followed by 45 cycles of 95 °C for 10 s, and 60 °C for 30 s (Applied Biosystems, USA) The ct (cycle threshold) values higher than 38 were considered as the negative results.
3. Results and discussion
Table 1 reveals the specifications of all collected samples of SARS-CoV-2 in the air of hospital wards, and Table 2 provides additional information on environmental status of patient rooms with confirmed COVID-19. As shown in Table 1, there were forty-four patients confirmed with COVID-19 that were receiving oxygen mask (n = 22) and intubation (n = 22) treatments. Given the Table 1, all air samples which were taken 2 to 5 m from the patients' beds were negative. Our results concur with one of the more recent World Health Organization report on March 27, 2020 (https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations) and a recent published article in the Journal of the American Medical Association (Ong et al., 2020) which indicated SARS-CoV-2 could not be transmitted by an airborne route, suggesting that airborne transmission is not driving the pandemic. However, in a recent preprint article by Joshua L. Santarpia et al. (2020) at University of Nebraska Medical Center, the viral RNA was found in air of eleven isolation rooms and in the hallway spaces. An average of 2.86 copies/L of air in room air samples was reported, suggesting the transportation of aerosols, potentially even in the absence of cough or aerosol generating procedures. Since the air samples were collected in the negative pressure equipped rooms, we assume air turbulence, causing by recirculating air in those isolation rooms could remarkably suspend the large droplets for a long period of time, and finally affect the results. Nevertheless, there are some other limitations in that study, e.g. the distance of air samplers from the patient beds was unclear which could limit interpretation for the importance of airborne transmission. It was also mentioned that the air samples in the hallways were taken during sampling activities and samplers were placed on the floor adjacent to rooms. The height of sampling is critically important once it comes to investigate the airborne aerosols. As copies/μL in recovered buffer from the personal air samplers was approximately in the range of that recovered for the floor under beds and from personal items, we presume personnel who wore the samplers likely had close contact with the patients during sampling activities (Santarpia et al., 2020). Neeltje and colleagues (2020) in their recent publication using laboratory nebulized SARS-CoV-2 showed that aerosol transmission of SARS-CoV-2 is plausible, as they found viable virus in aerosols hours post-nebulization by a Collison nebulizer (van Doremalen et al., 2020a; van Doremalen et al., 2020b). The size distribution of generated aerosols by a Collison nebulizer depends on the type and viscosity of nebulized fluid. Furthermore, multiples size distributions were reported for Collison nebulizer. For example, it was reported that Collison nebulizer produces high concentrations of aerosols which are relatively monodisperse with a mass median aerodynamic diameter between 1 and 2 μm (Swearengen, 2012). It was reported that COVID-19 patients are afflicted with the dry and short cough (Ghinai et al., 2020). Furthermore, the different cough mechanism could produce large carrier droplets compared to collision nebulizer that may not be within the airborne size range. It was accepted that one of the main limitations of laboratory carrier aerosols produced by nebulizers is that fail to adequately represent human aerosols in terms of the physical (size) and chemical characteristics of respiratory droplets (Vejerano and Marr, 2018). These properties could markedly change the final fate of a virus in a respiratory carrier droplet. Moreover, carrier aerosols from a patient's respiratory system could be spread by coughing, sneezing, speaking and normal breathing, but the profile and possibility of each mode of generation to produce airborne SARS-CoV-2 is unknown. A possible limitation of our study could be that the volume of air collected for each sample (90 L) influenced the ability to detect a PCR peak. As we had limited access to the wards, the number of collected samples might not be generalized to the all wards of hospital with confirmed COVID-19 patients. For the above discussed reasons, we suggest that in future in vivo experiments should be conducted using actual human cough, sneeze and breath aerosols in order to measure; first the possibility of generation of airborne carrier aerosols by each mode of aerosol production and second the number of viable and total viruses of those aerosols. Procedures such as intubation could provide the greatest chance of creating SARS-CoV-2 aerosols. As such health care workers have close contact to the patients, it is imperative that health care workers are protected by stringent levels of personal protective equipment.
Table 1.
SARS-CoV-2 in the air samples of hospital wards.
| # of samples | Hospital wards | # of COVID-19 patients (status) | # of staffs | SARS-CoV-2 in air sample |
| 1 | ICU-Thorax | 9 (Oxygen mask: 5, Intubated: 4) | 15 | Negative |
| 2 | Internal | 3 (Oxygen mask: 3, Intubated: 0) | 2 | Negative |
| 3 | ICU-General-Part 1 | 9 (Oxygen mask: 4, Intubated: 5) | 12 | Negative |
| 4 | Negative | |||
| 5 | ICU-General-Part 2 | 9 (Oxygen mask: 5, Intubated: 4) | 12 | Negative |
| 6 | Negative | |||
| 7 | ICU-Heart surgery - Part 1 | 8 (Oxygen mask: 3, Intubated: 5) | 7 | Negative |
| 8 | Negative | |||
| 9 | ICU-Heart surgery - Part 2 | 4 (Oxygen mask: 2, Intubated: 2) | 3 | Negative |
| 10 | ICU heart surgery | 2 (Oxygen mask: 0, Intubated: 2) | 2 | Negative |
Table 2.
Additional information on environmental status of patient rooms with confirmed COVID-19.
| # of samples | # of windows (status) | Area of patient rooms (m2) | VSa | Tb (°C) | RHc (%) | CO2 (ppm) | PNCd (min-max) |
|---|---|---|---|---|---|---|---|
| 1 | 4 (close) | 335 | Mechanical/Natural | 23.1 | 30.5 | 404 | 74,388 (56850–88,150) |
| 2 | 1 (close) | 20 | Natural | 23.5 | 32.3 | 438 | 75,555 (58200–140,750) |
| 3 | 6 (close) | 255 | Mechanical/Natural | 24.3 | 26.4 | 361 | 155,598 (91700–250,560) |
| 4 | 167,381 (125800–279,680) | ||||||
| 5 | 5 (close) | 215 | Mechanical/Natural | 28.4 | 27.0 | 362 | 152,135 (95100–256,770) |
| 6 | 110,026 (93650–183,750) | ||||||
| 7 | 4 (close) | 180 | Mechanical/Natural | 24.0 | 36.5 | 361 | 90,524 (74200–115,350) |
| 8 | 102,853 (90900–122,550) | ||||||
| 9 | 2 (open) | 96 | Mechanical/Natural | 24.0 | 28.0 | 403 | 107,884 (95700–126,600) |
| 10 | 2 (close) | 20 | Mechanical/Natural | 24.5 | 28.4 | 503 | 89,814 (70300–105,300) |
Ventilation system.
Temperature
Relative humidity
Particle number concentrations (# of particles/L).
4. Conclusion
The rapid expansion of the COVID-19 is indicative of more efficient person-to-person transmission and likely its pathway airborne. Consequently, we investigated ten air samples of patient rooms with confirmed COVID-19 in the largest clinical hospital in Iran to address the international challenge regarding the airborne pathway of the SARS-CoV-2 particles. All air samples were negative. As we collected these samples 2 to 5 m from the patients' beds, we suggest that all health-care workers at the clinical hospital who have close contact to the patients must adhere to national or international evidence based precautions.
CRediT authorship contribution statement
Sasan Faridi:Conceptualization, Methodology, Validation, Formal analysis, Resources, Writing - original draft, Writing - review & editing, Project administration.Sadegh Niazi:Conceptualization, Writing - original draft, Writing - review & editing.Kaveh Sadeghi:Conceptualization, Methodology, Validation, Formal analysis, Resources.Kazem Naddafi:Conceptualization.Jila Yavarian:Methodology, Validation, Formal analysis, Resources.Mansour Shamsipour:Investigation, Writing - review & editing.Nazanin Zahra Shafiei Jandaghi:Methodology, Validation, Formal analysis, Resources.Khosro Sadeghniiat:Writing - review & editing, Project administration.Ramin Nabizadeh:Writing - review & editing.Masud Yunesian:Writing - review & editing.Fatemeh Momeniha:Writing - review & editing.Adel Mokamel:Writing - review & editing.Mohammad Sadegh Hassanvand:Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing - original draft, Writing - review & editing, Supervision, Funding acquisition.Talat MokhtariAzad:Methodology, Validation, Formal analysis, Resources, Supervision.
Declaration of competing interest
The authors declared no conflicts of interests.
Acknowledgments
This study was financially supported by the Institute for Environmental Research (IER), Tehran University of Medical Sciences (grant number: 99-1-110-47133). We thank the staff of Imam Khomeini Hospital Complex and Department of Virology, School of Public Health, Tehran University of Medical Sciences.
Editor: Jay Gan
References
- Amirhossein Takian A.R., Kazempour-Ardebili Sara. COVID-19 battle during the toughest sanctions against Iran. Lancet. 2020;395(10229):1035–1036. doi: 10.1016/S0140-6736(20)30668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung J.C.-H., Ho L.T., Cheng J.V., Cham E.Y.K., Lam K.N. Staff safety during emergency airway management for COVID-19 in Hong Kong. Lancet Respir. Med. 2020:19. doi: 10.1016/S2213-2600(20)30084-9. April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart A., Thornburg J. Collection efficiencies of bioaerosol impingers for virus-containing aerosols. Atmos. Environ. 2008;42:828–832. [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D., Holbrook M., Gamble A., Williamson B. 2020. Aerosol and Surface Stability of HCoV-19 (SARS-CoV-2) Compared to SARS-CoV-1. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faridi S., Shamsipour M., Krzyzanowski M., Künzli N., Amini H., Azimi F. Long-term trends and health impact of PM2. 5 and O3 in Tehran, Iran, 2006–2015. Environ. Int. 2018;114:37–49. doi: 10.1016/j.envint.2018.02.026. [DOI] [PubMed] [Google Scholar]
- Ghinai I., McPherson T.D., Hunter J.C., Kirking H.L., Christiansen D., Joshi K. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395(10230):1137–1144. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.-J., Ni Z.-y., Hu Y., Liang W.-h., C-q Ou, He J.-x. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Zhang Q., Chen J., Xiang R., Song H., Shu S. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N. Engl. J. Med. 2020;382:1370–1371. doi: 10.1056/NEJMc2003717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y. Air, surface environmental, and personal protective equipment contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020 doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia J.L., Rivera D.N., Herrera V., Morwitzer M.J., Creager H., Santarpia G.W. medRxiv. 2020. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. [Google Scholar]
- Sohrabi C., Alsafi Z., O’Neill N., Khan M., Kerwan A., Al-Jabir A. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swearengen J.R. CRC Press; 2012. Biodefense Research Methodology and Animal Models. [Google Scholar]
- Vejerano E.P., Marr L.C. Physico-chemical characteristics of evaporating respiratory fluid droplets. J. R. Soc. Interface. 2018;15 doi: 10.1098/rsif.2017.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verreault D., Moineau S., Duchaine C. Methods for sampling of airborne viruses. Microbiol. Mol. Biol. Rev. 2008;72:413–444. doi: 10.1128/MMBR.00002-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith A., Chiew C.J., Lee V.J. Can we contain the COVID-19 outbreak with the same measures as for SARS? Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefian F., Faridi S., Azimi F., Aghaei M., Shamsipour M., Yaghmaeian K. Temporal variations of ambient air pollutants and meteorological influences on their concentrations in Tehran during 2012–2017. Sci. Rep. 2020;10:1–11. doi: 10.1038/s41598-019-56578-6. [DOI] [PMC free article] [PubMed] [Google Scholar]



