Abstract
Background
Cell-mediated immunity including T-cells (T helper and cytotoxic) plays an essential role in efficient antiviral responses against coronavirus disease-2019 (COVID-19). Therefore, in this study, we evaluated the ratio and expression of CD4 and CD8 markers in COVID-19 patients to clarify the immune characterizations of CD4 and CD8 T-cells in COVID-19 patients.
Methods
Peripheral blood samples of 25 COVID-19 patients and 25 normal individuals with similar age and sex as the control group were collected. White blood cells, platelets, and lymphocytes were counted and CD4 and CD8 T lymphocytes were evaluated by flow cytometry.
Results
The number of white blood cells, lymphocytes, and platelets were reduced significantly in COVID-19 patients (P < 0.05). The difference in CD4:CD8 ratio, CD4 T-cell frequency, CD8 T-cell frequency, and CD4 mean fluorescence intensity (MFI) was not significant between COVID-19 patients and healthy individuals (P > 0.05); however, the CD8 MFI increased significantly in COVID-19 infected patients (P < 0.05).
Conclusion
Although, there is no significant difference in the ratio of CD4 to CD8 between two groups, the expression level of CD8 in COVID-19 patients was significantly higher than the normal individuals. This result suggested that the cellular immune responses triggered by COVID-19 infection were developed through overexpression of CD8 and hyperactivation of cytotoxic T lymphocytes.
Keywords: Coronavirus, COVID-19, 2019-nCov, CD4 lymphocyte, CD8 lymphocyte
1. Introduction
The coronavirus disease 2019 (COVID-19) was isolated for the first time from a cluster of pneumonia patient's samples in China [1]. The virus spread rapidly in numerous countries around the world [2]. On March 11, 2020, because of the alarming levels of spread and severity of the virus, the WHO characterized COVID-19 as a pandemic disease [3].
Human coronavirus is characterized as the main pathogen of the respiratory system. There are two types of extremely pathogenic coronaviruses, SARS-CoV and MERS-CoV, which result in severe respiratory syndrome in humans and four other human coronaviruses, HCoVOC43, HCoV229E, HCoVNL63, and HCoVHKU which cause mild upper respiratory diseases [4,5]. The clinical manifestation of the disease includes fever, cough, shortness of breath, muscle ache, confusion, headache, sore throat, rhinorrhea, chest pain, diarrhea, nausea, and vomiting [6].
The host responses to the viral infections depend on the interactions between the innate and adaptive immune systems of the body. The T lymphocytes, including CD4 T lymphocytes and CD8 T lymphocytes, play a critical role in effective antiviral responses of the immune system against viruses [7]. Therefore, changes in the immunological state especially alterations in the normal CD4:CD8 ratio, in viral infected patients was observed due to the antiviral immune responses.
Zaunders et al. suggested that primary HIV-1 infection and infectious mononucleosis patients had inverted the CD4:CD8 ratio; this immune state was not observed in HIV-uninfected patients or HIV non-convertase [8]. Also, Sainz and coworkers have reported that the immune responses triggered by CMV and HIV are accompanied by a lower CD4:CD8 ratio resulting in a progressive decrease in immunity of the patients and suggesting a potential marker of immunosenescence [9]. Paltrinieri et al., in a study on the Birman cats with feline coronavirus, reported higher CD4+ T cell count and CD4:CD8 ratio compared to other breeds [10]. Several studies have suggested that the total number of lymphocytes has decreased significantly in Covid-19 patients [[11], [12], [13], [14], [15]].
According to previous studies, viral infections initiate T-cell responses, and this may impose some alterations in the immunological state in infected patients. Therefore, in the present study, after cell blood counting, we investigated the CD4:CD8 ratio and assessed the protein expression of CD4 and CD8 of the T cells through mean fluorescence intensity (MFI) evaluations in COVID-19 infected patients. The findings of this study may enhance our knowledge of the immunological responses to the COVID-19 infection and may have later medical and diagnostic implications.
2. Materials and methods
2.1. Study population
A number of 25 patients diagnosed with COVID-19 were included in the study. The patients were hospitalized in ICU with a serious conditions. All of the patients were initially diagnosed based on the clinical symptoms and later confirmed by quantitative RT-PCR (qRT-PCR) analysis of throat swab samples. Also, 25 healthy individuals with similar age and sex were selected as the control group. Patients with a history of previous chronic diseases especially who treated with immunosuppressive therapies before the onset of COVID-19 infection, as well as those who died of the disease, were excluded from the study. All of the subjects were informed of the objectives of the study and completed the consent forms. All of the samples were collected according to the laboratory testing of human suspected cases of novel coronavirus (nCoV) infection guideline [16] and approved by the Human Ethics Committee of Arak University of Medical Sciences, Arak, Iran [IR.ARAKMU.REC.1398.334].
2.2. Flow cytometry analysis
Venous blood sample (3 ml) was analyzed for counting total white blood cells (WBCs), platelets (Plt), and lymphocytes by hematology auto analyzer (Sysmex, KX-21N). For flow cytometry analysis, 50 μL of the samples were relocated into the round-bottom tubes to determine the CD4+, and CD8+ T cells count by flow cytometry (BD FACSCalibur, USA) according to the instructions. Cell surface antibodies were including peridinin chlorophyll protein (PerCP)-conjugated anti-CD4 and PerCP-conjugated anti-CD8 (BD Biosciences, MA, USA). A number of 10,000 leukocytes were counted for each sample and the results were analyzed using Flowjo software (Treestar, Ashland, OR, USA). Also, the MFI of CD4 and CD8 of T-lymphocytes were calculated.
2.3. Statistical analysis
Statistical analyses were performed in SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA) and presented as mean ± standard deviation (SD). t-test was used to assess significant differences between the groups. P-value of <0.05 was considered statistically significant.
3. Results
3.1. Blood cells analyzing
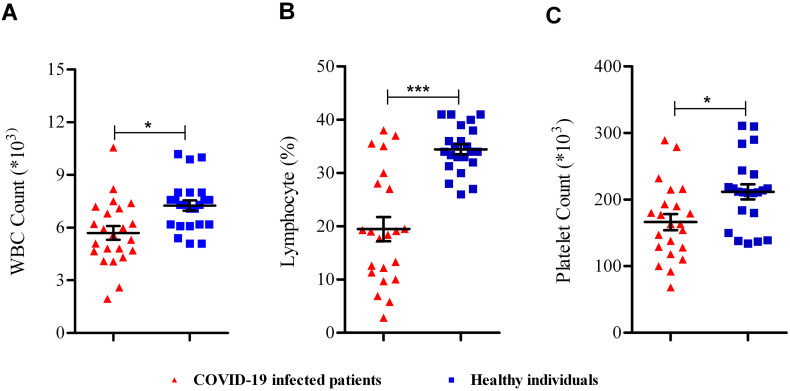
As Fig. 1 shows, WBC (Fig. 1A), lymphocyte (Fig. 1B), and Plt (Fig. 1C) numbers were reduced significantly (P < 0.05) in COVID-19 infected patients compared with healthy individuals.
Fig. 1.
Blood cells analyzing in COVID-19 infected patients. (A) WBC, (B) lymphocyte, and (C) platelet in COVID-19 infected patients compared to healthy individuals. *; P < 0.05, **; P < 0.01, ***; P < 0.001.
3.2. CD4:CD8 ratio and MFI analyzing
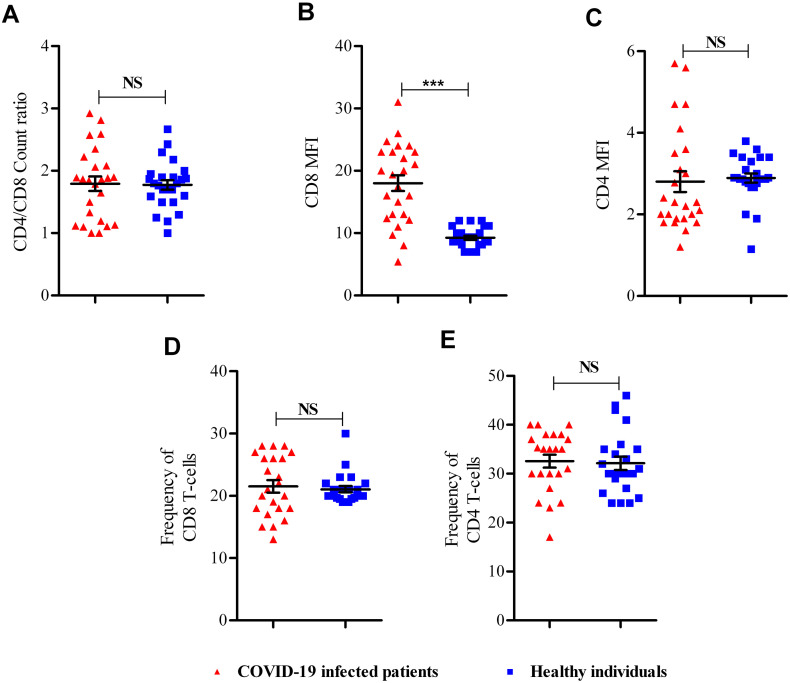
After calculating CD4:CD8 ratio and MFI analyzing (Fig. 2A–C), there is a significant increase of CD8 MFI in COVID-19 infected patients than healthy individuals (P = 0.001) (Fig. 3B); however, there was no significant difference in CD4:CD8 count ratio (Fig. 3A), CD4 MFI (Fig. 3C), CD8 T-cell frequency (Fig. 3D), and CD4 T-cell frequency (Fig. 3E) between studied groups (P > 0.05).
Fig. 2.
Flow cytometry analysis results. (A) Lymphocytes gating based on side and forward scatter and CD4 and CD8 counting, (B) CD8 and CD4 MFIs analysis of healthy individuals, (C) CD8 and CD4 MFIs analysis of COVID-19 infected patients.
Fig. 3.
Flow cytometry results. (A) CD4:CD8 count ratio, (B) CD8 MFI, and (C) CD4 MFI between COVID-19 infected patients and healthy individuals. NS; not significant; ***; P < 0.001.
4. Discussion
Response to viral infections is accompanied by activation of the innate and acquired immune system. The most effective response against a variety of viral infections is the activation of the cellular immune response especially T cell activation [7]. CD8 + cytotoxic T cells (CTLs) by secreting a number of molecules, including perforin, granzyme, and interferons (IFNs) can eliminate viruses from the host body [17]. CD4 helper T cells (Th) cells also help to eliminate viral infection by helping cytotoxic T cells and B cells [18].
As a feature of the immune system, the CD4:CD8 ratio in the normal state is about 2:1 [19]. But in some viral infections, as expected and based on research into the immune response in the human body, this ratio is disrupted and inverted CD4:CD8 ratio, <1:1 [20]. Based on the study by Sainz et al. finding this ratio can act as a potential diagnostic marker [9].
In the present study, flow cytometry studies were performed to measure the number of CD4 and CD8 T cells in peripheral blood samples of COVID-19 patients to measure the CD4:CD8 ratio in patients with COVID-19. Also, the MFI was calculated of these cellular markers to determine the expression of these markers.
The results of the present study indicated that the number of lymphocytes in the peripheral blood of COVID-19-infected individuals was significantly reduced, which is consistent with the results of previous studies [[11], [12], [13], [14], [15]]. Also, peripheral blood platelet counts in COVID-19 patients were significantly decreased. It is in harmony with Lippi et al. study that platelet count reduction is an indicator of worsening disease conditions [21].
In the patients with COVID-19, CD4:CD8 ratio was the same as the normal value of 2:1 that indicates no significant difference compared to the control group. Consistent with this finding, the results of Hou et al. study showed that in COVID-19 patients the CD4:CD8 ratio is in the normal range [22].
Also, the present study showed a significant increase in MFI of CD8 marker in the patient group compared to the healthy individuals, whereas MFI of CD4 marker did not show a significant difference between the patient and control groups. Unchanged CD4:CD8 ratio and a significant increase in CD8 MFI may indicate that the immune response of infected persons to COVID-19 virus is maintained by maintaining normal CD4:CD8 ratio and by increasing CD8 cell marker expression level. Since, CD8 molecule is important for T-cell activity, T lymphocytes try to increase their cytotoxic activity by increasing CD8 protein [23]. However, some viral diseases, such as AIDS, have associated with a decrease in CD8 molecule, which the difference could be due to the short duration of COVID-19 infection compared with the long duration of chronic infection of AIDS [23]. The findings of this study shed light on changes in immunologic markers in COVID-19 patients. Conducting such studies in larger populations and on other immunologic markers may open the way for diagnosing and treating this disease.
In conclusion, in COVID-19 infected patients, the alterations of CD4:CD8 ratio and CD4 MFI were not significant compared to the control group. However, the expression of CD8 on the CTLs in the patient group raised significantly. Therefore it can be inferred that the immune response to the COVID-19 infection is through overexpression of CD8 and hyperactivation of CTL antiviral responses and not by changes in CD4:CD8 ratio and CD4 MFI.
Funding
This study was supported by Arak University of Medical Sciences.
CRediT authorship contribution statement
Ali Ganji:Conceptualization, Methodology, Software.Iman Farahani:Data curation, Writing - original draft.Behzad Khansarinejad:Visualization, Investigation.Ali Ghazavi:Software, Validation.Ghasem Mosayebi:Supervision, Writing - review & editing.
Declaration of competing interest
The authors report no conflicts of interest.
Acknowledgments
We would like to thank Arak University of Medical Sciences for their support.
Editor: Mohandas Narla
References
- 1.Guarner J. Oxford University Press US; 2020. Three Emerging Coronaviruses in two Decades: The Story of SARS, MERS, and now COVID-19. [Google Scholar]
- 2.W.H. Organization . Vol. 3. 2020. Novel Coronavirus (2019-nCoV): Situation Report. [Google Scholar]
- 3.W.H. Organization . 2020. Coronavirus Disease (COVID-2019) Situation Report; p. 51. [Google Scholar]
- 4.Hu B., Zeng L.-P., Yang X.-L., Ge X.-Y., Zhang W., Li B., Xie J.-Z., Shen X.-R., Zhang Y.-Z., Wang N. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song H.-D., Tu C.-C., Zhang G.-W., Wang S.-Y., Zheng K., Lei L.-C., Chen Q.-X., Gao Y.-W., Zhou H.-Q., Xiang H. Cross-host evolution of severe acute respiratory syndrome coronavirus in palm civet and human. Proc. Natl. Acad. Sci. 2005;102:2430–2435. doi: 10.1073/pnas.0409608102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung M.-C., Pape G.R. Immunology of hepatitis B infection. Lancet Infect. Dis. 2002;2:43–50. doi: 10.1016/s1473-3099(01)00172-4. [DOI] [PubMed] [Google Scholar]
- 8.Zaunders J., Carr A., McNally L., Penny R., Cooper D.A. Effects of primary HIV-1 infection on subsets of CD4+ and CD8+ T lymphocytes. AIDS (London, England) 1995;9:561–566. doi: 10.1097/00002030-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Sainz T., Lee S., Hunt P. Conference on Retroviruses and Opportunistic Infections. 2014. CMV and HIV: a double hit on the CD4/CD8 ratio. [Google Scholar]
- 10.Paltrinieri S., Rossi G., Giordano A. Relationship between rate of infection and markers of inflammation/immunity in Holy Birman cats with feline coronavirus. Res. Vet. Sci. 2014;97:263–270. doi: 10.1016/j.rvsc.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G. medRxiv; 2020. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang B., Zhou X., Qiu Y., Feng F., Feng J., Jia Y., Zhu H., Hu K., Liu J., Liu Z. 2020. Clinical Characteristics of 82 Death Cases With COVID-19, medRxiv. [Google Scholar]
- 16.W.H. Organization . World Health Organization; 2020. Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases: Interim Guidance. 2 March 2020. [Google Scholar]
- 17.Mescher M.F., Curtsinger J.M., Agarwal P., Casey K.A., Gerner M., Hammerbeck C.D., Popescu F., Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol. Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J., Yamane H., Paul W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 2009;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owen J.P., Jenni, Stranford, Kuby Sharon. Immunology. 2013;40 [Google Scholar]
- 20.McBride J.A., Striker R. Imbalance in the game of T cells: what can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin. Chim. Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou J., Wan X., Shen Q., Leng Y., Xia Z., Zhao B., Zhu J., Lei S., He Y., Wu Y. 2020. Epidemiologic and Clinical Characteristics of Surgical Patients Infected With COVID-19 in Wuhan. Available at SSRN 3550044. [Google Scholar]
- 23.Jason J., Inge K.L. Modulation of CD8 and CD3 by HIV or HIV antigens. Scand. J. Immunol. 2001;53:259–267. doi: 10.1046/j.1365-3083.2001.00871.x. [DOI] [PubMed] [Google Scholar]