Abstract
Background
In late December 2019, an outbreak of acute respiratory illness, coronavirus disease 2019 (COVID-19), emerged in Wuhan, China. We aimed to study the epidemiology, clinical features and short-term outcomes of patients with COVID-19 in Wuhan, China.
Methods
We performed a single center, retrospective case series study in 221 patients with laboratory confirmed SARS-CoV-2 pneumonia at a university hospital, including 55 severe patients and 166 non-severe patients, from January 2, 2020 to February 10, 2020.
Results
Of the 221 patients with COVID-19, the median age was 55.0 years and 48.9% were male and only 8 (3.6%) patients had a history of exposure to the Huanan Seafood Market. Compared to the non-severe pneumonia patients, the median age of the severe patients was significantly older, and they were more likely to have chronic comorbidities. Most common symptoms in severe patients were high fever, anorexia and dyspnea. On admission, 33.0% patients showed leukopenia and 73.8% showed lymphopenia. In addition, the severe patients suffered a higher rate of co-infections with bacteria or fungus and they were more likely to developing complications. As of February 15, 2020, 19.0% patients had been discharged and 5.4% patients died. 80% of severe cases received ICU (intensive care unit) care, and 52.3% of them transferred to the general wards due to relieved symptoms, and the mortality rate of severe patients in ICU was 20.5%.
Conclusions
Patients with elder age, chronic comorbidities, blood leukocyte/lymphocyte count, procalcitonin level, co-infection and severe complications might increase the risk of poor clinical outcomes.
1. Introduction
In late December 2019, an outbreak of acute respiratory illness, now officially named as the COVID-19, or coronavirus disease 2019, emerged in Wuhan, China [1,2]. From bronchoalveolar lavage samples of the infected patients, a novel beta-coronavirus (SARS-CoV-2) was isolated and identified as the causative agent [3]. Current studies have demonstrated that the COVID-19 shares over 88% homology with two bat-derived severe acute respiratory syndrome (SARS)-related coronaviruses, bat-SL-CoVZC45 and bat-SL-CoVZC21, and that bats may be the most likely natural host [4]. However, whether the SARS-CoV-2 transmits directly from bats or through an intermediate host is still uncertain. Although epidemiological studies indicate a common link between the initially diagnosed patients and the Huanan Seafood Market in Wuhan [5], an increasing number of later confirmed cases have involved infected patients without a history of contacting the implicated market, nor traveling to Wuhan [6], and the family cluster type of infections were reported [7,8]. Human-to-human transmission has been confirmed [6], and respiratory droplets and contact are the main transmission routes, while recent reports also suggested the existence of the fecal-oral transmission route [6,9].
Based on a recent large-scale epidemiological survey, the latency period of the SARS-CoV-2 may extend up to 24 days, but the medium incubation period remains short at 3 days [6]. Reported illnesses have ranged from patients with little or no symptoms to patients being severely ill and dying [6]. The main clinical manifestations include fever, cough, fatigue, and dyspnea [5,10,11]. As compared to young and middle-aged patients with COVID-19, elder infected patients with chronic comorbidities have an increased risk of developing organ dysfunctions, including shock, acute respiratory distress syndrome (ARDS), acute cardiac injury, and acute kidney injury, resulting in a higher mortality rate [10,11]. However, the clinical features between severely affected and not severely affected cases have not yet been well described. Unlike SARS-CoV, the SARS-CoV-2 displays a highly contagious infectiousness even during the asymptomatic period [12].
In this study, we have performed a comprehensive exploration of the epidemiological, clinical, laboratory, and radiological characteristics of 221 hospitalized patients with laboratory-confirmed COVID-19, including 55 severely affected patients and 166 patients who were not severely affected of Zhongnan Hospital of Wuhan University, from January 2, 2020 to February 10, 2020.
2. Methods
2.1. Patients
For this retrospective, single-center study, we recruited 221 patients who were laboratory-confirmed and diagnosed as COVID-19 pneumonia according to WHO interim guidance [13], from January 2, 2020 to February 10, 2020 at Zhongnan Hospital of Wuhan University, Wuhan, China. This study was approved by the Medical Ethical Committee of Zhongnan Hospital of Wuhan University (NO. 2020020). Written informed consent was waived because of emerging infectious disease.
2.2. Laboratory confirmation
The presence of SARS-CoV-2 in pharyngeal swab specimens was detected by real-time reverse-transcriptase polymerase-chain-reaction (RT-PCR) using ORF1ab/N gene PCR kit (Biogerm, Cat# SJ-HX-226-1,2, Shanghai, China) according to the protocol described previously [11]. The diagnostic criteria for RT-PCR results were based on the recommendation by the National Institute for Viral Disease Control and Prevention (China) (http://ivdc.chinacdc.cn/kyjz/202001/t20200121_211337.html). Further details are applicable in the Supplementary Appendix.
2.3. Procedures and data collection
We reviewed clinical charts, nursing records, laboratory findings, and chest radiography for all patients with laboratory-confirmed COVID-19 pneumonia. The data of epidemiological, clinical, laboratory, and radiological features, treatments, and outcomes were obtained from standardized data collection forms and electronic medical records. The severity of COVID-19 was defined based on the international guidelines for community-acquired pneumonia [14]. For critically ill patients admitted to the intensive care unit (ICU), the Glasgow Coma Scale (GCS), Sequential (sepsis-related) Organ Failure Assessment (SOFA), and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores were monitored on the day of ICU admission. Two researchers independently reviewed data to double check the accuracy of collected data. For the data that were not available for records, the researchers directly communicated with patients and doctors to ascertain data integrity. Nucleic acid tests for SARS-CoV-2 were repeated twice and shown virus clearance before discharge of patients’. The clinical outcomes (i.e., discharges, mortality, and hospitalization) were followed up to February 15, 2020. More detailed procedures and relevant definitions are available in the<-- --> Supplementary Appendix.
2.4. Statistical analysis
All continuous variables were determined, the normality of distribution was determined by performing the Kolmogorov-Smirnov test, the normally distributed variables were described as the means ± standard deviation, and the skewed distributed variables were expressed as the median and interquartile range (IQR). We compared the normally distributed continuous variables by using the Student t-test and skewed distributed variables by using the Mann-Whitney U test. Comparisons of categorical variables between groups were conducted using the Pearson’s chi-squared test or Fisher’s exact test, as appropriate. All statistical analyses were performed using the IBM SPSS version 24.0 (IBM Corp., Armonk, NY, USA). P values less than 0.05 represented statistical significance and all reported P values were two-sided.
3. Results
3.1. Epidemiological and Clinical characteristics
The study population included a total of 221 admitted patients with confirmed COVID-19 infection in Zhongnan Hospital of Wuhan University, between January 2, 2020 and February 10, 2020. The median age was 55.0 years (IQR, 39.0–66.5; range, 20–96 years), and 108 of 221 (48.9%) were male. The number of patients with COVID-19 below the age of 45 years, between 45 and 65 years, and above 65 years were 73 (33.0%), 86 (38.9%), and 62 (28.1%) patients, respectively. Of these patients, 55 (24.9%) were severely affected patients and 166 (75.1%) patients were not severely affected by the virus (Table 1 ). Of the whole study population, only 8 (3.6%) patients with COVID-19 had a history of exposure to the Huanan Seafood Market, and 19 (8.6%) patients suffered a secondary infection during hospitalization (Table 1).
Table 1.
Demographics and baseline characteristics of patients with COVID-19.
| Characteristics | Total (n = 221) | Severe (n = 55) | Non-severe (n = 166) | P Value | |
|---|---|---|---|---|---|
| Age, years | 55.0 (39.0–66.5) | 62.0 (52.0–74.0) | 51.0 (36.0–64.3) | <0.001 | |
| <45 | 73 (33.0) | 6 (10.9) | 67 (40.4) | <0.001 | |
| 45–65 | 86 (38.9) | 25 (45.5) | 61 (36.7) | 0.251 | |
| >65 | 62 (28.1) | 24 (43.6) | 38 (22.9) | 0.003 | |
| Sex | 0.011 | ||||
| Male | 108 (48.9) | 35 (63.6) | 73 (44.0) | ||
| Female | 113 (51.1) | 20 (36.4) | 93 (56.0) | ||
| Huanan Seafood Market exposure | 8 (3.6) | 3 (5.5) | 5 (3.0) | 0.414 | |
| Infection during hospitalization | 19 (8.6) | 9 (16.4) | 10 (6.0) | 0.026 | |
| Comorbidity | 78 (35.3) | 40 (72.7) | 38 (22.9) | <0.001 | |
| Hypertension | 54 (24.4) | 26 (47.3) | 28 (16.9) | <0.001 | |
| Diabetes | 22 (10.0) | 7 (12.7) | 15 (9.0) | 0.428 | |
| Cardiovascular disease | 22 (10.0) | 13 (23.6) | 9 (5.4) | <0.001 | |
| Cerebrovascular disease | 15 (6.8) | 11 (20.0) | 4 (2.4) | <0.001 | |
| COPD | 6 (2.7) | 4 (7.3) | 2 (1.2) | 0.035 | |
| CKD | 6 (2.7) | 5 (9.1) | 1 (0.6) | 0.004 | |
| Chronic liver disease | 7 (3.2) | 4 (7.3) | 3 (1.8) | 0.066 | |
| Malignancy | 9 (4.1) | 4 (7.3) | 5 (3.0) | 0.231 | |
| Immunosuppression | 3 (1.4) | 1 (1.8) | 2 (1.2) | 1.000 | |
| Signs and symptoms | |||||
| Fever | 200 (90.5) | 55 (100.0) | 145 (87.3) | 0.006 | |
| Highest temperature ℃ | 38.5 (38.0–39.0) | 38.8 (38.5–39.0) | 38.3 (37.7–38.9) | <0.001 | |
| <37.3 °C | 21 (9.5) | 0 (0) | 21 (12.7) | 0.006 | |
| 37.3–38.0 °C | 57 (25.8) | 6 (10.9) | 51 (30.7) | 0.004 | |
| 38.1–39.0 °C | 104 (47.1) | 36 (65.5) | 68 (41.0) | 0.002 | |
| >39.0 °C | 39 (17.6) | 13 (23.6) | 26 (15.7) | 0.179 | |
| Fatigue | 156 (70.6) | 42 (76.4) | 114 (68.7) | 0.278 | |
| Cough | 136 (61.5) | 36 (65.5) | 100 (60.2) | 0.491 | |
| Anorexia | 80 (36.2) | 34 (61.8) | 46 (27.7) | <0.001 | |
| Dyspnea | 64 (29.0) | 35 (63.6) | 29 (17.5) | <0.001 | |
| Diarrhea | 25 (11.3) | 9 (16.4) | 16 (9.6) | 0.172 | |
| Pharyngalgia | 22 (10.0) | 8 (14.5) | 14 (8.4) | 0.189 | |
| Headache | 17 (7.7) | 4 (7.3) | 13 (7.8) | 1.000 | |
| Abdominal pain | 5 (2.3) | 2 (3.6) | 3 (1.8) | 0.429 | |
| Onset of symptom to hospital admission (d) | 7.0 (4.0–10.0) | 7.0 (6.0–10.0) | 7.0 (4.0–9.0) | 0.041 | |
| Onset of symptom to dyspnea (d) | 8.0(4.0–11.0) | 8.0 (5.0–11.0) | 5.0 (2.0–7.8) | 0.006 | |
| Heart rate (beats/min) | 84 (76–96) | 88 (80–105) | 81 (76–95) | <0.01 | |
| Respiratory rate | 20 (19–21) | 21 (20–26) | 20 (19–20) | <0.001 | |
| Mean arterial pressure (mmHg) | 90 (84–97) | 92 (80–97) | 89 (84–96) | 0.930 | |
Data are median (IQR), n (%). P values comparing severely affected patients and not severely affected patients are from χ² test, Fisher’s exact test, or Mann-Whitney U test. COPD = chronic obstructive pulmonary disease; CKD = chronic kidney disease; and COVID-19 = Corona Virus Disease 2019.
In total, 78 (35.3%) patients had 1 or more chronic comorbidities, including hypertension (54 [24.4%]), diabetes (22 [10.0%]), cardiovascular disease (22 [10.0%]), cerebrovascular disease (15 [6.8%]), chronic obstructive pulmonary disease (6 [2.7%]), chronic kidney disease (6 [2.7%]), chronic liver disease (7 [3.2%]), malignancy (9 [4.1%]), and patients with immunosuppression treatment (3 [1.4%]) (Table 1).
The symptom onset date of the first patient identified was Dec 25, 2019. The most common symptoms were fever (200 [90.5%]), followed by fatigue (156 [70.6%]), cough (136 [61.5%]), anorexia (80 [36.2%]), and dyspnea (64 [29.0%]). Less common symptoms included diarrhea (25 [11.3%]), pharyngalgia (22 [10.0%]), headache (17 [7.7%]), and abdominal pain (5 [2.3%]). The median duration from the onset of symptoms to hospital admission was 7.0 days (IQR, 4.0-10.0), to dyspnea was 8.0 days (IQR, 4.0-11.0), and to ICU admission was 10.0 days (IQR, 7.0–13.0) (Table 1, Table 3).
Table 3.
Treatments and prognosis of patients with COVID-19.<-- -->
| Treatments and Prognosis | Total (n = 221) | Severe (n = 55) | Non-severe (n = 166) | P Value |
|---|---|---|---|---|
| Complications | ||||
| ARDS | 48 (21.7) | 48 (87.3) | 0(0) | <0.001 |
| Arrhythmia | 24 (10.9) | 22 (40.0) | 2 (1.2) | <0.001 |
| Acute cardiac injury | 17 (7.7) | 16 (29.1) | 1 (0.6) | <0.001 |
| Shock | 15 (6.8) | 15 (27.3) | 0 (0) | <0.001 |
| AKI | 10 (4.5) | 8 (14.5) | 2 (1.2) | <0.001 |
| Treatment | ||||
| Antiviral therapy | 196 (88.7) | 50 (90.9) | 146 (88.0) | <0.001 |
| Glucocorticoid therapy | 115 (52.0) | 40 (72.7) | 75 (45.2) | <0.001 |
| CRRT | 5 (2.3) | 4 (7.3) | 1(0.6) | 0.016 |
| NIV | 27 (12.2) | 25 (45.5) | 2 (1.2) | <0.001 |
| IMV | 16 (7.2) | 16 (29.1) | 0 (0) | <0.001 |
| IMV + ECMO | 10 (4.5) | 10 (18.2) | 0 (0) | <0.001 |
| Prognosis | ||||
| Hospitalization | 167 (75.6) | 36 (65.5) | 131 (78.9) | 0.05 |
| Discharge | 42 (19.0) | 7 (12.7) | 35 (21.1) | 0.171 |
| Death | 12 (5.4) | 12 (21.8) | 0 (0) | <0.001 |
Data are n (%). P values comparing severely affected patients and not severely affected patients are from χ² test, Fisher’s exact test. ARDS = acute respiratory distress syndrome. AKI = acute kidney injury. CRRT = continuous renal replacement therapy; NIV = noninvasive ventilation; IMV = invasive mechanical ventilation; ECMO = extracorporeal membrane oxygenation; and COVID-19 = Corona Virus Disease 2019.
Compared to patients who were not severely affected (n = 166), the median age of the severely affected patients was significantly older (62.0 years [IQR, 52.0–74.0] vs 51.0 years [IQR, 36.0–64.3]; P < 0.001). In total, 35 (63.6%) of the severely affected patients were male, and 93 (56.0%) patients who were not severely affected were female. The severely affected patients were also more likely to have underlying comorbidities (40 [72.7%] vs 38 [22.9%]; P < 0.001), including hypertension (26 [47.3%] vs 28 [16.9%]; P < 0.001), cardiovascular disease (13 [23.6%] vs 9 [5.4%]; P < 0.001), and cerebrovascular disease (11 [20.0%] vs 4 [2.4%]; P < 0.001) (Table 1). A higher proportion of severely affected patients developed symptoms such as high fever (body temperature above 38.1 °C, 49 [89.1%] vs 94 [56.6%]; P < 0.001), anorexia (34 [61.8%] vs 46 [27.7%]; P < 0.001), and dyspnea (35 [63.6%] vs 29 [17.5%]; P < 0.001) (Table 1). Vital signs including heart rate (88 [80–107] vs 80 [74–90]; P < 0.01) and respiratory rate (21 [[20], [21], [22], [23], [24], [25], [26]] vs 20 [[19], [20]]; P < 0.001) were also significantly increased in severely affected patients compared to patients who were not severely affected (Table 1).
3.2. Laboratory findings in severely affected patients and patients not severely affected
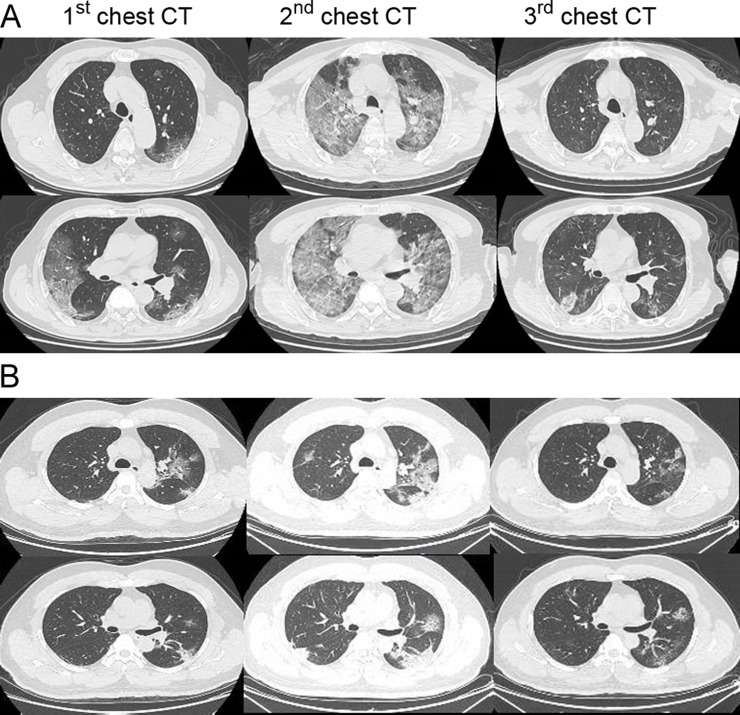
On admission, the blood counts of 73 (33.0%) of the 221 patients showed leukopenia (white blood cell count <3.5 × 109/L) and 163 (73.8%) showed lymphopenia (lymphocyte count <1.1 × 109/L). There were numerous laboratory parameters that were significantly increased in severely affected patients (Table 2 ), including the white blood cell and neutrophil, the prothrombin time, levels of D-dimer, hypersensitive troponin I, creatine kinase, creatine kinase-MB, lactate dehydrogenase, alanine, and aspartate aminotransferase (ALT/AST), total bilirubin, serum creatinine as well as procalcitonin (Table 2, P < 0.001). Additionally, the lymphocyte count was significantly decreased in severely affected patients compared to the patients who were not severely affected (Table 2, P < 0.001)). Of the 221 patients with COVID-19, a total of 215 (97.3%) showed bilateral and the rest (6, [2.7%]) showed unilateral chest radiograph abnormalities, characterized by multiple patchy, ground-grass opacities and multiple lobes of consolidation (the 1st and 2nd chest CT in Fig. 1 A) or honeycomb-like thickening of the interlobular septa and subsegmental areas of consolidation (the 1st and 2nd chest CT in Fig. 1B). Pathogenic analyses show that the severely affected patients suffered a significantly higher rate of coinfections with bacteria (14 [25.5%] vs 3 [1.8%]; P < 0.001) and fungus (6 [10.9%] vs 1 [0.6%]; P = 0.001) compared to patients who were not severely affected (Table 2).
Table 2.
Laboratory tests of patients with COVID-19 on admission to hospital. Data are median (IQR), n (%). P values comparing severely affected patients and not severely affected patients are from χ² test, Fisher’s exact test, or Mann-Whitney U test. COVID-19 = Corona Virus Disease 2019.
| Laboratory parameters | Total (n = 221) | Severe (n = 55) | Non-severe (n = 166) | P Value |
|---|---|---|---|---|
| White blood cell count (×109 /L; normal range 3·5–9·5) | 4.4 (3.2–6.6) | 6.2 (4.1–9.4) | 4.1 (3.1–5.8) | <0.001 |
| <3.5 | 73 (33.0) | 11 (20.0) | 62 (37.3) | 0.018 |
| 3.5–9.5 | 125 (56.6) | 31 (56.4) | 94 (56.6) | 0.973 |
| >9.5 | 23(10.4) | 13(23.6) | 10(6.0) | <0.001 |
| Neutrophil count (×109 /L; normal range 1.8–6.3) | 3.0 (1.9–5.1) | 5.4 (2.8–8.4) | 2.6 (1.8–4.0) | <0.001 |
| Lymphocyte count (×109 /L; normal range 1.1–3.2) | 0.8 (0.6–1.1) | 0.7 (0.4–0.9) | 0.9 (0.6–1.2) | <0.001 |
| <0.5 | 39(17.6) | 18(32.7) | 21(12.7) | 0.001 |
| 0.5–1.1 | 124 (56.2) | 30 (54.5) | 94 (56.6) | 0.788 |
| >1.1 | 58 (26.2) | 7 (12.7) | 51 (30.7) | 0.009 |
| Monocyte count (×109 /L; normal range 0.1-0.6) | 0.4(0.3-0.5) | 0.4(0.2-0.5) | 0.4(0.3-0.5) | 0.381 |
| Platelet count (×109 /L; normal range 125–350) | 175 (127–209) | 169 (111–202) | 175 (136–213) | 0.050 |
| Prothrombin time (s; normal range 9.4-12.5) | 12.9 (12.1–13.6) | 13.4 (12.3–14.8) | 12.7 (12.1–13.4) | <0.001 |
| Activated partial thromboplastin time (s; normal range 25.1–36.5) | 31.1 (29.1–33.1) | 31.1 (29.0–34.9) | 31.1 (29.1–33.0) | 0.666 |
| D-dimer (ng/mL; normal range 0–500) | 227 (129–485) | 443 (211–1304) | 184 (118–324) | <0.001 |
| Hypersensitive troponin I (pg/mL; normal range <26.2) | 7.6 (3.6–21.5) | 14.9 (6.9–55.3) | 5.4 (2.2–9.7) | <0.001 |
| Creatine kinase (U/L; normal range <171) | 87 (55–143) | 121 (73–268) | 75 (53–122) | <0.001 |
| Creatine kinase-MB (U/L; normal range <25) | 13 (10–17) | 18 (14–35) | 12 (10–15) | <0.001 |
| Lactate dehydrogenase (U/L; normal range 125–243) | 227 (174–367) | 424 (287–591) | 204 (167–290) | <0.001 |
| Alanine aminotransferase (U/L; normal range 9–50) | 23 (16–39) | 32 (22–57) | 22 (14–33) | <0.001 |
| Aspartate aminotransferase (U/L; normal range 15–40) | 29 (22–49) | 51 (29–78) | 27 (20–38) | <0.001 |
| Total bilirubin (mmol/L; normal range 5–21) | 10.0 (8.0–14.2) | 11.4 (8.6–17.4) | 9.6 (7.9–13.8) | 0.034 |
| Blood urea nitrogen (mmol/l; normal range 2.8–7.6) | 4.3 (3.4–5.6) | 5.8 (4.3–8.5) | 4.0 (3.3–5.0) | <0.001 |
| Creatinine (μmol/L; normal range 64-104) | 69(56-84) | 75(64-108) | 67(55-79) | <0.001 |
| Procalcitonin (ng/mL; normal range <0.05) | ||||
| <0.05 | 150 (67.9) | 15 (27.3) | 135 (83.1) | <0.001 |
| 0.05–1.00 | 58 (26.2) | 28 (50.9) | 30 (18.1) | <0.001 |
| >1.00 | 13 (5.9) | 12 (21.8) | 1 (0.6) | <0.001 |
| Bilateral involvement of chest radiographs | 215 (97.3) | 55 (100.0) | 160 (96.4) | 0.340 |
| Coinfection | ||||
| Other viruses | 33 (14.9) | 16 (29.1) | 17 (10.2) | 0.001 |
| Bacteria | 17 (7.7) | 14 (25.5) | 3 (1.8) | <0.001 |
| Fungus | 7 (3.2) | 6 (10.9) | 1 (0.6) | 0.001 |
Fig. 1.
Transverse chest CT images of the patients with COVID-19. Case A: Transverse chest CT images from a 72-year-old man severely affected with severe COVID-19. The first chest CT shows multiple ground-glass opacities in bilateral lungs on day 8 after symptom onset. The second chest CT shows progressive bilateral ground-glass opacities and multiple lobes of consolidation on day 14 after symptom onset, and the third CT image was obtained after ECMO supportive therapy in the ICU showing recovery status on day 30 after symptom onset. Case B: Transverse chest CT images from a 44-year-old man with mild COVID-19. The first chest CT shows bilateral multiple lobular and subsegmental areas of consolidation on day 7 after symptom onset. The second chest CT shows bilateral ground-glass opacity and subsegmental areas of consolidation on day 10 after symptom onset, and the third chest CT shows improved status on day 18 after symptom onset.
Of the 55 patients who were severely affected with COVID-19, 44 (80%) of them were admitted to the ICU due to combined moderate or severe ARDS, requiring noninvasive or invasive mechanical ventilation therapy. The median time from the onset of symptoms to ICU admission was 10.0 days (IQR, 7–13)<-- --> (Supplementary Table). On the day of ICU admission, the median GCS, APACHE II, and SOFA scores were 15 (IQR, 11–15), 17 (IQR, 12–22), and 5 (IQR, 4–8), respectively (Supplementary Table), indicating critical illness. The median PaO2 level of patients in ICU was 66 mmHg (IQR, 53–87) and the median of P/F (PaO2 to FiO2) ratio was 113 mmHg (IQR, 84–190).
3.3. Complications, Treatment, and Prognosis
Common complications among the total 221 subjects included ARDS, arrhythmia, acute cardiac injury, acute kidney injury (AKI), and shock. Compared to patients who were not severely affected, the percentages of severely affected patients with complications were significantly increased, including ARDS (48 [87.3%] vs 0; P < 0.001), arrhythmia (22 [40.0%] vs 2 [1.2%]; P < 0.001), acute cardiac injury (16 [29.1%] vs 1 [0.6%]; P < 0.001), shock (15 [27.3%]) vs 0; P < 0.001), and AKI (8 [14.5%] vs 2 [1.2%]; P < 0.001) (Table 3).
Most patients (196 [88.7%]) received antiviral therapy, and a total of 64 (49.6%) patients were given glucocorticoid treatment. The severely affected patients receiving antiviral therapy (50 [90.0%] vs 146 [88.0%]; P < 0.001) and glucocorticoid treatment (40(72.7%) vs 75 [45.2%]; P < 0.001) were significantly higher than those patients who were not severely affected (Table 3).
Among all the severely affected patients, 25 patients (45.5%) received noninvasive ventilation and 26 patients (47.3%) received IMV, which were significantly higher than those patients who were not severely affected, respectively (45.5% vs 1.2% and 47.3% vs 0; P < 0.001) (Table 3). In addition, 48 (87.3%) severely affected patients developed ARDS, and 10 (18.2%) of them were treated with IMV plus ECMO support and 2 AKI patients underwent CRRT. Of 10 severely affected patients receiving IMV + ECMO support, 2 patients had clinical benefits and had been discharged and 3 of them were nonsurvivors. The remaining 5 patients were still under treatment at the time of data collection. Fig. 1A shows that the chest CT was significantly improved after receiving IMV + ECMO support.
We also analyzed the outcome of the 44 severely affected patients in the ICU (Table 4 ). Of these patients, 23 of them (52.3%) had symptomatic relief and were transferred to the general wards, while 9 of them (20.5%) were dead, and the remaining patients were still under treatment. The patients with higher scores of APACHE II, SOFA, and increased levels of PCT, showed a worse prognosis (Table 4). The elder and male patients had an increased mortality rate. The dose and duration of intravenous glucocorticoid treatment showed no difference in outcomes of symptomatic relief and death (Table 4). Intriguingly, patients in the death group received a significantly enlarged volume of fluid balance per day (483 [IQR, 333∼717] vs 60 [IQR, −164∼133]; P < 0.001), and cumulative fluid volume in total (4800 [IQR, 2500–8996] vs 270 [IQR, −1150∼1200]; P < 0.001), compared to patients in the ICU-to-Ward transfer group.
Table 4.
Comparison of clinical parameters based on ICU outcomes: Death vs Transfer.
| Parameters | ICU-to-Ward Transfers (n = 23) | Death in ICU (n = 9) | P Value |
|---|---|---|---|
| Age, years | 62.0 (49.0–71.0) | 76.0 (57.5–81.5) | 0.093 |
| Sex | 0.681 | ||
| Male | 15 (65.2) | 7 (77.8) | |
| Female | 8 (34.8) | 2 (22.2) | |
| APACHE II on ICU admission | 13.0 (10.0–16.0) | 19.0 (15.0–31.5) | 0.003 |
| SOFA on ICU admission | 4.0 (3.0–5.0) | 7.0 (4.0–11.0) | 0.009 |
| PCTmax (ng/ml) | 0.17 (0.05–1.06) | 1.89 (1.53–8.67) | 0.001 |
| Coinfection | |||
| Other viruses | 2 (8.7) | 4 (44.4) | 0.038 |
| Bacteria | 6 (26.1) | 5 (55.6) | 0.213 |
| Fungus | 3 (13.0) | 4 (44.4) | 0.076 |
| Onset of symptom to dyspnea (d) | 10.0 (7.0–12.0) | 10.0 (8.0–10.5) | 0.914 |
| Onset of symptom to ICU admission (d) | 10.0 (7.0–12.0) | 11.0 (8.0–12.0) | 0.832 |
| Onset of symptom to IMV (d) | 11.0 (9.3–14.3) | 11.0 (9.0–13.5) | 0.646 |
| Onset of symptom to glucocorticoid therapy (d) | 9.5 (7.0–11.5) | 11.0 (8.5–15.5) | 0.206 |
| Maximum methylprednisolone dosage (mg/d) | 80 (60–80) | 80 (50–100) | 0.856 |
| Duration of glucocorticoid therapy (d) | 6.5 (4.0–12.0) | 8.0 (2.5–12.0) | 0.776 |
| Cumulative fluid balance in ICU (ml) | 270 (−1150∼1200) | 4800 (2500∼8996) | <0.001 |
| Mean daily fluid balance in ICU (ml/d) | 60 (−164∼133) | 483 (333∼717) | <0.001 |
| ICU length of stay (d) | 8.0 (5.0–13.0) | 11.0 (4.5–14.5) | 0.737 |
Data are median (IQR), n (%). P values comparing severely affected patients and not severely affected patients are from χ² test, Fisher’s exact test, or Mann-Whitney U test. COVID-19 = Corona Virus Disease 2019. ICU = intensive care unit; APACHE II = Acute Physiology and Chronic Health Evaluation II score; SOFA = Sequential Organ Failure Assessment score; PCT = procalcitonin; and Cumulative fluid balance = total fluid input minus output in ICU.
As of Feb 15, 2020, a total of 42 (19.0%) patients had been discharged, 12 (5.4%) patients had died, and 167 (75.6%) patients were still hospitalized. Of the 55 severely affected patients, 36 (65.5%) were still hospitalized, 7 (12.7%) had been discharged, and 12 (21.8%) were dead. The mortality rate was significantly higher in the severely affected patients compared to that of patients who were not severely affected (12 [21.8%] vs 0 [0.0%]; P < 0.001, Table 3).
4. Discussion
This retrospective, single-center study included a total of 221 SARS-CoV-2 pneumonia cases from Jan 2, 2020 to Feb 10, 2020. Only 8 (3.6%) patients had a history of exposure to the Huanan Seafood Market, while a majority of patients without the exposure history indicate the rapid human-to-human transmission. Based on a recent review, the estimated mean R0, an indicator of virus transmissibility, for the SARS-CoV-2 is around 3.28, which is higher than the WHO estimation at 1.95 [15], and is also higher than that of the SARS-CoV outbreak in 2003, with R0 approximately at 3.0 [16].
Clinical characteristic analysis shows a significantly increased age as well as elevated numbers of underlying comorbidities in severely affected patients than those who are not severely affected, indicating that the age and comorbidity may be important risk factors for poor outcome. Contradictory to the previous report showing a higher incidence of COVID-19 in male patients [16], recent data including our data, consistently showed a similar proportion of male and female patients with COVID-19 [11].
The patients with COVID-19 also demonstrated a decreased lymphatic count when compared with healthy people, and it was significantly decreased in severely affected patients as compared to those patients who were not severely affected. Our data show that lymphocytopenia occurred in more than 80% of severely affected patients, consistent with the result of a recent cohort study [17]. Lymphocytopenia is also a prominent feature of severely affected patients with SARS-CoV and MERS infection, which attribute to the necrosis or apoptosis of lymphocyte caused by invasive viral particles [18,19]. We also analyzed the absolute number of different subsets of lymphocytes in ICU patients. Compared to the health population, the total number of T cells (312 [231–558] vs [805–4459]; number per μl), CD4+ T cells (245 [148–297] vs [345–2350]), CD8+ T cells (81 [64–230] vs [345–2350]), total B cells (81 [47–106] vs [240–1317]) as well as natural killer cells (51 [21–115] vs [210–1514]) were dramatically decreased in severely affected patients (data not shown), suggesting that the severity of lymphocytopenia reflects the severity of COVID-19. A recent study showed a significantly reduced total number of T cells, CD4+ and CD8+ T cells in older and severely affected patients with COVID-19 [20], and revealed one possible mechanism by which the aberrant cytokine signaling including TNF-a, IL-6, and IL-10 may be mediated in T cell pro-apoptosis [20]. In addition, abnormal laboratory findings in severely affected patients also included prolonged thrombin time, increased AST/AST, hypersensitive troponin I, and serum creatine, suggesting aberrant coagulation pathway, hepatic injury, myocardial injury, and kidney injury, respectively. The levels of PCT, a marker suggesting bacterial infection, were not elevated in patients with COVID-19, suggesting viral-mediated pneumonia rather than bacteria. These laboratory abnormalities are similar to those previously observed in patients with MERS-CoV and SARS-CoV infection [[21], [22], [23]].
The rates of coinfection, including other viruses, bacteria, and fungus, were significantly increased in severely affected patients with COVID-19 than those patients who were not severely affected. The coinfection rate was also higher in the death group than that of the ICU-to-Ward transfer group, despite no significantly statistical difference due to a small number of cases being involved. The main reasons for the increased hospital-acquired infections were due to lymphocytopenia and the reduced host immune functions in critically ill patients. These patients who have invasive catheters including endotracheal tube, arteriovenous catheters, urinary, and gastric tubes, result in increased susceptibility to secondary infections of nosocomial multidrug-resistant pathogens, such as Acinetobacter baumannii, Escherichia coli, Pseudomonas aeruginosa and Enterococcus. We found that 5 (55.6%) of COVID-19 patients coinfected with carbapenem-resistant A. baumanni (CRAB) in the ICU death group, which was much higher than those (4 [17.4%]) in the ICU-to-Ward transfer group (data not shown). The higher rate of CRAB infection poses difficulties in antibiotic treatment, resulting in increased possibility to develop septic shock and death [24].
At present, there is no evidence showing specific drug treatment against the new coronavirus in suspected or confirmed cases. The principles of treatment include improvement of the symptoms and underlying diseases, active prevention of potential complications, and secondary infections. Like other viruses, the SARS-CoV-2 enters cells through receptor-mediated endocytosis [25]. Studies showed that the SARS-CoV-2 may infect alveolar epithelial cells in the lung through the angiotensin-converting enzyme II receptor, which is also expressed in other tissues, such as kidney, blood vessels, and heart [25,26]. Researchers using an artificial intelligence predicted that Baricitinib, an inhibitor of AP2-associated protein kinase 1, might be useful to interrupt the entrance of virus to cells as well as the intracellular assembly of virus particles [4,27]. In addition, a case report showed that remdesivir, an adenosine analogue, has shown survival benefits in one severely affected patient with COVID-19 pneumonia [28]. The effectiveness has been verified in vitro [29]. Now a couple of clinical trials focus on the efficacy of remdesivir, as well as other therapeutic strategies, such as immunoglobulins, Vitamin C infusion, mesenchymal stem cell treatment, arbidol hydrochloride plus interferon atomization, ritonavir combined with oseltamivir, lopinavir plus ritonavir and arbidol hydroxychloroquine and methylprednisolone [30].
The corticosteroid therapy regarding the onset therapeutic time, the dosage, and duration were still controversial in the treatment of severe SARS or SARS-CoV-2 pneumonia [31]. Corticosteroid therapy was used to treat patients with refractory high fever, exacerbation of wheezing symptoms, increased interstitial exudation based on chest radiology, and high levels of inflammatory mediators to inhibit the "cytokine storm". Corticosteroid therapy (methylprednisolone 1–2 mg/kg/day) is recommended for severely ill patients with ARDS, for as short a duration of treatment as possible [32,33]. The results showed that the early onset use of corticosteroid may have clinical benefits, but more cases and multivariate correlation analysis regarding the safety and efficacy were needed to be verified. Our data also suggested that excessive fluid resuscitation may increase the risk of death. One principle of the ARDS treatments is the restricted fluid resuscitation strategy to prevent the exacerbation of pulmonary edema [34].
The limitation of this study is that among the 221 cases, most of the patients were still hospitalized at the time of data collection. It is incomplete to assess risk factors for outcomes. Continued observation and dynamic clinical datasets are required.
5. Conclusion
In this single-center case series of 221 hospitalized patients with confirmed COVID-19 in Wuhan, China, 55 severely affected patients (24.9%) with older age and chronic comorbidities, developed more than one complication. In all, 44 (80%) of them received ICU care, and 52.3% of them were transferred to the general wards due to relieved symptoms, and the mortality rate of severely affected patients in ICU was 20.5%. Of the 166 patients who were not severely affected, 21.1% of them were cured and discharged and no patients died. Older and male patients with higher APACHE II and SOFA scores, elevated PCT level, excessive fluid volume input, as well as the delayed use of corticosteroid might increase the risk of death.
Author contributions
HQ Pan conceptualized the paper. C Hu analyzed the data with input from GQ Zhang, F Fang, YF Chen, JG Li, and ZY Peng. GQ Zhang, HQ Pan, and LJ Luo wrote the initial draft with all authors providing critical feedback and edits to subsequent revisions. All authors approved the final draft of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Declaration of Competing Interest
The authors declared that they have no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 81700493 to Dr. Pan).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jcv.2020.104364.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Lu H., Stratton C.W., Tang Y.W. Outbreak of Pneumonia of Unknown Etiology in Wuhan China: the Mystery and the Miracle. Journal of Medical Virology. 2020;92(Apr (4)):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC): Coronavirus Disease . 2019. (COVID-19) Situation Summary.https://wwwcdcgov/coronavirus/2019-ncov/summaryhtml [Google Scholar]
- 3.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine. 2020;382(Feb (8)):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020;395(Feb (10224)):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(Feb (10223)):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.W-j Guan, Z-y Ni, Hu Y. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv. 2020 doi: 10.1101/2020.02.06.20020974. 2020.02.06.20020974. [DOI] [Google Scholar]
- 7.JF-W Chan, Yuan S., Kok K.-H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet. 2020;395(Feb (10223)):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin Y.-H., Cai L., Cheng Z.-S. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Military Medical Research. 2020;7(1):4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H., Kang Z., Gong H. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. bioRxiv. 2020 doi: 10.1101/2020.01.30.927806. [DOI] [Google Scholar]
- 10.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D., Hu B., Hu C. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothe C., Schunk M., Sothmann P. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. New England Journal of Medicine. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO . 2020. Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance.https://www.who.int/internal-publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Jan 28. (accessed February 5th, 2020. [Google Scholar]
- 14.Metlay J.P., Waterer G.W., Long A.C. Diagnosis and treatment of adults with community-acquired pneumonia: An official clinical practice guideline of the American Thoracic Society and Infectious Disease Society of America. Am J Respir Crit Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y., Gayle A.A., Wilder-Smith A. The reproductive number of COVID-19 is higher compared to SARS coronavirus. Journal of Travel Medicine. 2020;27(2) doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization . Available: http://www.who.int/csr/sars/en/WHOconsensus.pdf; 2003. Consensus document on the epidemiology of severe acute respiratory syndrome (SARS) [Google Scholar]
- 17.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory Medicine. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu H., Zhou J., Wong B.H.-Y. Middle East respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. The Journal of infectious diseases. 2016;213(6):904–914. doi: 10.1093/infdis/jiv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu W.J., Zhao M., Liu K. T-cell immunity of SARS-CoV: Implications for vaccine development against MERS-CoV. Antiviral research. 2017;137:82–92. doi: 10.1016/j.antiviral.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diao B., Wang C., Tan Y. Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19) medRxiv. 2020 doi: 10.1101/2020.02.18.20024364. 2020.02.18.20024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das K.M., Lee E.Y., Jawder S.E.A. Acute Middle East respiratory syndrome coronavirus: temporal lung changes observed on the chest radiographs of 55 patients. American Journal of Roentgenology. 2015;205(3) doi: 10.2214/AJR.15.14445. W267-S74. [DOI] [PubMed] [Google Scholar]
- 22.Müller N.L., Ooi G.C., Khong P.L. High-resolution CT findings of severe acute respiratory syndrome at presentation and after admission. American Journal of Roentgenology. 2004;182(1):39–44. doi: 10.2214/ajr.182.1.1820039. [DOI] [PubMed] [Google Scholar]
- 23.Nicolaou S., Al-Nakshabandi N.A., Müller N.L. SARS: imaging of severe acute respiratory syndrome. American Journal of Roentgenology. 2003;180(5):1247–1249. doi: 10.2214/ajr.180.5.1801247. [DOI] [PubMed] [Google Scholar]
- 24.Gao H.-N., Lu H.-Z., Cao B. Clinical findings in 111 cases of influenza A (H7N9) virus infection. New England Journal of Medicine. 2013;368(24):2277–2285. doi: 10.1056/NEJMoa1305584. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y., Zhao Z., Wang Y. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. BioRxiv. 2020 doi: 10.1101/2020.01.26.919985. [DOI] [Google Scholar]
- 26.Zhou P., Yang X.-L., Wang X.-G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson P., Griffin I., Tucker C. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. The Lancet. 2020;395(10223):e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 novel coronavirus in the United States. New England Journal of Medicine. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Research. 2020:1–3. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avaiable from: https://clinicaltrials.gov/ct2/results?cond=Coronavirus&term=&cntry=&state=&city=&dist=.
- 31.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. The Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peter J.V., John P., Graham P.L. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. BMJ. 2008;336(7651):1006–1009. doi: 10.1136/bmj.39537.939039.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khilnani G.C., Hadda V. Corticosteroids and ARDS: A review of treatment and prevention evidence. Lung India. 2011;28(2):114–119. doi: 10.4103/0970-2113.80324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roch A., Guervilly C., Papazian L. Fluid management in acute lung injury and ards. Ann Intensive Care. 2011;1(1) doi: 10.1186/2110-5820-1-16. 16-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.