Graphical abstract
Keywords: Cryptosporidiosis, Weight, Calves, Production impact
Highlights
-
•
Cryptosporidium parvum is a major cause of enteric disease in neonatal calves.
-
•
Clinically severe cryptosporidiosis in neonatal calves significantly reduces weight gain.
-
•
The impact of reduced weight gain in calves is seen over a 6 month period.
Abstract
Cryptosporidiosis can have a devastating effect in neonatal calves, resulting in diarrhoea, dehydration and, in severe cases, death of the animal. The disease is caused by Cryptosporidium spp. and is one of the most common causes of calf enteritis in the UK. The parasite is very difficult to remove from the farm, as the oocysts have a tough outer wall which enables the parasite to survive for several months in moist temperate environmental conditions and it is difficult to kill oocysts with common disinfectants used on a farm. If appropriate management practises are applied, the disease is usually self-limiting and most calves will recover. It has been shown, in studies with children and in lambs, that severe clinical cryptosporidiosis can result in long-term growth and cognitive impairment compared with individuals with no obvious signs of the disease. This study measured the long-term growth rate of beef calves on farm by comparing groups of animals that had suffered differing degrees of clinical severity of cryptosporidiosis as neonates. A group of 27 beef calves were enrolled in the study and monitored from birth to 6 months of age. The calves were scored for severity of cryptosporidiosis and weighed at regular intervals. The average difference in weight gain, at 6 months, between a group of calves that had severe cryptosporidiosis as neonates and a group of calves with no clinical signs of infection was 34 kg. Those calves that had experienced severe cryptosporidiosis as neonates showed a significantly reduced live weight gain compared with those calves showing no clinical signs of infection (P = 0.034). Therefore, the impact of severe cryptosporidiosis in neonatal calves has longer term effects on weight gain and production efficiency, resulting in the parasite having a greater impact on cattle production than previously thought.
1. Introduction
Cryptosporidiosis in calves, most commonly caused by the apicomplexan parasite Cryptosporidium parvum, is an important cause of enteric disease in neonatal and pre-weaned calves under 6 weeks of age (Thomson et al., 2017). The parasite is the most commonly diagnosed pathogen found in diarrheic neonatal calves in both beef and dairy systems in the UK (www.gov.uk/government/collections/animal-disease-surveillance-reports) and in many other countries worldwide (Wang et al., 2017). Clinical signs include watery and sometimes bloody diarrhoea, nutrient malabsorption, dehydration and, in severe cases, mortality (Naciri et al., 1999). The economic impact of enteric disease has been estimated to cost in the region of GBP 32 per affected calf (Gunn and Stott, 1997) and approximately GBP 11 million annually in UK (Bennet and Ijpelaar, 2005), although no figures are currently available on the economic impact of Cryptosporidium specifically. The parasite is difficult to remove from the environment as the oocysts have a very tough outer shell, enabling the parasite to survive for long periods of time in the environment, withstand a wide range of temperatures (−22 °C to 60 °C) (Robertson et al., 1992), and resist the effects of many of the commonly used farm disinfectants (Casemore, 1990). The infectious dose for neonatal calves can be as low as 17 oocysts (Zambrinski et al., 2013), making it very difficult to control exposure to the parasite on the farm. The disease is normally self-limiting in neonatal calves where sufficient colostral antibodies have been absorbed, and animals are kept warm and dry with supportive treatments administered where required, and when there are no co-infections with other gastrointestinal pathogens (Thomson et al., 2017). Currently there are no vaccines available and only two licenced products for the prevention and treatment of cryptosporidiosis in calves in the UK (Grinberg et al., 2002, Innes et al., 2011, Trotz-Williams et al., 2011).
Studies looking at the impact of severe cryptosporidiosis in very young children has shown that infection with the parasite can impair growth (Checkley et al., 1998, Guerrant et al., 1999, Kotloff et al., 2013). A review of the Global Burden of Diseases, Injuries and Risk Factors study (GBD; http://www.healthdata.org/gbd), together with previously published and unpublished data, found that diarrhoea caused by cryptosporidiosis was associated with a decrease in average height-for-age, weight-for-age and weight-for-height in children younger than 5 years old (Khalil et al., 2018). This study also estimated that cryptosporidiosis was responsible for an additional 7.85 million disability adjusted life years (DALYs).
This pattern of long-term growth effects due to cryptosporidiosis have also been seen in a study conducted in lambs on extensive sheep farms in Australia, where lambs were sampled from 2 to 6 weeks of birth on five occasions up to slaughter at 7–8 months (Sweeny et al., 2011). Lambs positive for Cryptosporidium in faecal samples on at least one occasion over the study period had significantly reduced carcass weight and dressing percentage (Sweeny et al., 2011). A further study, also conducted on lambs, found that animals positive for Cryptosporidium were between 2.31 and 4.52 kg lighter over three sampling occasions at 12, 19 and 29 weeks of age and showed both a reduced carcass weight and dressing percentage (Jacobson et al., 2016).
It has been shown that diarrhoea in neonatal calves increases the risk of mortality (Gulliksen et al., 2009) and reduces both growth rate and carcass quality (Pardon et al., 2013). Very little is currently known about the extent to which calves with diarrheal disease recover and experience catch up growth with their healthy cohorts. A study in dairy calves in New York state, USA, did not find a significant effect on growth when calves that had experienced enteric disease were followed for a 3 month period (Virtala et al., 1996). A further study examining intestinal functions in calves following infection with C. parvum, found that all functions except retinyl-palmitate absorption were significantly reduced at day 14 p.i. (Klein et al., 2008). Infected calves showed reduced intestinal absorptive capacity and a high elevation of intestinal permeability with intestinal recovery observed by day 21 p.i. and infected calves showed a reduced growth rate over this period (Klein et al., 2008). A further study conducted in Brazil monitored calves infected with Cryptosporidium spp. from birth to 7 months of age and found an association of infection with lower live weight gain and poorer production performance (Bueno da Silva et al., 2019).
Due to the lack of specific data, and some conflicting reports, on Cryptosporidium infection and its potential effect on the long-term growth of calves, the aim of this study was to address this knowledge gap and provide data to help evaluate the impact of cryptosporidiosis on the efficiency of beef production.
2. Materials and methods
2.1. Animals and farm history
Twenty-seven Limousin cross Belgian Blue calves were scored for severity of cryptosporidiosis every second day from their birth until they reached 16 days of age. This study was an observational study which took place during the spring calving of 2017 on a commercial beef suckler farm in Scotland, where there was a history of clinical cryptosporidiosis in neonatal calves. The calves were housed individually with their mother until at least 48 h of age and the calf had been visualised suckling. Afterwards, they were housed in group pens with their mothers. Group housed calves included both males and females ranging from 48 h to 3 months of age. At 3 months, adult cattle and their calves were moved to pasture. Preliminary visits and an interview with the farmer confirmed that calves commonly showed signs of cryptosporidiosis between 6–10 days old. These calves were naturally infected, thus the infectious dose was unknown. This farm had no historical veterinary diagnoses of other gastrointestinal pathogens (Rotavirus, coronavirus, Escherichia coli or Salmonella) in the calves, making it a suitable candidate to examine Cryptosporidium as the main cause of enteritis in a natural farm setting. Calf health was assessed by the farmer, who consulted vets when required. Severely affected calves were treated with HALOCUR® and rehydration therapy.
2.2. Scoring severity of cryptosporidiosis
The scoring system used to assess the severity of cryptosporidiosis in the calves is described in Table 1 and includes faecal consistency and demeanour. Calves were scored for a period of 16 days to cover the time period most calves would show signs of cryptosporidiosis. A daily score was attributed to each animal by multiplying the faecal consistency score by a factor of 2 and adding the demeanour score. An overall score was assigned after the 16-day period by taking an average of the worst daily score, the preceding score, and the following score; this score then providing an indication of disease duration (G. Innocent, BiOSS, Personal Communication).
Table 1.
Scoring system for severity of cryptosporidiosis in calves.
| Scoring factor | Description | Score |
|---|---|---|
| Faecal consistency | Firm | 0 |
| Semi-formed | 1 | |
| Loose but stays on top of the bedding | 2 | |
| Loose and sifts through the bedding | 3 | |
| Demeanour | Standing, happy to rise, ears and eyes normal | 0 |
| Standing, happy to rise Suffering one or more of: lethargic, ear droop, licked back |
1 | |
| As above including hunched over, head down | 2 | |
| Reluctant to rise with one or more of: lethargic, ear droop, licked back | 3 | |
| As above including hunched over, head down | 4 | |
| Unable to rise, lethargic, sunken eyes, ear droop | 5 | |
Overall clinical severity scores were used to assign each animal into one of three groups:
-
1.
High: severe clinical signs included animals that had severe diarrhoea (score 2 or 3 in Table 1) for three or more days with a poor demeanour (score 3 or over in Table 1). An overall score of 5–7 was assigned to indicate high severity of disease.
-
2.
Medium: mid-range clinical signs included animals that had severe diarrhoea (score 2 or 3 in Table 1) for less than 3 days and a demeanour score of 0 or 1. An overall score of 2–4 was assigned to indicate medium disease severity.
-
3.
Low: low clinical signs included animals that had no diarrhoea, although they may have had a demeanour score of 1 on two occasions or less. An overall score of 0–1 was assigned to indicate low disease severity.
2.3. Calf weights
All calves on the farm were weighed at birth using a small scale designed for sheep (IAE Ltd, UK) and then at 3, 4 and 6 months of age using an aluminium cattle platform (Allied Weighing, UK, http://www.alliedweighing.co.uk). Scales were calibrated at the start of each weighing session and weighed calves in units of 0.5 kg.
2.4. Diagnosis and genotyping of Cryptosporidium
All calves were tested for Cryptosporidium between 3 and 6 days of age which is the reported age when calves typically start to shed C. parvum (Dinler and Ulutas, 2017). Cryptosporidium spp. were identified using a multiplex PCR (Thomson et al., 2016), amplifying the 18S region, including specific primers for species commonly found in cattle, namely C. parvum, Cryptosporidium bovis, Cryptosporidium ryanae and Cryptosporidium andersoni. Any C. parvum-positive samples were genotyped at the gp60 gene and loci MM5, MM18, MM19 and TP14 (Morrison et al., 2008, Hotchkiss et al., 2015) to determine the multilocus genotypes of the affected calves.
During this 6 month period, animal health was monitored to ensure there were no other concurrent infections that might cause enteritis. All 27 calves had faecal samples tested for Cryptosporidium, Coronavirus, Rotavirus and E. coli F5 (K99) using the EXPERTISTM Rainbow calf scour diagnostic kit (MSD Animal Health, UK).
2.5. Statistical analysis
Data were analysed using Minitab (19.2.0.0, US) using a one-way ANOVA and post-hoc Tukey test. A normality test (Kolmogorov–Smirnov) confirmed the data to be parametric. Mean weight gain was calculated by subtracting mean birth weight from mean 6 month weight for each severity group. P values of 0.05 or less were considered significant.
3. Results
3.1. Clinical scoring of calves
All calves were assigned a faecal score and a demeanour score every second day until they reached 16 days of age, or 20 days of age if diarrhoea was present at day 16, until two consecutive zero scores were obtained. Therefore, each animal was scored between 8 and 10 times. All animals in this study tested negative on all occasions for Coronavirus, Rotavirus and E. coli F5 (K99).
Twenty-seven calves were placed into one of three groups based on the scoring system described in the section 2 (see Table 1). The split between the three groups was eight calves with a severe infection, 10 with a mid-range infection and nine with no signs of clinical cryptosporidiosis (Table 2 ). The average score for severely infected, mid-range infection and no clinical disease was 5.8, 3.4 and 0.2, respectively.
Table 2.
Calf overall scores and severity group to which they were assigned.
| Calf ID | Overall score | Severity group |
|---|---|---|
| 1 | 7 | High |
| 2 | 6 | High |
| 3 | 6 | High |
| 4 | 6 | High |
| 5 | 6 | High |
| 6 | 5 | High |
| 7 | 5 | High |
| 8 | 5 | High |
| 9 | 4 | Medium |
| 10 | 4 | Medium |
| 11 | 4 | Medium |
| 12 | 4 | Medium |
| 13 | 3 | Medium |
| 14 | 3 | Medium |
| 15 | 3 | Medium |
| 16 | 3 | Medium |
| 17 | 3 | Medium |
| 18 | 3 | Medium |
| 19 | 1 | Low |
| 20 | 1 | Low |
| 21 | 0 | Low |
| 22 | 0 | Low |
| 23 | 0 | Low |
| 24 | 0 | Low |
| 25 | 0 | Low |
| 26 | 0 | Low |
| 27 | 0 | Low |
3.2. Weight of calves over 6 month period
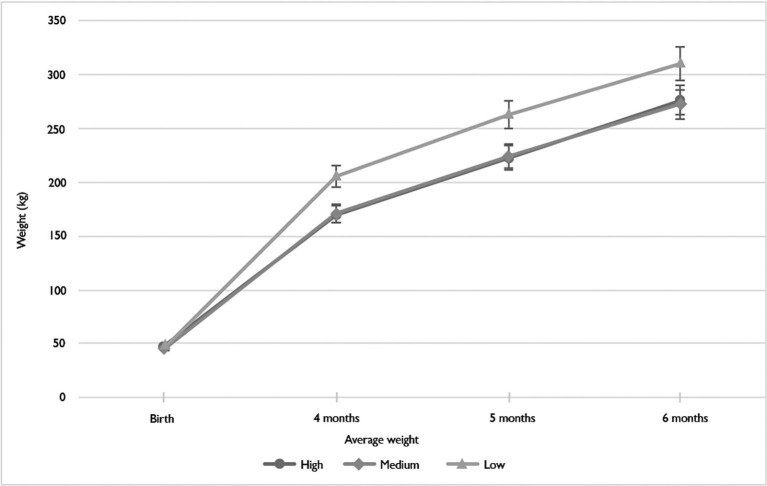
The mean weights of calves at birth and at 4, 5 and 6 months of age were calculated for each of the severity groups and are shown in Table 1. Fig. 1 illustrates the average weight of the calves in each severity group, at each of the weighing time points. The results show a mean increase in weight gain for all three severity groups at each weighing point. There was a statistically significant difference in the weight between the different groups with different severity levels of cryptosporidiosis at 6 months of age as determined by one-way ANOVA (F(2,25) = 3.89, P = 0.034). A Tukey post-hoc test (see Table 3 ) revealed that there was a significant difference between the high and low severity groups, but not between the medium severity and the other two groups. There was also a significant difference between the weight gain from birth to 6 months of age between the different cryptosporidiosis severity groups (F(2,25) = 4.20, P = 0.028). A significant difference was found between the high and low groups, but no difference was found between the medium and the other two groups. Fig. 1 illustrates that the calves in the high and medium severity groups do not demonstrate any ‘catch-up’ growth in the first 6 months of life compared with those in the low severity group.
Fig. 1.
Average weights of calves over a 6 month period based on their cryptosporidiosis severity level. Error bars represent 95% confidence interval of the mean.
Table 3.
Mean weights of calves in each disease severity group at birth, 4 months, 5 months and 6 months with mean weight gains.
| Severity group | Mean birth weight (kg) | Mean weight 4 months (kg) | Mean weight 5 months (kg) | Mean weight 6 months (kg) | Mean weight gain (6 months – birth (kg) | S.D. Mean weight (6 months) | Tukey grouping |
|---|---|---|---|---|---|---|---|
| High | 48.3 | 170.3 | 222.6 | 276.1 | 227.8 | 21.4 | A |
| Medium | 45.5 | 170.6 | 224.3 | 272.3 | 226.7 | 47.5 | AB |
| Low | 46.5 | 205.1 | 262.6 | 310.1 | 263.6 | 21.6 | B |
A and B are post-hoc pairwise comparison groups. As the mean of the high group is significantly different from that of the low group, they have been assigned different Tukey groups (A and B). However as the mean of the medium group is not significantly different from either the high or low group, it has been assigned as AB.
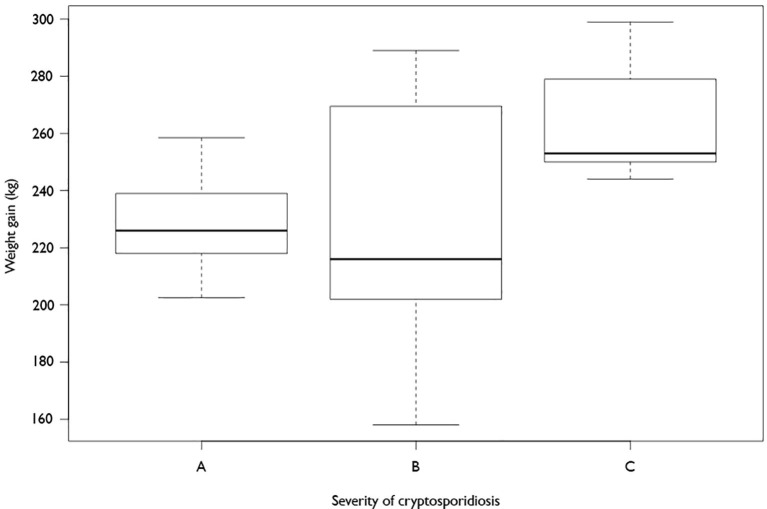
The weight gain from birth to 6 months of age in calves according to their differing levels of severity of cryptosporidiosis, assigned to them during the first 16 days of life, can be seen in Fig. 2 , which takes the birth weight of each calf into account when calculating weight gain. Those calves with the highest severity of disease as neonates show the lowest weight gain after 6 months, being significantly lighter (P = 0.034) by 34 kg compared with those calves in the low severity group. Those animals which were in the medium group showed no statistically significant difference compared with the other two groups. Fig. 2 shows that the weight gain in animals in the medium severity group spanned a wide range of scores, but the data spread indicates that there were no outliers which could skew the average weight gain shown in Fig. 1.
Fig. 2.
Comparison of weight gain from birth to 6 months in calves with different levels of cryptosporidiosis. (A) Severe clinical disease. (B) Mid-range disease. (C) Low clinical disease. The rectangle represents the second and third quartiles, the horizontal line inside indicates the median value and the lower and upper quartiles are shown as vertical lines either side of the rectangle.
3.3. Cryptosporidium diagnosis
Twenty-seven calves (100%) which were scored in the first 16–20 days of life tested positive for C. parvum only. Of those, 24 were successfully genotyped using markers gp60, MM5, MM18, MM19 and TP14 (Table 4 ). All calves were infected with multilocus genotype MLG 10, as described in Hotchkiss et al. (2015), and one calf had a mixed infection with MLG 10 and a different genotype, which was picked up through amplification of the MM5 locus (Calf no 20).
Table 4.
Cryptosporidium parvum genotypes in calves.
| Calf ID | GP60 | MM5 | MM18 | MM19 | TP14 | MLG |
|---|---|---|---|---|---|---|
| 1–19 and 21–24 | IIaA17G1R1 | 235 bp | 288 bp | 292 bp | 296 bp | 10 |
| 20 | IIaA17G1R1 | 235 bp + 225 bp | 288 bp | 292 bp | 296 bp | 10 |
GP60, hyper-variable 60 kDa glycoprotein gene; MM5, MM18, MM19 and TP14, microsatellite markers, MLG, multilocus genotype.
4. Discussion
Previously, it was unclear whether or not infection with Cryptosporidium could have a long term production effect in calves, with contrasting results in the very few published studies available. The findings from this study indicate that severe clinical cryptosporidiosis at a young age significantly reduces the long-term growth rate, which concurs with some studies looking at the effect of diarrhoea (Pardon et al., 2013) and the short-term or longer term effect of cryptosporidiosis (Klein et al., 2008, Da Silva et al., 2019) on growth rate in calves. The current study relates cryptosporidiosis to economic losses and is one of the first papers to describe the long-term effects of cryptosporidiosis on calf growth. Cryptosporidium infection has a direct impact on growth rates in calves during the acute infection, as shown by Klein et al. (2008) but this paper monitored the calves for only 28 days and therefore did not determine whether there was any long-term weight reduction in calves due to the infection. The study described here clearly shows that the weight lost during the acute infection in neonatal calves is not regained during the subsequent 6 months, indicating that cryptosporidiosis may have a much more significant economic impact to the cattle industry than was previously thought. To date only one other study (Bueno da Silva et al., 2019) has reported long-term growth effects due to cryptosporidiosis in neonatal calves, therefore the data presented in the current study is highly significant. There are also key differences in the study designs between the two long-term growth studies, which are important to highlight. The study reported by Bueno da Silva et al. (2019) was carried out in Brazil on an experimental farm, using a more subjective method of condition scoring to determine the effect of cryptosporidiosis on calf growth. The current study is, to our knowledge, the first one to measure long-term growth effects on a beef suckler commercial farm in Scotland and measured calf growth by weight from birth to 6 months of age. This therefore provides an objective data set, enabling economic analysis of disease impact. In this paper, a scoring system of taking an average of the worst score, the preceding and following score was used, which was more accurate than an average score of 16 days where many of the initial and final scores would have been likely to total zero. This method ensured exclusion of milk scour, which neonatal calves may have in a single bout of diarrhoea, confirming the focus was on more significant clinical disease. This method therefore captured the severity of the disease by including only the disease timeframe (G. Innocent, BiOSS, Personal Communication). In addition, Bueno da Silva et al. (2019) used microscopy to determine oocyst concentration, whereas the current study used 18S nested species-specific PCR (Thomson et al., 2016), allowing speciation of Cryptosporidium and determination that the oocysts present were C. parvum.
The results from this study are in contrast to a study looking at growth rates in dairy calves with and without diarrhoea in New York (Virtala et al., 1996), which found no significant difference in growth rates between the two groups of calves. This particular study did not identify Cryptosporidium as the main cause of the diarrhoea, so it could be that it was not the main causative agent. Although the current study focused on a single farm, there were several advantages to this. This farm practised good biosecurity and attention to detail regarding calf health. Climatic regional patterns meant that the farm calved indoors in the spring and retained cows and calves inside until weather and grass availability allowed turnout, usually 3 months post-calving. The long period of time that the cattle spent indoors meant that the farm had a history of problems with cryptosporidiosis. Cryptosporidium was identified as being the sole diagnosed cause of the diarrhoea observed in the calves. Gastro-intestinal pathogens such as Rotavirus and Coronavirus are considered to be widespread in calves (Cho and Yoon, 2014), however in the study presented here none of the calves tested positive for Rotavirus, Coronavirus or E. coli F5 (K99). As the calves were on a single farm, a similar grazing and management scheme was followed for all groups, giving confidence that the weight effect seen was due to the initial Cryptosporidium infection.
A critical factor in health and resilience of neonatal calves is to ensure that they receive adequate quantities of good quality colostrum in the first few hours of life (Megank et al., 2014) and this is likely to have a significant influence on the ability of the calf to resist disease following infection with Cryptosporidium spp. parasites. In the study all calves were observed to suckle during the first 24–48 h after birth but no data on colostrum quality and absorption by the calves was obtained.
The average cattle prices at Scottish abattoirs according to the Quality Meat Scotland market report (2018) is GBP 3.77 per kg. Therefore an average difference of 34 kg in animal weight at 6 months of age means an animal with severe cryptosporidiosis as a neonate, could result in an average loss of approximately GBP 128.18 per animal to the farmer in direct sales. There are also the potential increased costs in extra feeding and husbandry of animals that have been affected by clinical cryptosporidiosis to get them to their market weight. Although no treatment intervention costs were applied in this study, there are likely to be additional costs to farmers in dealing with severe cryptosporidiosis including veterinary costs, diagnostics and the purchase and application of licenced therapeutics.
Factors involved in determining the clinical severity of Cryptosporidium infection will involve both host and parasite aspects (Thomson et al., 2017). These may include the dose of the parasites that the calf receives (Zambrinski et al., 2013); the immune resilience of the calves; the uptake and quality of the colostrum (Megank et al., 2014); the nutritional status of the calf; the virulence of the parasite (Bouzid et al., 2013) and the occurrence of co-infections in the calves (Cho and Yoon, 2014).
Cryptosporidium parasites have the ability to survive many commonly used farm disinfectants and temperature extremes from −22 °C to 60 °C (Casemore, 1990, Fujino et al., 2002), thus it is likely that a high proportion of animals would be exposed to the infection if the parasite is present on the farm.
The multilocus genotype of C. parvum was consistent in all calves with little evidence of mixed infections. This predominant genotype present in all the calves in this study shows that this was the consistent Cryptosporidium challenge on this farm and that the differences in the clinical severity observed in the calves in the study may reflect differences in calf resilience and immunity, or reflect the different doses of parasite that the calves were exposed to. The infectious dose that each calf received was unknown as calves were studied in a natural farm setting. It is known that clinically affected calves can shed very high numbers (1 × 1010) of infectious Cryptosporidium oocysts in their faeces (Nydam et al., 2001) and further experimental studies have shown that it only requires 17 oocysts to cause clinical infection in neonatal calves (Zambrinski et al., 2013). The use of individual pens for calving on beef farms could lead to a build-up of Cryptosporidium oocysts and other pathogens which are shed by cows and their calves over the duration of the calving season, thereby exposing calves born later in the season to a higher infectious dose. This higher infectious dose would increase the likelihood of the calf developing diarrhoea (Blanchard, 2012). In Sweden it has been found that there is no association between C. parvum shedding and diarrhoea in calves and therefore there are more factors at play which affect severity of disease rather than oocyst shedding alone (Silverlas et al., 2010). Calf housing, frequency of cleaning and disinfectant used all have an effect on the prevalence of Cryptosporidium on farm (Castro-Hermida et al., 2002) however as this study was done on one farm with a single management regime, these are not the likely reason for the differences in severity of cryptosporidiosis.
In conclusion, this study has shown calves which are affected with severe cryptosporidiosis in the first 16 days of life have a significantly reduced weight gain over a 6 month period. On average, a calf with severe disease weighed 34 kg less than a calf which showed no clinical signs of cryptosporidiosis. The direct losses associated with this reduced weight gain related to sales is approximately GBP 130 per affected calf, however further costs such as increased feed and husbandry costs to get cattle to their market weights, additional labour involved in looking after sick calves, together with veterinary and treatment costs, make cryptosporidiosis a significant economic burden to the cattle industry. Management strategies to help reduce the impact of cryptosporidiosis should be applied to improve the health and welfare of cattle, increase production efficiency and reduce contamination of the farm environment with infectious Cryptosporidium oocysts.
Acknowledgements
This project was funded by the Agriculture and Horticulture Development Board (AHDB), England and the Scottish Government Rural and Environment Science and Analytical Services. The authors would like to thank Giles Innocent (BiOSS, UK), Darren Shaw (Roslin Institute, UK) Miriam Thavarajah and Nathan Thavarajah (University Centre Reaseheath, UK) for helpful advice on the statistical analysis, to the Moredun Foundation, UK, for help to arrange access to the farm used in the study, and especially to the farmer involved for all his valuable help and access to his cattle and farm. Roslin Institute is core-funded by the UK Biotechnology and Biological Sciences Research Council.
References
- Bennet R., Ijpelaar J. Updated estimates of the costs associated with thirty four endemic livestock diseases in Great Britain: a note. J. Agric. Econ. 2005;56:135–144. [Google Scholar]
- Blanchard P.C. Diagnostics of dairy and beef cattle diarrhea. Vet. Clin. N. America: Food Anim. Prac. 2012;28:443–464. doi: 10.1016/j.cvfa.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzid M., Hunter P.R., Chalmers R.M., Tyler K.M. Cryptosporidium pathogenicity and virulence. Clin. Microbiol. Rev. 2013;26:115–134. doi: 10.1128/CMR.00076-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casemore D.P. Epidemiological aspects of human cryptosporidiosis. Epidemiol. Infect. 1990;104:1–28. doi: 10.1017/s0950268800054480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Hermida J.A., González-Losada Y.A., Ares-Mazás E. Prevalence of and risk factors involved in the spread of neonatal bovine cryptosporidiosis in Galicia (NW Spain) Vet. Parasitol. 2002;106:1–10. doi: 10.1016/S0304-4017(02)00036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checkley W., Epstein L.D., Gilman R.H., Black R.E., Cabrera L., Sterling C.R. Effects of Cryptosporidium parvum infection in Peruvian children: growth faltering and subsequent catch-up growth. Am. J. Epidemiol. 1998;148:497–506. doi: 10.1093/oxfordjournals.aje.a009675. [DOI] [PubMed] [Google Scholar]
- Cho Y.I., Yoon K.J. An overview of calf diarrhoea: infectious etiology, diagnosis and intervention. J. Vet. Sci. 2014;15:1–17. doi: 10.4142/jvs.2014.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva B.A., Pires L., Santos K., Luz C., Oliviera M., Severino J. Occurance of Cryptosporidium spp. and its association with ponderal development and diarrhoea episodes in Nellore mixed breed cattle. Acta. Vet. Bras. 2019;13:24–29. [Google Scholar]
- Dinler C., Ulutas B. Cryptosporidiosis in ruminants: uptake and current therapeutic approaches. Am. J. Anim. Vet. Sci. 2017;12:96–103. [Google Scholar]
- Fujino T., Matsui T., Kobayashi F., Haruki K., Yoshino Y., Kajima J., Tsuji M. The effect of heating against Cryptosporidium oocysts. J. Vet. Med. Sci. 2002;64:199–200. doi: 10.1292/jvms.64.199. [DOI] [PubMed] [Google Scholar]
- Grinberg A., Markovics A., Galindez J., Lopez-Villalohos N., Kosak A., Tranquillo V.M. Controlling the onset of natural cryptosporidiosis in calves with paramomycin sulphate. Vet. Rec. 2002;151:606–608. doi: 10.1136/vr.151.20.606. [DOI] [PubMed] [Google Scholar]
- Guerrant D.I., Moore S.R., Lima A.A., Patrick P.D., Schorling J.B., Guerrant R.L. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four-seven years later in a poor urban community in northeast Brazil. Am. J. Trop. Med. Hyg. 1999;61:707–713. doi: 10.4269/ajtmh.1999.61.707. [DOI] [PubMed] [Google Scholar]
- Gulliksen S.M., Lie K.I., Loken T., Osteras O. Calf mortality in Norwegian dairy herds. J Dairy Sci. 2009;92:2782–2795. doi: 10.3168/jds.2008-1807. [DOI] [PubMed] [Google Scholar]
- Gunn G., Stott A. A comparison of economic losses due to calf enteritis and calf pneumonia in northern Scotland. Epidemiologie et Sante Animal. 1997:31–32. [Google Scholar]
- Hotchkiss E.J., Gilray J.A., Brennan M.L., Christley R.M., Morrison L.J., Jonsson N.N., Innes E.A., Katzer F. Development of a framework for genotyping bovine-derived Cryptosporidium parvum, using a multilocus fragment typing tool. Parasit. Vec. 2015;8:500. doi: 10.1186/s13071-015-1107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes E.A., Bartley P.M., Rocchi M., Benevides-Silvan J., Burrells A., Hotchkiss E., Chianini F., Canton G., Katzer F. Developing vaccines to control protozoan parasites in ruminants: dead or alive? Vet. Parasitol. 2011;180:155–163. doi: 10.1016/j.vetpar.2011.05.036. [DOI] [PubMed] [Google Scholar]
- Jacobson C., Williams A., Yang R., Ryan U., Carmichael I., Campbell A.J., Gardner G.E. Greater intensity and frequency of Cryptosporidium and Giardia oocyst shedding beyond the neonatal period is associated with reductions in growth, carcase weight and dressing efficiency in sheep. Vet. Parasitol. 2016;228:42–51. doi: 10.1016/j.vetpar.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Khalil I.A., Troeger C., Rao P.C., Blacker B.F., Brown A., Brewer T.G., Colombara D.V., DeHostos E.L., Engmann C., Guerrant R.L., Haque R., Houpt E.R., Kamg G., Korpe P.S., Kotloff K., Lima A.A.M., Petri W.A., Platt-Mills J.A., Shoultz D.A., Forouzanfor M.H., Hay S.I., Reiner R.C., Mokdal A.H. Morbidity, mortality and long term consequences associated with diarrhoea from Cryptosporidium infection in children younger than 5 years: a meta-analysis study. Lancet Glob. Health. 2018;6:758–768. doi: 10.1016/S2214-109X(18)30283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P., Kleinova T., Volek Z., Simunek J. Effect of Cryptosporidium parvum infection on the absorptive capacity and paracellular permeability of the small intestine in neonatal calves. Vet. Parasitol. 2008;152:53–59. doi: 10.1016/j.vetpar.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloff K.L., Nataro J.P., Blackwelder W.C., Nasrin D., Faraq T.H., Panchalingam S., Wu Y., Sow S.O., Sur D., Breiman R.F., Faraque A.S.G., Zaidi A.K.M., Saha D., Alonso P.L., Tamboura B., Sanago D., Onwuchekwa U., Manna B., Ramamurthy T., Kanugo S., Ochieng J.B., Omore R., Oundo J.O., Hussain A., Dag S.K., Ahmed S., Qureshi S., Quadri F., Adegbda A., Antonio M., Hassain M.J., Akinsala A., Mandomando I., Nhampossa T., Acacio S., Biswas K., O’Reilly C.E., Mintz E.D., Berkely L.Y., Muhsen K., Sommorfelt H., Robins-Browne R.M., Levine M.M. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study GEMS): a prospective, case control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- Megank V., Hoflack G., Opsomer G. Advances in prevention and therapy of neonatal dairy calf diarrhoea: a systematical review with emphasis on colostrum management and fluid therapy. Acta. Vet. Scand. 2014;56:75. doi: 10.1186/s13028-014-0075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison L.J., Mallon M.E., Smith H.V., Macleod A., Xiao L., Tait A. The population structure of Cryptosporidium parvum in Scotland: a complex picture. Infect. Genet. Evol. 2008;8:121–129. doi: 10.1016/j.meegid.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naciri M., Paul Lefay M., Mancassola R., Poirier P., Chermette R. Role of Cryptosporidium parvum as a pathogen in neonatal diarrhoea complex in suckling and dairy calves in France. Vet. Parasitol. 1999;85:245–257. doi: 10.1016/S0304-4017(99)00111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nydam D.V., Wade S.E., Schaaf S.L., Mohammad H.O. Number of Cryptosporidium parvum oocysts or Giardia spp. cysts shed by dairy calves after natural infection. Am. J. Vet. Res. 2001;62:1612–1615. doi: 10.2460/ajvr.2001.62.1612. [DOI] [PubMed] [Google Scholar]
- Pardon B., Hostens M., Duchateau L., Dewulf J., De Bleecker K., Deprez P. Impact of respiratory disease, diarrhea, otitis and arthritis on mortality and carcass traits in white veal calves. BMC Vet. Res. 2013;9:79. doi: 10.1186/1746-6148-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson L.J., Campbell A.T., Smith H.V. Survival of Cryptosporidium parvum oocysts under various environmental pressures. Appl. Environ. Microbiol. 1992;58:3494–3500. doi: 10.1128/aem.58.11.3494-3500.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverlas C., Naslund K., Björkman C., Mattsson J.G. Molecular characterisation of Cryptosporidium isolates from Swedish dairy cattle in relation to age, diarrhoea and region. Vet. Parasitol. 2010;169:289–295. doi: 10.1016/j.vetpar.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Sweeny J.P., Ryan U.M., Robertson I.D., Jacobson C. Cryptosporidium and Giardia associated with reduced lamb carcase productivity. Vet. Parasitol. 2011;182:127–139. doi: 10.1016/j.vetpar.2011.05.050. [DOI] [PubMed] [Google Scholar]
- Thomson S., Hamilton C.A., Hope J.C., Katzer F., Mabbott N.A., Morrison L.J., Innes E.A. Bovine cryptosporidiosis: impact, host-parasite interaction and control strategies. Vet. Res. 2017;48:42. doi: 10.1186/s13567-017-0447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson S., Innes E.A., Jonsson N.N., Katzer F. A multiplex PCR test to identify four common cattle adapted Cryptosporidium species. Parasitol. Open. 2016;2:E5. doi: 10.1017/pao.2016.2. [DOI] [Google Scholar]
- Trotz-Williams I.A., Jarvie B.D., Peregrine A.S., Duffield T.F., Leslie K.E. Efficacy of halofuginone lactate in the prevention of cryptosporidiosis in dairy calves. Vet. Rec. 2011;168:509. doi: 10.1136/vr.d1492. [DOI] [PubMed] [Google Scholar]
- Virtala A.M., Mechor G.D., Grohn Y.T., Erb H.N. The effect of calfhood diseases on growth of female dairy calves during the first 3 months of life in New York State. J. Dairy Sci. 1996;79:1040–1049. doi: 10.3168/jds.S0022-0302(96)76457-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Zhao G., Gong Y., Zhang L. Advances and perspectives on the epidemiology of bovine cryptosporidium in China in the past 30 years. Front. Microbiol. 2017;8(1823):1–6. doi: 10.3389/fmicb.2017.01823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrinski J.A., Nydam D.V., Wilcox Z.J., Bowman D.D., Mohammed H.O., Liotta J.L. Cryptosporidium parvum: determination of ID50 and the dose-response relationship in experimentally challenged dairy calves. Vet. Parasitol. 2013;197:104–112. doi: 10.1016/j.vetpar.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]