Highlights
-
•
In post-transplantation cyclophosphamide-based allogeneic hematopoietic stem cell transplantation, graft cryopreservation was not associated with significantly higher mortality.
-
•
Cryopreserved grafts were not associated with significantly delayed hematopoietic recovery or increased risk of acute graft-versus-host disease (GVHD).
-
•
Cryopreserved grafts were associated with lower risk of chronic GVHD and inferior disease-free survival, but these differences were of only borderline statistical significance.
Key Words: Cryopreservation, ptCY, Allogeneic hematopoietic stem cell transplantation, COVID-19
Abstract
The COVID-19 pandemic has created significant barriers to timely donor evaluation, cell collection, and graft transport for allogeneic hematopoietic stem cell transplantation (allo-HCT). To ensure availability of donor cells on the scheduled date of infusion, many sites now collect cryopreserved grafts before the start of pretransplantation conditioning. Post-transplantation cyclophosphamide (ptCY) is an increasingly used approach for graft-versus-host disease (GVHD) prophylaxis, but the impact of graft cryopreservation on the outcomes of allo-HCT using ptCY is not known. Using the Center for International Blood and Marrow Transplant Research (CIBMTR) database, we compared the outcomes of HCT using cryopreserved versus fresh grafts in patients undergoing HCT for hematologic malignancy with ptCY. We analyzed 274 patients with hematologic malignancy undergoing allo-HCT between 2013 and 2018 with cryopreserved grafts and ptCY. Eighteen patients received bone marrow grafts and 256 received peripheral blood stem cell grafts. These patients were matched for age, graft type, disease risk index (DRI), and propensity score with 1080 patients who underwent allo-HCT with fresh grafts. The propensity score, which is an assessment of the likelihood of receiving a fresh graft versus a cryopreserved graft, was calculated using logistic regression to account for the following: disease histology, Karnofsky Performance Score (KPS), HCT Comorbidity Index, conditioning regimen intensity, donor type, and recipient race. The primary endpoint was overall survival (OS). Secondary endpoints included acute and chronic graft-versus-host disease (GVHD), non-relapse mortality (NRM), relapse/progression and disease-free survival (DFS). Because of multiple comparisons, only P values <.01 were considered statistically significant. The 2 cohorts (cryopreserved and fresh) were similar in terms of patient age, KPS, diagnosis, DRI, HCT-CI, donor/graft source, and conditioning intensity. One-year probabilities of OS were 71.1% (95% confidence interval [CI], 68.3% to 73.8%) with fresh grafts and 70.3% (95% CI, 64.6% to 75.7%) with cryopreserved grafts (P = .81). Corresponding probabilities of OS at 2 years were 60.6% (95% CI, 57.3% to 63.8%) and 58.7% (95% CI, 51.9% to 65.4%) (P = .62). In matched-pair regression analysis, graft cryopreservation was not associated with a significantly higher risk of mortality (hazard ratio [HR] for cryopreserved versus fresh, 1.05; 95% CI, .86 to 1.29; P = .60). Similarly, rates of neutrophil recovery (HR, .91; 95% CI, .80 to 1.02; P = .12), platelet recovery (HR, .88; 95% CI, .78 to 1.00; P = .05), grade III-IV acute GVHD (HR, .78; 95% CI, .50 to 1.22; P = .27), NRM (HR, 1.16; 95% CI, .86 to 1.55; P = .32) and relapse/progression (HR, 1.21; 95% CI, .97 to 1.50; P = .09) were similar with cryopreserved grafts versus fresh grafts. There were somewhat lower rates of chronic GVHD (HR, 78; 95% CI, .61 to .99; P = .04) and DFS (HR for treatment failure, 1.19; 95% CI, 1.01 to 1.29; P = .04) with graft cryopreservation that were of marginal statistical significance after adjusting for multiple comparisons. Overall, our data indicate that graft cryopreservation does not significantly delay hematopoietic recovery, increase the risk of acute GVHD or NRM, or decrease OS after allo-HCT using ptCY.
INTRODUCTION
Donor hematopoietic stem and progenitor cells for allogeneic hematopoietic stem cell transplantation (allo-HCT) are generally collected and infused fresh (ie, without cryopreservation) [1]. Limited data in patients undergoing HLA-matched related donor (MRD) allo-HCT using bone marrow (BM) as the graft source suggest that cryopreservation of the harvested marrow product does not impact hematopoietic recovery or the risk of graft-versus-host disease (GVHD) 2, 3, 4, 5. Among recipients of peripheral blood (PB) allografts, some [6], but not all, studies [7] have reported delayed platelet recovery with cryopreserved grafts, but these studies have shown no impact of cryopreservation of PB allografts on neutrophil recovery, GVHD or survival outcomes.
The emergence of coronavirus disease 2019 (COVID-19) in Wuhan, China in December 2019 [8] and its rapid evolution into a pandemic not only has caused a serious healthcare crisis, but also has impacted the world economy and disrupted travel across international borders and within countries. These travel restrictions, combined with potentially reduced HCT donor availability (due to infection, quarantine, and constraints on travel to collection centers) and complex allograft processing logistics (ie, donor assessment, collection, and on-schedule delivery for fresh infusion), directly impact a transplantation center's ability to infuse fresh donor cells into intended recipients on the scheduled day of transplantation. Recognizing these challenges, both the American Society for Transplantation and Cellular Therapy (ASTCT) [9] and the National Marrow Donor Program (NMDP) [10] initially issued a strong recommendation that all unrelated donor (URD) products be delivered and cryopreserved at transplantation centers before initiation of patient conditioning. On March 23, 2020, the NMDP informed transplantation centers that starting on March 30, 2020, cryopreservation of URD grafts would be required before initiating conditioning in transplant recipients [10]. Many transplantation centers have instituted similar practices for HCT using cells from related donors.
Although published studies (all with limited patient numbers) suggest no significant impact of graft cryopreservation on outcomes of allo-HCT using conventional GVHD prophylaxis platforms (eg, calcineurin inhibitor-based), no data are available on whether this strategy is feasible for HCT using post-transplantation cyclophosphamide (ptCY)-based GVHD prophylaxis. Using the CIBMTR database, we evaluated the outcomes of patients undergoing ptCY-based allo-HCT for hematologic malignancies with either fresh or cryopreserved grafts, to inform clinical practice during the ongoing COVID-19 pandemic.
METHODS
Data Sources
The CIBMTR is a working group of more than 380 transplantation centers worldwide that contribute detailed data on HCT to a central coordinating center managed by the NMDP and the Medical College of Wisconsin. Participating centers are required to report all transplantations consecutively, and compliance is monitored by onsite audits. Computerized checks for discrepancies, physicians’ review of submitted data, and onsite audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. The NMDP’s Institutional Review Board, which is the board of record for the CIBMTR's database protocols, approved this study.
The CIBMTR collects data at 2 levels: transplant essential data (TED) and comprehensive report form (CRF) data. TED include disease type, age, sex, pre-HCT disease stage and chemotherapy responsiveness, date of diagnosis, graft type, conditioning regimen, post-transplantation disease progression and survival, development of a new malignancy, and cause of death. All CIBMTR centers contribute TED. More detailed disease and pretransplantation and post-transplantation clinical information is collected from a subset of registered patients selected for CRF data by a weighted randomization scheme. TED- and CRF-level data are collected pretransplantation, 100 days and 6 months post-transplantation, and annually thereafter or until death. Data for the current analysis were retrieved from CIBMTR (TED and CRF) report forms, considering all patients for whom a CRF 2006 form (collecting details of graft manipulation and composition) was submitted.
Patients
Included in this analysis are adults (age ≥18 years) undergoing allo-HCT between 2013 and 2018 for hematologic malignancies with ptCY (with or without calcineurin inhibitor and/or mycophenolate mofetil) as the GVHD prophylaxis. Diagnosis was limited to acute leukemias in first or second complete remission (CR1/CR2), chronic leukemias, or myelodysplastic syndrome (with <5% blasts at HCT) and lymphomas. Donors included MRDs, haploidentical related donors, matched URDs, and mismatched URDs. Umbilical cord blood grafts were not included because of universal cryopreservation.
Definitions and Study Endpoints
The primary study endpoint was overall survival (OS); death from any cause was considered an event and surviving patients were censored at last contact. Secondary endpoints included hematopoietic recovery, acute and chronic GVHD, non-relapse mortality (NRM), progression/relapse and disease-free survival (DFS). NRM was defined as death without evidence of disease relapse/progression; relapse was considered a competing risk. Relapse/progression was defined as morphologic, cytogenetic, or molecular disease recurrence for leukemias and myeloid malignancies and as progressive lymphoma after HCT or lymphoma recurrence after a CR; NRM was considered a competing risk. For DFS, a patient was considered a treatment failure at the time of relapse/progression or death from any cause. Patients alive without evidence of disease relapse or progression were censored at last follow-up.
Neutrophil recovery was defined as the first of 3 successive days with absolute neutrophil count ≥500/µL after the post-transplantation nadir. Platelet recovery was considered to have occurred on the first of 3 consecutive days with platelet count ≥20,000/µL in the absence of platelet transfusion for 7 consecutive days. For neutrophil and platelet recovery, death without the event was considered a competing risk. The intensity of allo-HCT conditioning regimens was categorized as myeloablative (MAC) or reduced-intensity/nonmyeloablative conditioning (RIC/NMA) using consensus criteria [11]. Disease Risk Index (DRI) was assigned as reported previously [12]. Acute GVHD [13] and chronic GVHD [14] were graded using standard criteria. For calculation of acute and chronic GVHD incidences, death without the event was considered a competing risk.
Statistical Analysis
A total of 277 patients were identified who met the aforementioned eligibility criteria and who received cryopreserved grafts and 4083 patients who met eligibility criteria and received fresh graft infusion. A mixed method of direct matching and propensity score matching was applied before the analyses to obtain a control groups with similar clinical characteristics. The propensity score is the probability of a given patient to receive the cryopreserved graft, based on that patient's observed covariates. The propensity score was predicted for each patient using logistic regression accounting for following risk factors: disease histology (acute myelogenous leukemia versus acute lymphocytic leukemia versus chronic myelogenous leukemia versus chronic lymphocytic leukemia versus myelodysplastic syndrome versus non-Hodgkin lymphoma versus Hodgkin lymphoma), Karnofsky Performance Score (KPS) (≥90% versus <90%), HCT-Comorbidity Index (HCT-CI) (0 versus 1 to 2 versus ≥3), conditioning intensity (MAC versus RIC/NMA), donor type (MRD versus haploidentical related donor versus 8/8 matched URD versus ≤7/8 URD) and recipient race. Two patients with equal propensity scores meant they had similar probabilities of receiving a cryopreserved graft. The distributions of estimated propensity scores between cryopreserved and fresh grafts were examined. We then matched each recipient of a cryopreserved graft with controls receiving fresh grafts, by matching on 4 covariates: graft type (BM versus PB), DRI (low risk versus intermediate risk versus high risk), recipient age (within 5 years), and propensity score (within 1 standard deviation from the pooled sample). The following procedure was adopted to find a maximum of 4 fresh graft controls for each cryopreserved graft case:
-
1.
Identify all potential matched controls for each case.
-
2.
Assign the control with the smallest age difference with the case. If there were multiple controls with the same age difference, assign one at random.
-
3.
Repeat steps 1 and 2 four times, to identify 4 controls.
We matched a total of 1080 controls to 274 cases, including 266 cases matched to 4 controls, 3 cases matched to 3 controls, 2 cases matched to 2 controls, and 3 cases matched to a single control. Three cases with no matched controls were excluded.
Patient-, disease-, and transplantation-related factors were compared between matched cases and controls using the chi-square test for categorical variables and the Mann-Whitney U test for continuous variables. The Kaplan-Meier estimator was used to evaluate the probability of OS and DFS [15]. Cumulative incidence rates were calculated for hematopoietic recovery, GVHD, NRM, and relapse while accounting for competing events [16]. The marginal Cox model was applied to evaluate the main treatment effect while adjusting for the potential correlation within each matched pair. The assumption of proportional hazards for the main risk factor (cryopreserved graft versus fresh graft) for each outcome was tested by adding a time-dependent covariate. Hazard ratios (HRs) with 95% confidence intervals (CIs) and P values were reported for each clinical outcome of interest, comparing the cryopreserved graft treatment group with the fresh graft group. Because of the large number of comparisons performed, only Pvalues <.01 were considered statistically significant a priori. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Baseline Characteristics
A total of 1354 patients were included in the analysis, of whom 1080 received fresh grafts and 274 received cryopreserved grafts. The baseline patient-, disease-, and transplantation-related characteristics are summarized in Table 1 . The 2 cohorts were similar in terms of median patient age, sex, race, KPS, diagnosis, DRI, HCT-CI, donor/graft source, conditioning intensity, and donor-recipient cytomegalovirus serostatus. Acute leukemias constituted the most common diagnoses, and haploidentical related donors were the most common donor source in both cohorts. BM was the graft source in only ~6% of the procedures. The median follow-up of survivors was 24 months (range, 3 to 77 months) in the fresh graft cohort and 23 months (range, 3 to 68 months) in the cryopreserved graft cohort.
Table 1.
Baseline Characteristics of the Propensity Score-Matched Patient Population (2013 to 2018)
| Characteristic | Fresh Grafts | Cryopreserved Grafts | P Value |
|---|---|---|---|
| Number of patients | 1080 | 274 | |
| Age at transplantation, yr, median (range) | 52 (19-80) | 55 (22-75) | .33 |
| Male sex, n (%) | 620 (57.4) | 163 (59.5) | .53 |
| Karnofsky performance score ≥90, n (%) | 536 (49.6) | 137 (50) | .71 |
| Not reported, n (%) | 13 (1.2) | 5 (1.8) | |
| Race, n (%) | .98 | ||
| Caucasian | 738 (68.3) | 187 (68.2) | |
| African-American | 205 (19) | 53 (19.3) | |
| Others | 66 (6.1) | 15 (5.5) | |
| Not reported | 71 (6.6) | 19 (6.9) | |
| Disease, n (%) | .66 | ||
| Acute myelogenous leukemia | 431 (39.9) | 107 (39.1) | |
| Acute lymphoblastic leukemia | 228 (21.1) | 55 (20.1) | |
| Chronic leukemias | 78 (7.2) | 28 (10.2) | |
| Myelodysplastic syndrome | 172 (15.9) | 46 (16.8) | |
| Lymphoma | 171 (15.9) | 38 (13.8) | |
| Disease risk index, n (%) | 1.00 | ||
| Low | 114 (10.6) | 29 (10.6) | |
| Intermediate | 715 (66.2) | 181 (66.1) | |
| High | 251 (23.2) | 64 (23.4) | |
| HCT-CI, n (%) | .17 | ||
| 0 | 192 (17.8) | 36 (13.1) | |
| 1-2 | 324 (30) | 90 (32.8) | |
| ≥3 | 564 (52.2) | 148 (54) | |
| Donor/recipient CMV serostatus, n (%) | .41 | ||
| -/+ | 289 (26.8) | 76 (27.7) | |
| Other combinations | 786 (72.8) | 197 (71.9) | |
| Not reported | 5 (.5) | 1 (.4) | |
| Conditioning intensity, n (%) | .80 | ||
| Myeloablative | 515 (47.7) | 133 (48.5) | |
| Reduced-intensity/nonmyeloablative | 565 (52.3) | 141 (51.5) | |
| Donor type, n (%) | .18 | ||
| Matched related donor | 152 (14.1) | 49 (17.9) | |
| Haploidentical related donor | 659 (61) | 169 (61.7) | |
| 8/8 unrelated donor | 182 (16.9) | 34 (12.4) | |
| ≤7/8 unrelated donor | 87 (8.1) | 22 (8) | |
| Graft type, n (%) | 1.00 | ||
| BM | 71 (6.6) | 18 (6.6) | |
| PB | 1009 (93.4) | 256 (93.4) | |
| TNC dose infused in BM grafts, × 108/kg recipient body weight, median (range) | 3.1 (1.2-26.3) | 2.9 (1.8-4.6) | .85 |
| CD34+ cell dose infused in PB grafts, × 106/kg recipient body weight, median (range) | 5.3 (1-24.5) | 5.2 (1.1-13.7) | .03 |
CMV indicates cytomegalovirus; TNC, total nucleated cell.
OS
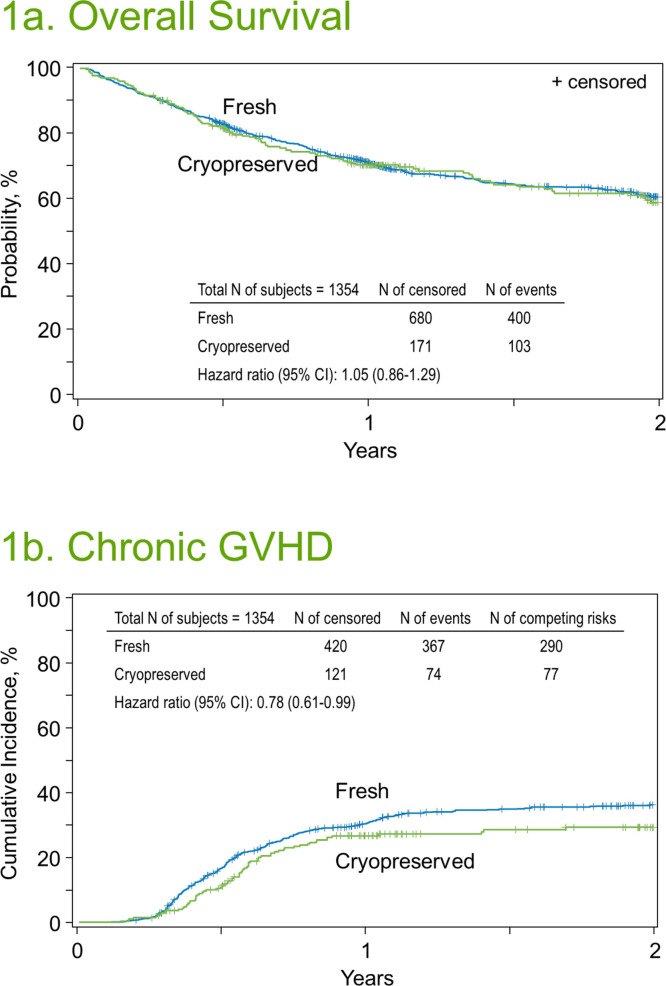
Two-year OS rates were 60.6% (95% CI, 57.3% to 63.8%) in the fresh graft cohort and 58.7% (95% CI, 51.9% to -65.4%) in the cryopreserved graft cohort (P= .62) (Figure 1 A, Table 2 ). In matched pair regression analysis, graft cryopreservation was not associated with a significantly higher risk of mortality (HR for cryopreserved versus fresh, 1.05; 95% CI, .86% to 1.29%; P= .60) (Table 3 ).
Figure 1.
Outcomes of ptCY-based allogeneic HCT with either fresh or cryopreserved grafts. (A) OS. (B) Cumulative incidence of chronic GVHD.
Table 2.
Univariate Outcomes of the Matched Population
| Outcomes | Fresh (N = 1080) | Cryopreserved (N = 274) | |||
|---|---|---|---|---|---|
| n | Prob (95% CI), % | n | Prob (95% CI), % | P Value | |
| Neutrophil recovery | 1075 | 270 | |||
| 28 days | 93.8 (92.3-95.1) | 93.3 (90-96) | .80 | ||
| Platelet recovery | 1076 | 270 | |||
| 100 days | 88.8 (86.8-90.6) | 87.7 (83.4-91.4) | .62 | ||
| Grade II-IV acute GVHD | 1040 | 271 | |||
| 100 days | 31.3 (28.5-34.1) | 34 (28.5-39.8) | .40 | ||
| Grade III-IV acute GVHD | 1040 | 271 | |||
| 100 days | 9.4 (7.7-11.3) | 6.3 (3.7-9.5) | .07 | ||
| Chronic GVHD | 1077 | 272 | |||
| 1 year | 30.7 (27.9-33.5) | 26.8 (21.5-32.5) | .22 | ||
| 2 years | 36.4 (33.4-39.6) | 29.5 (23.8-35.5) | .04 | ||
| Relapse/progression | 1062 | 273 | |||
| 1 year | 24.1 (21.6-26.8) | 24.7 (19.7-30.1) | .85 | ||
| 2 years | 30.7 (27.7-33.7) | 36.3 (29.9-42.9) | .13 | ||
| NRM | 1062 | 273 | |||
| 1 year | 15.8 (13.7-18.1) | 16.9 (12.6-21.7) | .67 | ||
| 2 years | 19 (16.5-21.5) | 22 (16.8-27.7) | .32 | ||
| DFS | 1062 | 273 | |||
| 1 year | 60 (57-63) | 58.4 (52.4-64.3) | .63 | ||
| 2 years | 50.4 (47-53.7) | 41.7 (35-48.6) | .03 | ||
| OS | 1080 | 274 | |||
| 1 year | 71.1 (68.3-73.8) | 70.3 (64.6-75.7) | .81 | ||
| 2 years | 60.6 (57.3-63.8) | 58.7 (51.9-65.4) | .62 | ||
Table 3.
Matched-Pair Analysis by Marginal Cox Model
| Outcomes | Cryopreserved Grafts, HR (95% CI) | Fresh Grafts, HR (Reference) | P Value |
|---|---|---|---|
| Neutrophil recovery | .91 (.80-1.02) | 1 | .12 |
| Platelet recovery | .88 (.78-1.00) | 1 | .05 |
| Grade II-IV acute GVHD | 1.10 (.87 -1.38) | 1 | .43 |
| Grade III-IV acute GVHD | .78 (.50-1.22) | 1 | .27 |
| Chronic GVHD | .78 (.61-.99) | 1 | .04 |
| Relapse/progression | 1.21 (.97-1.50) | 1 | .09 |
| NRM | 1.16 (.86-1.55) | 1 | .32 |
| DFS | 1.19 (1.01 -1.40) | 1 | .04 |
| OS | 1.05 (.86-1.29) | 1 | .60 |
Hematopoietic Recovery and GVHD
The day +28 cumulative incidence of neutrophil recovery was 93.8% (95% CI, 92.3% to 95.1%) in the fresh graft cohort and 93.3% (95% CI, 90% to 96%) in the cryopreserved graft cohort (P = .80) (Table 2). The corresponding median times to neutrophil recovery were 16 days (range, 5 to 69 days) and 17 days (range, 8 to 48 days; P = .05). The respective day +100 cumulative incidences of platelet recovery were 88.8% (95% CI, 86.8% to 90.6%) and 87.7% (95% CI, 83.4% to 91.4%) (P = .62; Table 2). The corresponding median times to platelet recovery were 24 days (range, 1 to 321 days) and 26 days (range, 7 to 351 days; P = .007). In matched-pair regression analysis, graft cryopreservation was not associated with significantly delayed neutrophil recovery (HR, .91; 95% CI, .80 to 1.02; P = .12) or platelet recovery (HR, .88; 95% CI, .78 to 1.00; P = .05; Table 3).
The cumulative incidence of grade II-IV acute GVHD at day +100 (Table 2) was 31.3% (95% CI, 28.5% to 34.1%) in the fresh graft group and 34% (95% CI, 28.5% to 39.8%) in the cryopreserved graft group (P = .40). Corresponding rates of grade III-IV acute GVHD were 9.4% (95% CI, 7.7% to 11.3%) and 6.3% (95% CI, 3.7% to 9.5%) (P = .07). In matched-pair regression analysis (Table 3), the 2 cohorts had similar risks of grade II-IV (HR, 1.10; 95% CI, .87 to 1.38; P = .43) and grade III-IV acute GVHD (HR, .78; 95% CI, .50 to 1.22; P = .27). The cumulative incidence of chronic GVHD at 1 year was 30.7% (95% CI, 27.9% to 33.5%) in the fresh graft cohort and 26.8% (95% CI, 21.5% to 32.5%) in the cryopreserved graft cohort (P = .22; Figure 1B, Table 2). In matched-pair regression analysis (Table 3), cryopreserved grafts were associated with a lower risk of chronic GVHD (HR, .78; 95% CI, .61% to .99%) but this was of only borderline statistical significance (P = .04).
NRM, Relapse/Progression, and DFS
The 2-year rate of NRM was 19.0% (95% CI, 16.5% to 21.5%) in the fresh graft cohort and 22.0% (95% CI, 16.8% to 27.7%) in the cryopreserved graft cohort (P = .32). The corresponding rates of relapse/progression were 30.7% (95% CI, 27.7% to 33.7%) and 36.3% (95% CI, 29.9% to 42.9%) (P = .13), and corresponding rates of DFS were 50.4% (95% CI, 47% to 53.7%) and 41.7% (95% CI, 35% to 48.6%) (P = .03) (Table 2). In matched-pair regression analysis, the HRs for NRM (1.16; 95% CI, .86 to 1.55; P = .32) and relapse/progression (1.21; 95% CI, .97 to 1.50; P = .09) were not statistically significant, whereas the HR for treatment failure (inverse of DFS) was of borderline significance (1.19; 95% CI, 1.01 to 1.29; P = .04) (Table 3).
DISCUSSION
Prospective, randomized data comparing outcomes of ptCY-based allo-HCT using fresh versus cryopreserved grafts are not available. Using the CIBMTR database, we evaluated the 2 approaches retrospectively, using available data to adjust for known covariates. The most important finding of our analysis is that OS out to 2 years was virtually identical with fresh grafts and cryopreserved grafts. Second, there was no evidence of significantly delayed hematopoietic recovery or higher risks of either acute or chronic GVHD with cryopreservation. Marginal increases in relapse/progression and marginal decreases in chronic GVHD and DFS are of uncertain significance given the multiple comparisons in the study and the fact that this was not a randomized study. In fact, a key piece of information was unavailable to us, which is why these grafts were cryopreserved. One can reasonably assume that this was not a random decision. Although some delays might be precipitated by donor scheduling issues, many were likely influenced by clinical events necessitating requiring a delay in transplantation, and these events themselves might be indicators of prognosis (eg, delay because of the need for chemotherapy to achieve better pretransplantation disease control). Given this background, the similar survival outcomes are particularly reassuring.
A rapidly growing body of literature shows good outcomes of ptCY-based allo-HCT in patients with both myeloid 17, 18, 19, 20 and lymphoid malignancies 21, 22, 23, 24, 25, validating the seminal observations from the Johns Hopkins group [20]. Administration of ptCY potentially mitigates the risk of GVHD by targeting alloreactive T cells that are rapidly proliferating early after stem cell infusion, and by relatively sparing regulatory T cells and leaving the nondividing hematopoietic stem and progenitor cells unaffected [20]. Whether the proliferation kinetics of thawed alloreactive T cells different from those of fresh cells is not known. Murine data suggest that freezing and thawing of regulatory T cells results in loss of CD62L expression and a reduced capacity to protect against GVHD [26]. In addition, limited data indicate that cryopreservation can increase the sensitivity of porcine PB mononuclear cells (stimulated by phorbol myristate acetate) for IFN-γ production, but not for IL-6 production [27]. Despite these preclinical observations, our analysis did not reveal any clinically relevant differences in hematopoietic recovery kinetics, acute GVHD risk, or OS between fresh versus cryopreserved grafts for patients undergoing ptCY-based allo-HCT. Limited data in allo-HCT (with non-ptCY-based GVHD prophylaxis) using either BM 2, 3, 4, 5 or PB [6,7] as the graft source also show no impact of cryopreservation on hematopoietic recovery, GVHD, or survival outcomes. Although of uncertain clinical significance, the observations of lower chronic GVHD and DFS warrant further investigation, especially probing the impact on the freeze-thaw cycle on functional profile of immune effector cells. However, in the ongoing COVID-19 pandemic, necessitating cryopreservation of all URD grafts and the majority of related donor products, our data do not show a net safety signal against the use of ptCY-based platforms with frozen products. In this ongoing global outbreak, the ability to cryopreserve allografts has obvious logistical advantages, the most important being the ability to secure a graft before myeloablative therapy in a transplant recipient. Even under normal circumstances, it is sometimes advantageous to ensure the availability of an optimal stem cell dose before the start of conditioning (eg, in the setting of major donor/recipient weight discrepancy and/or advanced donor age).
Our current analysis also underscores the value of observational transplant registries like CIBMTR that can be quickly leveraged to examine critical clinical questions to inform practice and improve patient care, even in unexpected emergencies.
We must acknowledge the limitations of our analysis. Despite propensity score matching, our analysis cannot adjust for unknown clinical parameters influencing the decision to use cryopreservation. We cannot assess the impact of cryopreservation on graft viability (compared with a fresh graft) or examine functional characteristics of thawed immune effector cells. We also acknowledge that chronic GVHD rates across both cohorts in the current analysis are higher than those originally reported with ptCY [20], likely a reflection of increased use of PB grafts in clinical practice [28]. BM grafts were underrepresented in our study, and thus we suggest caution in interpreting these results.
In conclusion, our analysis provides evidence that for patients undergoing ptCY-based allo-HCT, cryopreservation of donor allografts, although not fully understood, appear to be safe and thus suitable for patients during the current worldwide crisis, and perhaps in other settings more broadly.
ACKNOWLEDGMENTS
Financial support: The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children's Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick's Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the US Government.
*Corporate members
Conflict of interest statement: M.H. has received research support/funding from Takeda Pharmaceutical and Spectrum Pharmaceuticals; served as a consultant for Incyte, ADC Therapeutics, Pharmacyclics, Omeros, and Tenebio; has served on speaker's bureaus for AstraZeneca and Sanofi Genzyme. S.C. has received honoraria from Takeda Pharmaceutical (advisory board).
Authorship statement: Study conception and design: M. Hamadani and M. Horowitz; collection and assembly of data: M.-J.Z., X.T., M.F.; data analysis: M.-J.Z., X.T., M.F.; data interpretation: all authors. M.H. wrote the first draft of the manuscript. All authors revised the manuscript and approved the final manuscript for publication.
Footnotes
Financial disclosure: See Acknowledgments on page 1317.
REFERENCES
- 1.Frey N.V., Lazarus H.M., Goldstein S.C. Has allogeneic stem cell cryopreservation been given the “cold shoulder”? An analysis of the pros and cons of using frozen versus fresh stem cell products in allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;38:399–405. doi: 10.1038/sj.bmt.1705462. [DOI] [PubMed] [Google Scholar]
- 2.Eckardt J.R., Roodman G.D., Boldt D.H. Comparison of engraftment and acute GVHD in patients undergoing cryopreserved or fresh allogeneic BMT. Bone Marrow Transplant. 1993;11:125–131. [PubMed] [Google Scholar]
- 3.Stockschläder M., Hassan H.T., Krog C. Long-term follow-up of leukaemia patients after related cryopreserved allogeneic bone marrow transplantation. Br J Haematol. 1997;96:382–386. doi: 10.1046/j.1365-2141.1997.d01-2032.x. [DOI] [PubMed] [Google Scholar]
- 4.Lasky L.C., Van Buren N., Weisdorf D.J. Successful allogeneic cryopreserved marrow transplantation. Transfusion. 1989;29:182–184. doi: 10.1046/j.1537-2995.1989.29289146840.x. [DOI] [PubMed] [Google Scholar]
- 5.Shinkoda Y., Ijichi O., Tanabe T. Identical reconstitution after bone marrow transplantation in twins who received fresh and cryopreserved grafts harvested at the same time from their older brother. Clin Transplant. 2004;18:743–747. doi: 10.1111/j.1399-0012.2004.00273.x. [DOI] [PubMed] [Google Scholar]
- 6.Medd P., Nagra S., Hollyman D., Craddock C., Malladi R. Cryopreservation of allogeneic PBSC from related and unrelated donors is associated with delayed platelet engraftment but has no impact on survival. Bone Marrow Transplant. 2013;48:243–248. doi: 10.1038/bmt.2012.118. [DOI] [PubMed] [Google Scholar]
- 7.Kim D.H., Jamal N., Saragosa R. Similar outcomes of cryopreserved allogeneic peripheral stem cell transplants (PBSCT) compared to fresh allografts. Biol Blood Marrow Transplant. 2007;13:1233–1243. doi: 10.1016/j.bbmt.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Society for Transplantation and Cellular Therapy. ASTCT resources for COVID-19. Available at: https://www.astct.org/communities/public-home?CommunityKey=d3949d84-3440-45f4-8142-90ea05adb0e5. Accessed March 30, 2020.
- 10.National Marrow Donor Program (Be The Match). Response to COVID-19. Available at: https://network.bethematchclinical.org/news/nmdp/be-the-match-response-to-covid-19/. Accessed March 30, 2020.
- 11.Bacigalupo A., Ballen K., Rizzo D. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armand P., Kim H.T., Logan B.R. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–3671. doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Przepiorka D., Weisdorf D., Martin P. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 14.Shulman H.M., Sullivan K.M., Weiden P.L. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X., Loberiza F.R., Klein J.P., Zhang M.J. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88:95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X., Zhang M.J. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Programs Biomed. 2011;101:87–93. doi: 10.1016/j.cmpb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCurdy S.R., Zhang M.J., St Martin A. Effect of donor characteristics on haploidentical transplantation with posttransplantation cyclophosphamide. Blood Adv. 2018;2:299–307. doi: 10.1182/bloodadvances.2017014829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rashidi A., Hamadani M., Zhang M.J. Outcomes of haploidentical vs matched sibling transplantation for acute myeloid leukemia in first complete remission. Blood Adv. 2019;3:1826–1836. doi: 10.1182/bloodadvances.2019000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciurea S.O., Zhang M.J., Bacigalupo A.A. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126:1033–1040. doi: 10.1182/blood-2015-04-639831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luznik L., O'Donnell P.V., Symons H.J. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh N., Karmali R., Rocha V. Reduced-intensity transplantation for lymphomas using haploidentical related donors versus HLA-matched sibling donors: a Center for International Blood and Marrow Transplant Research analysis. J Clin Oncol. 2016;34:3141–3149. doi: 10.1200/JCO.2015.66.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed S., Kanakry J.A., Ahn K.W. Lower graft-versus-host disease and relapse risk in post-transplant cyclophosphamide-based haploidentical versus matched sibling donor reduced-intensity conditioning transplant for Hodgkin lymphoma. Biol Blood Marrow Transplant. 2019;25:1859–1868. doi: 10.1016/j.bbmt.2019.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietrich S., Finel H., Martinez C. Post-transplant cyclophosphamide-based haplo-identical transplantation as alternative to matched sibling or unrelated donor transplantation for non-Hodgkin lymphoma: a registry study by the European Society for Blood and Marrow Transplantation. Leukemia. 2016;30:2086–2089. doi: 10.1038/leu.2016.125. [DOI] [PubMed] [Google Scholar]
- 24.Dreger P., Sureda A., Ahn K.W. PTCy-based haploidentical vs matched related or unrelated donor reduced-intensity conditioning transplant for DLBCL. Blood Adv. 2019;3:360–369. doi: 10.1182/bloodadvances.2018027748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanate A.S., Mussetti A., Kharfan-Dabaja M.A. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood. 2016;127:938–947. doi: 10.1182/blood-2015-09-671834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Florek M., Schneidawind D., Pierini A. Freeze and thaw of CD4+CD25+Foxp3+ regulatory T cells results in loss of CD62L expression and a reduced capacity to protect against graft-versus-host disease. PLoS One. 2015;10 doi: 10.1371/journal.pone.0145763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X., Zhong Z., Liang S., Wang X., Zhong F. Effect of cryopreservation on IL-4, IFN-gamma, and IL-6 production of porcine peripheral blood lymphocytes. Cryobiology. 2009;59:322–326. doi: 10.1016/j.cryobiol.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Bashey A., Zhang M.J., McCurdy S.R. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell-replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017;35:3002–3009. doi: 10.1200/JCO.2017.72.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]